1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57
z
Biological Chemistry & Chemical Biology
Rapid, Efficient, and Green Synthesis of Coumarin
Derivatives via Knoevenagel Condensation and
Investigating Their Biological Effects
Leila Dinparast,
[a]Salar Hemmati,
[b]Gokhan Zengin,
[c]Ali Akbar Alizadeh,
[a]Mir Babak Bahadori,
[d]Hossein Samadi Kafil,
[b]and Siavoush Dastmalchi*
[a, e, f] Coumarins as naturally occurring heterocycles are chemicallyand biologically attractive compounds due to their diverse pharmacological properties. This study aimed to design a green method for the synthesis of coumarin derivatives followed by their biological investigations. To do so, coumarins were synthesized with excellent yields using a one-pot procedure under solvent-free conditions at room temperature with very short reaction times. 1-hexyl-3-methylimidazolium bromide was used as a reaction medium and an alternative for common toxic solvents. The structure of coumarins was confirmed using spectroscopic techniques as well as elemental analysis. The
cytotoxicity of coumarins was evaluated against A549 cancer-ous cells and was found to be non-cytotoxic in nature. Also, their abilities for inhibition of acetylcholinesterase, tyrosinase, and α-glucosidase were assessed. The results showed that some derivatives have mild to moderate inhibitory activity (IC50=3.64-5.96 mm) against acetylcholinesterase. The tested
coumarins have also moderately inhibited tyrosinase (IC50=
3.95-13.96 mm). The results of this study could be useful for the design and development of green and effective methods for the synthesis of new drugs with the core structure of coumarin.
Introduction
Coumarins (2H-chromen-2-one) are important heterocyclic compounds which consist of fused benzene and α-pyrone rings (Scheme 1). Both of synthetic and natural coumarins are valuable structures in drug design and development, due to broad spectrum of biological properties including anti-HIV,[1]
antimicrobial,[2]
anticancer,[3]
anti-inflammatory,[4]
anti-Alzheimer,[5]and antidepressant[6]activities. Several well-known
natural and synthetic drugs with the core structure of coumarin are used clinically (Scheme 2). Also, such structures are used in cosmetics products,[7] food industries,[8] laser dyes,[9]
lumines-cent materials,[10]
as well as florescent dyes and probes.[11]
Various synthetic routs have been reported for the syn-thesis of coumarin derivatives such as Pechmann,[12]Perkin,[13]
[a] L. Dinparast, A. A. Alizadeh, S. Dastmalchi
Biotechnology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
E-mail: dastmalchi.s@tbzmed.ac.ir [b] S. Hemmati, H. S. Kafil
Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
[c] G. Zengin
Department of Biology, Science Faculty, Selcuk University, Konya, Turkey [d] M. B. Bahadori
Medicinal Plants Research Center, Maragheh University of Medical Sciences, Maragheh, Iran
[e] S. Dastmalchi
School of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran [f] S. Dastmalchi
Faculty of Pharmacy, Near East University, POBOX:99138, Nicosia, North Cyprus, Mersin 10, Turkey
Supporting information for this article is available on the WWW under https://doi.org/10.1002/slct.201901921
Scheme 1. Coumarin
Scheme 2. Structures of clinically used well-known natural and synthetic drugs with coumarin scaffold.
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 Knoevenagel,[14] Reformatsky,[15] Heck,[16] and Wittig[17] reactions. Despite the existence of several publications which report the synthetic procedures for coumarins, still there is a considerable demand for designing of efficient, rapid, one-pot, one-step, solvent-free, and environmentally benign methodologies for the synthesis of such valuable compounds with high biological and industrial potential. In this context, shorter reaction times as well as easy work-up and purification steps are some of the advantages for the solvent-free methods.[18]
Design of new compounds to inhibit key enzymes is one of the important techniques for the development of effective treatment or prevention of disorders such as diabetes mellitus (α-glucosidase and α-amylase), Alzheimer’s disease (choliester-ases), skin disorders (tyrosinase), inflammation (cyclooxyge-nase), and obesity (lipase).[19]
Nowadays, one of the critical health problems in the world is cancer.[20]
Although there are numerous reports about the synthetic and natural anti-cancer drugs, the investigation for the design, discovery, and synthesis of potent anti-cancer drugs free from the problems associated with the current commercial drugs including toxicity and drug resistance is warranted.
Here, the effective, rapid, one-pot, and solvent-free syn-thesis of coumarins (oxo-2H-chromene-3-carboxylate and 2-oxo-2H-chromene-3-carbonitrile derivatives) in the presence of ionic liquid is reported. These important heterocycles were synthesized using a green method in excellent yields at room temperature via Knoevenagel reaction. Some bioassays were performed to shed light on their biological effects. In this regard, the antiproliferative activity of coumarins was evaluated on A549 cancerous cells. Also, the enzyme inhibitory activity, antioxidant, and antimicrobial effects of these compounds were studied. The results of the current work could be useful for future drug design studies, green synthesis, and pharmaco-logical investigations on coumarin derivatives.
Results and Discussion
1. Chemistry
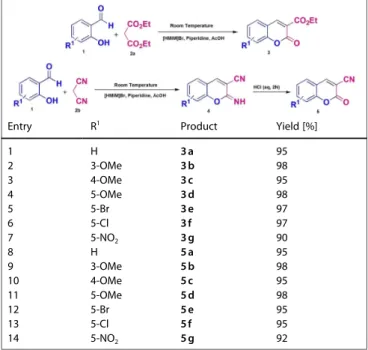
To find the optimized reaction condition for the synthesis of the reported compounds (3 a-3 g and 5 a-5 g), 2-hydroxy-3-methoxy benzaldehyde (1), and diethyl malonate (2 a)/malono-nitrile (2 b) were reacted in various conditions. As a model, 2-hydroxy-3-methoxy benzaldehyde (1, 1 mmol) and diethyl malonate (2 a) or malononitrile (2 b, 1 mmol) were mixed in absolute ethanol. Then, the catalytic amount of piperidine and acetic acid were added to the reaction mixture at room temperature. The progress of reactions was followed by TLC. The reactions were proceed very slow. Work-up of the reaction mixture after 2 h, afforded the compounds 3 b and 5 b with 30– 40% yields. Moreover, the formation of by-products was observed in this reaction condition. Study of these reactions at higher temperatures showed that just the yields of products were increased. Base on the our previous experiences about the use of ionic liquids as an effective reaction medium, 1-hexyl-3-methylimidazolium bromide ([HMIM]Br) was used for the synthesis of coumarins. The reactions were tried in different
conditions using [HMIM]Br. Interestingly, in the presence of the mentioned ionic liquid, the reactions were proceed very rapidly at room temperature and also, the products were formed in excellent yields (> 90%) immediately after addition of salicylal-dehyde. It should be noted that the best yield of product was achieved using 1.2/1 molar ratios of diethyl malonate (2 a)/ malononitrile (2 b) to salicylaldehyde derivatives. However, the optimized reaction conditions for the synthesis of coumarins (3 a-3 g and 5 a-5 g) have been mentioned in details at the experimental section. The synthetic rout for compounds 3 a-3 g and 5 a-5 g was represented in Table 1. It should be noted that,
from the cyclization between salicylaldehyde derivatives (1) and malononitrile (2 b), the iminocoumarin derivatives were produced (4 a-4 g) with excellent yields (near to 100%). The iminocoumarins were hydrolyzed by acidic extraction in work-up step (as mentioned in experimental section) to produce coumarin derivatives (5 a-5 g).
2. Cytotoxicity
Literature survey shows the existence of several publications which report the anticancer properties of both synthetic and natural coumarins.[21] Despite the existence of numerous
anticancer drugs, still there is a considerable demand for the discovery of new therapeutic anticancer agents with high efficiency and limited side effects. To this aim, the antiprolifer-ative properties of coumarins presented in this work were evaluated using MMT assay. Human lung cancer cells (A549) was treated by the studied compounds and the results of the assay did not reveal any significant cytotoxic effects at the used concentrations.
Table 1. Green synthesis of coumarin derivatives in the presence of ionic liquid at room temperature via Knoevenagel condensation.
Entry R1 Product Yield [%]
1 H 3 a 95 2 3-OMe 3 b 98 3 4-OMe 3 c 95 4 5-OMe 3 d 98 5 5-Br 3 e 97 6 5-Cl 3 f 97 7 5-NO2 3 g 90 8 H 5 a 95 9 3-OMe 5 b 98 10 4-OMe 5 c 95 11 5-OMe 5 d 98 12 5-Br 5 e 95 13 5-Cl 5 f 95 14 5-NO2 5 g 92
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57
3. Acetylcholinesterase inhibition assay
Alzheimer’s disease is known as a progressive neurodegener-ative disorder that affects aged people and causes loss of memory and decreases the cognitive abilities. The inhibition of acetyl/butyrylcholinesterase is one of the successful therapeutic strategies for the treatment of Alzheimer’s disease. It should be mentioned that among the developed various pharmaceuticals as AChE inhibitors such as donepezil, rivastigmine, galant-amine, ensaculine, and propidium, and tacrine, there is at least one drug, namely ensaculine, which encompasses the coumar-in coumar-in its structure. Moreover, several reports demonstrate the inhibitory effects of coumarins against AChE.[5,22]
In the present work, 14 coumarins were evaluated for their AChE inhibitory effects. As shown in Table 2, four out of 14 coumarins showed
moderate AChE inhibitory activity (IC50=3.64-5.96 mm)
com-pared with galantamine as a standard drug (IC50=0.01 mm),
but the rest of the compounds were inactive. Ethyl 6-bromo-2-oxo-2H-chromene-3-carboxylate (3 e) was the most active coumarin in this respect. Based on the obtained results, the correlation between the observed activities and the responsible structural features in the structures of the active coumarins was not clearly apparent. Preparing more derivatives with diverse structural moieties can help to elucidate structure activity relationship for the AChE inhibitory activity of these com-pounds.
4. Tyrosinase inhibition assay
Tyrosinase inhibitors are clinically used as significant therapeu-tic agents for skin diseases. Moreover, these compounds are attractive agents in cosmetic industries, food sciences, and agriculture.[23] Several synthetic and natural tyrosinase
inhib-itors were reported in literatures, but due to the safety
problems just few of them are used clinically as a drug, in cosmetic and food industry, as well as agriculture.[24]
On the other hand, there is a demand for design, discovery, and synthesis of novel effective tyrosinase inhibitors with limited side effects. The synthesized coumarins (3 a-3 g and 5 a-5 g) were investigated for their tyrosinase inhibitory activity. The IC50 values obtained for these compounds are represented in
Table 2. Compared with the kojic acid as a positive control (IC50
= 0.91 mm), coumarins showed moderate inhibitory activity against tyrosinase (3.95-13.96 mm). The most active compound was 6-bromo-2-oxo-2H-chromene-3-carbonitrile (5 e) among the tested coumarins. The obtained results could be related to the structure of tyrosinase active site which composed of two copper atoms. The ability of target compound in formation of complex with these copper atoms may be causes to tyrosinase lose its catalyzing activity. In a recent study, tyrosinase inhibitory potential of some synthesized coumarin derivatives were evaluated.[25]
The results showed that ethyl 2-oxo-2H-chromene-3-caboxylate derivatives have low or no activities for inhibition of tyrosinase. But, the most potent tyrosinase inhibitory potential was observed for 2-(1-(coumarin-3-yl) ethylidene)hydrazinecarbothioamide derivatives bearing thio-semicarbazide group (IC50= 3.44-114.68 μm). They suggested
that those activities might be related to the existence of sulfur atom in the structure of these compounds which could be chelated and formed the complex with the copper atoms in the active site.
5. α-Glucosidase inhibition assay
One of the important human health problems is diabetes mellitus which is growing in the whole world. Control of the blood glucose level of diabetic patients could be performed by the inhibition of carbohydrate-hydrolyzing enzymes such as α-glucosidase and α-amylase. α-Glucosidase inhibitory activity of several synthetic and natural coumarin derivatives was re-ported in the literatures. Zhao et al. evaluated the α-amylase and α-glucosidase inhibitory activity of two monomers, five dimers, and one trimer of coumarins isolated from the flowers of Edgeworthia gardneri.[17] The obtained IC
50values for these
coumarins were in the range of 18.7-780 μg/mL. In another study, a series (44 compounds) of 3-aryl coumarins were synthesized and evaluated for their α-glucosidase inhibition activity.[26] The results showed that only 8 compounds have
moderate to excellent (1.37-16.39 μm) inhibitory activity com-pared with acarbose (0.05 μm) as a standard drug. In the present study, for investigation of anti-diabetic properties of coumarins, the α-glucosidase inhibitory activity of these compounds was demonstrated. The obtained results showed that these coumarins could not inhibit the α-glucosidase activity.
6. Antimicrobial assay
The antimicrobial properties of coumarins were assessed against both gram-positive and gram-negative bacteria and also, fungal strains by disc diffusion method. The obtained Table 2. Acetylcholinesterase (AChE), tyrosinase (TYR), and α-glucosidase
(GLU) inhibitory activities of the synthesized coumarins (mm � SEM).
Entry Compound AChE TYR GLU
1 3 a 4.72 � 0.04 13.42 � 0.41 na 2 3 b na[a] -[c] na 3 3 c na 7.13 � 0.16 na 4 3 d na 10.40 � 0.80 na 5 3 e 3.64 � 0.10 6.14 � 0.27 na 6 3 f na 12.50 � 0.03 na 7 3 g na 10.33 � 0.22 na 8 5 a na 5.90 � 0.17 na 9 5 b 5.86 � 1.14 13.96 � 4.02 na 10 5 c na 4.67 � 0.54 na 11 5 d na 7.45 � 0.74 na 12 5 e 5.96 � 2.16 3.95 � 0.27 na 13 5 f na 6.12 � 0.34 na 14 5 g na 4.95 � 0.09 na 15 Galantamine 0.01 � 0.003 nt nt 16 Kojic acid nt[b] 0.91 � 0.07 nt 17 Acarbose nt nt 7.90 � 0.03
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57
results of this investigations revealed that these compounds do not have any antibacterial and antifungal activity against all the tested microorganisms. Previously, in vitro antimicrobial activity of the novel coumarin-1,2,3-triazole conjugates was tested against the yeast and both positive as well as gram-negative bacteria strains by Maslyk et al..[27]
They reported that most of the tested coumarin-triazole derivatives have not considerable antimicrobial activity against the tested micro-organisms and just 6 out of 26 compounds showed significant antibacterial activity against Enterococcus faecalis (MIC = 12.5-50.0 μg/mL). In another study, Gali et al. synthesized coumarin derivatives and investigated their antimicrobial properties.[28]
All of the tested compounds had the moderate antibacterial activity (MIC = 150 μg/mL), but did not display antifungal property.
7. Antioxidant activity
Oxidant agents and radicals are responsible for oxidative stress and many diseases in human body. On the other hand, antioxidant and radical scavengers are helpful agents that protect the body against these compounds. In this study, the synthesized coumarins were subjected to further antiradical evaluation using DPPH assay. Any activity was not observed for coumarin derivatives in this assay. The observed results might be related to the absence of phenolic hydroxyl groups on the structure of tested coumarins. Prenyloxy coumarin derivatives were synthesized and evaluated for their antioxidant activities using DPPH assay by Kavetsou et al..[29]
They also reported that the tested coumarins did not have any significant antiradical activity.
Conclusions
Green synthesis of a series of coumarin derivatives using ionic liquid was reported. The mild reaction condition, excellent yields, avoiding toxic solvents, one-pot procedure, and easy work-up and purification process were some of the advantages of the presented method for synthesis of coumarins. Surpris-ingly, the reactions proceed very fast in the presence of ionic liquid at room temperature. These coumarin compounds were evaluated for their possible bioactivities for the first time. The antiproliferative activities of the coumarins were evaluated against A549 cells. Also, the enzyme inhibitory assays were performed to investigate the capacity of coumarins for inhibition of acetylcholinesterase, tyrosinase, and α-glucosi-dase. Compared with the standard drug, moderate acetylcholi-nesterase inhibitory activity was detected for some derivatives. Also, the derivatives showed moderate inhibitory activities on tyrosinase. Antimicrobial and antioxidant assays revealed that this series of coumarins have no significant antibacterial, antifungal, and antiradical properties.
Supporting Information Summary
The Experimental section and spectral data of synthesized compounds are given in Supporting Information.
Acknowledgements
The authors would like to acknowledge the Ministry of Health and Medical Education, and also, Biotechnology Research Center at Tabriz University of Medical Sciences for the financial support.
Conflict of Interest
The authors declare no conflict of interest.
Keywords: Biological activity · Coumarin · Enzyme inhibition ·
Green chemistry · Ionic liquid
[1] A. M. Hamdy, Z. Khaddour, N. A. Al-Masoudi, Q. Rahman, C. Hering-Junghans, A. Villinger, P. Langer, Biorg. Med. Chem. 2016, 24, 5115–5126. [2] S. N. Mangasuli, K. M. Hosamani, H. C. Devarajegowda, M. M. Kurjogi,
S. D. Joshi, Eur. J. Med. Chem. 2018, 146, 747–756.
[3] A. Thakur, R. Singla, V. Jaitak, Eur. J. Med. Chem. 2015, 101, 476–495. [4] H. N. Lv, S. Wang, K. W. Zeng, J. Li, X. Y. Guo, D. Ferreira, J. K. Zjawiony,
P.-F. Tu, Y. Jiang, J. Nat. Prod. 2015, 78, 279–285.
[5] S. Singla, P. Piplani, Biorg. Med. Chem. 2016, 24, 4587–4599.
[6] K. V. Sashidhara, R. K. Modukuri, S. Singh, K. B. Rao, G. A. Teja, S. Gupta, S. Shukla, Bioorg. Med. Chem. Lett. 2015, 25, 337–341.
[7] a) K. Kinga, E.-P. Anna, B. Elzbieta, Lett. Drug Des. Discov. 2016, 13, 465– 474; b) B.-C. Lee, S. Y. Lee, H. J. Lee, G.-S. Sim, J.-H. Kim, J.-H. Kim, Y.-H. Cho, D.-H. Lee, H.-B. Pyo, T.-B. Choe, D. C. Moon, Y. P. Yun, J. T. Hong,
Arch. Pharmacal Res. 2007, 30, 1293.
[8] Y. H. Wang, B. Avula, N. D. Nanayakkara, J. Zhao, I. A. Khan, J. Agric. Food
Chem. 2013, 61, 4470–4476.
[9] I. Esnal, G. Duran-Sampedro, A. Agarrabeitia, J. Bañuelos, I. García-Moreno, M. Macías, E. Peña-Cabrera, I. López-Arbeloa, S. De La Moya, M. J. Ortiz, PCCP 2015, 17, 8239–8247.
[10] S. J. Bullock, C. E. Felton, R. V. Fennessy, L. P. Harding, M. Andrews, S. J. Pope, C. R. Rice, T. Riis-Johannessen, Dalton T. 2009, 10570–10573. [11] aJ. Li, C.-F. Zhang, S.-H. Yang, W.-C. Yang, G.-F. Yang, Anal. Chem. 2014,
86, 3037–3042; bR. M. Christie, K. M. Morgan, M. S. Islam, Dyes Pigments
2008, 76, 741–747.
[12] a) S. A. Popova, O. G. Shevchenko, I. Y. Chukicheva, A. V. Kutchin, Chem.
Biodivers. 2019, 16, e1800317; b) A. S Zambare, F. A Kalam Khan, S. P
Zambare, S. D Shinde, J. N Sangshetti, Curr. Org. Chem. 2016, 20, 798– 828.
[13] J. K. Augustine, A. Bombrun, B. Ramappa, C. Boodappa, Tetrahedron
Letters 2012, 53, 4422–4425.
[14] a) M. R. Shakil, A. G. Meguerdichian, H. Tasnim, A. Shirazi-Amin, M. S. Seraji, S. L. Suib, Inorg. Chem. 2019, 58, 5703–5714; b) R. H. Vekariya, H. D. Patel, Synth. Commun. 2014, 44, 2756–2788.
[15] B. Zhao, M. j. Fan, Z. Liu, L.-f. Hu, B. Song, L. y. Wang, Q. g. Deng, J.
Chem. Res. 2012, 36, 393.
[16] D. A. Barancelli, A. G. Salles Jr, J. G. Taylor, C. R. D. Correia, Org. Lett. 2012, 14, 6036–6039.
[17] D. G. Zhao, A.-Y. Zhou, Z. Du, Y. Zhang, K. Zhang, Y. Y. Ma, Fitoterapia 2015, 107, 122–127.
[18] a) H. Valizadeh, L. Dinparast, S. Noorshargh, M. M. Heravi, CR. CHIM. 2016, 19, 395–402; b) L. Dinparast, H. Valizadeh, MONATSH. CHEM. 2015,
146, 313–319.
[19] a) B. Asghari, S. Mafakheri, M. Zarrabi, S. Erdem, I. Orhan, M. Bahadori, S.
Afr. J. Bot. 2019, 120, 191–197; b) M. B. Bahadori, B. Kirkan, C. Sarikurkcu,
O. Ceylan, Ind. Crop. Prod. 2019, 131, 85–89; c) M. B. Bahadori, L. Dinparast, G. Zengin, C. Sarikurkcu, S. Bahadori, B. Asghari, N. Movahhedin, Int. J. Food Prop. 2017, 20, 1761–1772; d) L. Dinparast, H. Valizadeh, M. B. Bahadori, S. Soltani, B. Asghari, M.-R. Rashidi, J. Mol.
Struct. 2016, 1114, 84–94.
[20] a) M. B. Bahadori, M. Eskandani, M. De Mieri, M. Hamburger, H. Nazemiyeh, Food Chem. Toxicol. 2018, 120, 155–163; b) H. Hamishehkar, M. B. Bahadori, S. Vandghanooni, M. Eskandani, A. Nakhlband, M. Eskandani, J. Drug Deliv. Sci. Technol. 2018, 45, 272–280.
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57
[21] a) S. S. Abd El-Karim, Y. M. Syam, A. M. El Kerdawy, T. M. Abdelghany,
Bioorg. Chem. 2019, 86, 80–96; b) M. Kumar, R. Singla, J. Dandriyal, V.
Jaitak, Anti-Cancer Agents Med. Chem. 2018, 18, 964–984; c) D. Cao, Y. Liu, W. Yan, C. Wang, P. Bai, T. Wang, M. Tang, X. Wang, Z. Yang, B. Ma, J.
Med. Chem. 2016, 59, 5721–5739.
[22] a) L. G. de Souza, M. N. Rennó, J. D. Figueroa-Villar, Chem. Biol. Interact. 2016, 254, 11–23; b) P. Anand, B. Singh, N. Singh, Biorg. Med. Chem. 2012, 20, 1175–1180.
[23] S. Parvez, M. Kang, H. S. Chung, H. Bae, Phytother. Res. 2007, 21, 805– 816.
[24] S. Y. Lee, N. Baek, T.-g. Nam, J. Enzyme inhib. Med. Chem. 2016, 31, 1–13. [25] J. Liu, F. Wu, L. Chen, L. Zhao, Z. Zhao, M. Wang, S. Lei, Food Chem.
2012, 135, 2872–2878.
[26] Y. Hu, B. Wang, J. Yang, T. Liu, J. Sun, X. Wang, J. Enzyme Inhib. Med.
Chem. 2019, 34, 15–30.
[27] P. López-Rojas, M. Janeczko, K. Kubiński, Á. Amesty, M. Masłyk, A. Estévez-Braun, Molecules 2018, 23, 199.
[28] R. Gali, J. Banothu, R. Bavantula, J. Heterocycl. Chem. 2015, 52, 641–646. [29] E. Kavetsou, L. Gkionis, G. Galani, C. Gkolfinopoulou, L. Argyri, E. Pontiki, A. Chroni, D. Hadjipavlou-Litina, A. Detsi, Med. Chem. Res. 2017, 26, 856– 866.
Submitted: May 27, 2019 Accepted: August 5, 2019