Suppressor capacity of copper
nanoparticles biosynthesized using
Crocus sativus L. leaf aqueous
extract on methadone‑induced
cell death in adrenal
phaeochromocytoma (PC12) cell
line
peng Zhang
1, Jian cui
2*, Shirin Mansooridara
3, Atoosa Shahriyari Kalantari
4,
Akram Zangeneh
5,6, Mohammad Mahdi Zangeneh
5,6, nastaran Sadeghian
7, parham taslimi
8,
Ramazan Bayat
9& Fatih Şen
9in this research, we prepared and formulated a neuroprotective supplement (copper nanoparticles in aqueous medium utilizing Crocus sativus L. Leaf aqueous extract) for determining its potential against methadone-induced cell death in PC12. The results of chemical characterization tests i.e., fe‑SeM, ft‑iR, XRD, eDX, teM, and UV–Vis spectroscopy revealed that the study showed that copper nanoparticles were synthesized in the perfect way possible. in the teM and fe‑SeM images, the copper nanoparticles were in the mean size of 27.5 nm with the spherical shape. In the biological part of the present research, the Rat inflammatory cytokine assay kit was used to measure the concentrations of inflammatory cytokines. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) test was used to show DNA fragmentation and apoptosis. Caspase-3 activity was assessed by the caspase activity colorimetric assay kit and mitochondrial membrane potential was studied by Rhodamine123 fluorescence dye. Also, the cell viability of PC12 was measured by trypan blue assay. Copper nanoparticles-treated cell cutlers significantly (p ≤ 0.01) decreased the inflammatory cytokines concentrations, caspase-3 activity, and DNA fragmentation and they raised the cell viability and mitochondrial membrane potential in the high concentration of methadone‑ treated PC12 cells. The best result of neuroprotective properties was seen in the high dose of copper nanoparticles i.e., 4 µg. According to the above results, copper nanoparticles containing C. sativus leaf aqueous extract can be used in peripheral nervous system treatment as a neuroprotective promoter and central nervous system after approving in the clinical trial studies in humans.
open
1Department of Neurosurgery, Henan Provincial People’s Hospital, People’s Hospital of Zhengzhou University,
Medical College of Henan University, Zhengzhou 450003, Henan, China. 2Department of Neurosurgery, Xi’an
No. 1 Hospital, No. 30 South Street Powder Lane, Beilin District, Xi’an 710002, Shaanxi, China. 3Medical
Sciences Research Center, Faculty of Medicine, Tehran Medical Sciences Branch, Islamic Azad University, Tehran, Iran. 4Department of Neurology, Faculty of Medicine, Tehran Medical Sciences, Islamic Azad University,
Tehran, Iran. 5Department of Clinical Sciences, Faculty of Veterinary Medicine, Razi University, Kermanshah,
Iran. 6Biotechnology and Medicinal Plants Research Center, Ilam University of Medical Sciences, Ilam,
Iran. 7Department of Chemistry, Faculty of Science, Atatürk University, 25240 Erzurum, Turkey. 8Department of
Biotechnology, Faculty of Science, Bartin University, 74100 Bartin, Turkey. 9Sen Research Group, Department of
www.nature.com/scientificreports/
In the last two decades, attention to nanomaterials, especially metal nanomaterials, has grown significantly due to their interesting and unique properties1–5. The properties of nanoparticles in nano sizes have created unique features that have made their application in various sciences attractive6–10. Meanwhile, metal oxide nanomaterials, especially copper oxide nanoparticles, have shown more unique properties, which has increased their use in vari-ous sciences11–15. The demand for conventional healthcare treatment is rising every day. Iranian traditional medi-cine is one of the traditional and accepted methods of conventional medimedi-cine all over the world16–22. Numerous medicinal supplements and drugs are developed per annum and manufactured from traditional medicinal plants. One of these plants is Crocus sativus L. C. sativus is from Plantae kingdom, Magnoliophyta division, Liliopsida class, Asparagales order, Iridaceae family, and Crocus genus23. The most antioxidant compounds of C. sativus are crocin, picrocrocin, and safranal. C. sativus is used in the medicine for its antioxidant, anti-inflammatory, antinociceptive, antibacterial, antifungal, antiviral, anti-parasitic, gastro-protective, entro-protective, hepato-protective, nephron-hepato-protective, spleno-hepato-protective, anticancer, and especially anxiolytic and relaxant properties. As a neuroprotective supplement, C. sativus is involved in the treatment of nervous disorders in conventional medicine23. An in vitro study displayed the inhibitory impact of C. sativus on amyloid beta-peptide fibrillogenesis and its defensive function against the toxicity of H2O2-triggered human neuroblastoma cells24. In addition, C.
sativus was administered for one week to normal and aged mice (60 mg/kg body weight), and markedly increased memory and learning25. In addition, in vitro studies in amnesic and ischemic rat models have verified the neu-roprotective effects of C. sativus and its components26. C. sativus has been declared to inhibit peroxidized lipid formation in cultured adrenal pheochromocytoma (PC12) cells, in moderation restored superoxide dismutase (SOD) activity and preserved morphology of neurons. Although C. sativus antioxidant impact was comparable to that of α-tocopherol, at certain concentrations it was much more prominent25.
Methadone is an artificial, long-acting opioid with qualitatively similar morphine-like pharmacological actions and is active through oral and parenteral administration. Methadone is a small molecule that contains hydrophobic and hydrophilic units. Methadone can be considered a small amphipathic agent for mitochondria which acts as an ion carrier26,27. N-methyl-d-aspartate (NMDR) and μ opioid receptors are considered to have a greater impact on the treatment of chronic pain and neuropathic pain caused by the activity of methadone28–32. In a study on the SH-SY5Y cell line, this undifferentiated human neuroblastoma cell line led to severe cell deaths using methadone at high concentrations33. Increasing the production of free radicals and increasing the amount of calcium affect increasing cell deaths. Methadone does not directly produce reactive oxygen species, however, an increment in intracellular calcium can cause toxicity26. The decomposition activity of methadone may result from the ability of small ions to transfer to the phospholipid membrane. In addition, methadone, like other opioids, has been shown to leak into the membranes of lipophlipids made up of phospholipids and form pores27,34. Methadone-exposed cells lie behind the mitochondrial outer membrane and BAX transfer ’s high permeability and also release cytochrome C from mitochondria33. A study conducted on rat liver mitochondria that have been treated using methadone, methadone induced mitochondrial disruption and also showed that it has the ability ions transferred, therefore, it causes cellular death due to remarkably reducing cellular ATP levels35,36. One group of materials that can remove the neurotoxicity activities of the psychedelic drugs such as methadone is the metallic nanoparticles synthesized by plants37. Recently, scientists have used the neuroprotec-tive potentials of medicinal plants for synthesizing the metallic nanoparticles containing natural compounds. So far, the neuroprotective effects of Salvia Officinalis, Hypericum perforatum, Lavandula angustifolia, Opuntia ficus-indica, Curculigo orchioides, Ficus religiosa, Angelica sinensis, Cassia fistula, Dichrostachys cinerea, Panax ginseng, Aerva lanata, Juglans regai, Crocus sativus, Pongamia pinnata, Polygala paniculata, Cipura paludosa, Carum carvi L., Cymbopogon winteri-anus, Mentha spicata L., Cassia siamea, Galanthus nivalis L., Thymus vulgaris L., and Curcuma longa have been proved38.
Attention to nanotechnology and synthesis of different types of nanomaterials increase in recent years2,4–6,39. This increasing is relative to unique properties and wide range application of nanomaterails7–9,13,40. In between, attention to metal oxide nanoparticles show more interest between researchers due to large surface area and catalytic activity10–12,41,42. Copper nanoparticles (CuNPs) have a special role between all metallic nanoparticles39,40. CuNPs have been recently described as having significant neuroprotective potential and being able to treat the central and peripheral nervous systems37. One study investigated the positive activity of CuNPs on cerebral microvascular endothelial cells of rats. Previous research, copper nanoparticles 1.56–50 µg/ml exposing at con-centrations increases proliferation of rat cerebral microvascular endothelial cells. Copper nanoparticles (40 and 60 nm) the release of prostaglandin E2 was increased noteworthy manner (3 times, 8 h). There were no changes in the extracellular levels of both IL-1b and TNF-a after exposure to copper nanoparticles Rat brain as an indi-cation of increased permeability of the microvascular endothelial cells, P-an aspect ratio greater than about twofold for copper nanoparticles37. In addition, neurotoxic agents in the peripheral nervous system and central nervous system may be removed by copper nanoparticles37. About the neuroprotective properties of CuNPs green-synthesized by plants, the neuroprotective effects of Achillea biebersteinii leaf aqueous extract synthesized CuNPs against methamphetamine-induced cell death in PC12 were investigated by Wang et al. (2020). They indicated when copper salt is combined with plant extract, herbal nanoparticle with significant antioxidant activities is synthesized and probably neuroprotective properties of copper nanoparticles green-synthesized by plants are related to these antioxidant effects43.
Based on the neuroprotective properties of C. sativus and CuNPs, we have attempted to determine the CuNPs effects comprising C. sativus leaf aqueous extract on cellular and molecular variables like viability and cell death index in methadone-induced PC12 cell death. In these cells, the activity of caspase-3, inflammatory cytokine concentration, and mitochondrial membrane potential were also examined to effectively figure out the function-ing mechanism of the CuNPs.
experimental
Material.
Antimycotic antibiotic solution, decamplmaneh fetal bovine serum, Dulbecco’s Modified Eagle Medium (DMED), carbazole reagent, 2,2-diphenyl-1-(2,4,6-trinitrophenyl)hydrazyl (DPPH), 4-(Dimethyl-amino)benzaldehyde, phosphate buffer solution (PBS), borax-sulphuric acid mixture, Ehrlich solution, hydro-lysate, and dimethyl sulfoxide (DMSO), all of this was obtained from Sigma-Aldrich, USA.Synthesis of copper nanoparticles.
The C. sativus used in this study was obtained in Kermanshah, Iran, at the elevation of 1,400 m hereinabove sea level, in spring 2019, after it was identified. Extraction was obtained by dissolving 250 g of C. sativus leaf powder in 2,500 mL of distilled water and holding it in an orbital shaker for 48 h. It then concentrated the extract in a rotary evaporator. In addition, galenic extracts were lyophilized for 24 h at − 48 °C and stored for later use37. With a 20 mL of Cu(NO3)2·3H2O reaction mixture in the concentration
of 0.05 M and 200 mL of aqueous extract solution of C. sativus leaf (20 μg/mL) in the proportion 1:10 in a coni-cal flask, the green synthesis of the copper nanoparticles was launched. The solution mixture was held at room temperature under a magnetic stirring for 12 h. The dark green colored solution of Cu was produced at last time of reaction. The mixture obtained at the end of the reaction was centrifuged at 10 × 103 rpm for 15 min. The
sedi-ment was washed with water at three times, then centrifuged44,45.
The that organic chemistry techniques, i.e., for the study of CuNPs FE-SEM, FT-IR, TEM, and UV–Vis spec-troscopy was used. TEM and FE-SEM microscopic methods analyzed the morphological properties of CuNPs in terms of shape and size. In addition, CuNPs were primarily tested utilizing UV–Vis spectroscopy at a scanning range of 450–750 nm of the FT-IR spectrophotometer wavelength to classify biomolecules involved in CuNP reduction. The XRD pattern of CuNPs was obtained in the 2θ range of 30°–80° by a GNR EXPLORER device at 40 kV, 30 mA, and Cu-Kα radiation (1.5406 Å).
neuroprotective analyses of copper nanoparticles.
Cell culture. PC12 (ATCC CRL-1721TM) cells used in this experiment were cultured according to protocol rules in the Gibco RPMI1640 cell culture environ-ment. 100 IU/mL penicillin (Sigma), 100 µg/mL streptomycin and 10% fetal bovine serum supported in cell cultures and standard case in T-25 cm2 tissue culture bottles (5% incubated at 37 °C in CO2). It led to changes
in the morphology of methadone cells, so apoptotic cells led to the cell being treated with diluted methadone with 100 mM water. For Cu(NO3)2, C. sativus leaf aqueous extract, CuNPs solutions in the RPMI1640 water cell
cultural environment were first dissolved in DMSO and added to the cultural environment with a final volume of 0.1%. The cells were then washed by PBS (37 °C) 12 h after the plating and divided into eight classes, including I) Methadone: Cell culture contains 100 μM methadone; II) Control: Cell culture medium without methadone, Cu(NO3)2, C. sativus leaf aqueous extract, and CuNPs; III) T1: Cell culture contains 100 μM methadone and
2 μg of Cu(NO3)2; IV) T2: Cell culture contains 100 μM methadone and 4 μg of Cu(NO3)2; V) T3: Cell culture
contains 100 μM methadone and 2 μg of C. sativus leaf aqueous extract; VI) T4: Cell culture contains 100 μM methadone and 4 μg of C. sativus leaf aqueous extract; VII) T5: Cell culture contains 100 μM methadone and 2 μg of CuNPs; VIII) T6: Cell culture contains 100 μM methadone and 4 μg of CuNPs.
Caspase-3 activity. For plating PC12 cells, the well cell culture plate containing the PRMI1640 medium was used. After 12 h, the plate was washed by PBS. Then, the different treatments of I-VII were added to the cells. Trypsin was used for separating the cells from the flask. For removing the supernatant, all samples were centri-fuged for 10 min, then the centrifuging was done by adding lysate buffer and final they were transferred to the well cell culture plate. Afterwards, 5 µl of N-acetyl-Asp-Glu-Val-Asp-p-nitroaniline Caspase-3 Substrate DEVD-pNA, the wells cell culture plate was added and was allowed within 2 h of incubation at 37 °C. Subsequently, the release of pNA on the result of caspase-3 activity was measured and recorded43.
Secretion of inflammatory cytokines. Pro-inflammatory cytokines TNFa, IL-6 and IL-1β concentrations were measured using the Rat V-Plex Kit.
Cell death index. For determining of PC12 cell death index in the different treatments of I-VII, TUNEL stain-ing was used. Eight random wells with a fluorescence microscope were chosen to count TUNEL positive cells. The ratio of cell death index to apoptotic cells to total cells is equal43.
Cell viability. Trypan blue was used for assessing cell viability. Vital Dye, whose membranes penetrate broken damaged or Dead Cells, is seen by penetrating dead cells in the Blue lobes in the Neubauer Lamela. For 12 h, 96 wells with a 5 × 104 cell/mL density were coated on the cultural plate, then, they were cultured with various I-VII treatments and incubated at 37 °C at 5% CO2 for 48 h. Since the cells were trypsinized, Neubauer Lamela
suspended 200 μL of cell suspension 2–3 min since combining with 0.4% 40 μL of Trypan blue. The cell viability of all samples was determined by following the formula below46:
Mitochondrial membrane potential (MMP). For half an hour different treatment were exposed to 10 mg/mL rhodamine-123. The cell was then washed using PBS. 900 μL Triton X-100 was subsequently added to each well and held for 2 h at 4 °C. The solutions were taken into microtubes to be centrifuged at 16,000 rpm for 20 min. Fluorescent absorbing in cells was performed using a fluorescent microplate reader (488 nm excitation and 520 nm emissions)47.
www.nature.com/scientificreports/
evaluation of the antioxidant activity of copper nanoparticles with DppH.
Throughout the start of the study, 100 mL of methanol (50%) was applied to DPPH’s 39.4 g. Also reported were some amounts of Cu(NO3)2, C. sativus leaf aqueous sample, and CuNPs, i.e., 0–1,000 μg/mL. The following DPPH has beenapplied to Cu(NO3)2, C. sativus leaf aqueous sample, and CuNPs at different concentration. At a temperature
of 37 °C all samples were transferred to an incubator. For this analysis, the negative and positive controls were methanol (50%) and butylated hydroxytoluene (BHT), respectively. The antioxidant properties of Cu(NO3)2, C.
sativus leaf aqueous extract, and CuNPs were defined according to the following formula44,48,49:
Statistical analysis.
The data obtained at the end of the study were fed to the SPSS-22 program and the Tukey post-hoc test (p ≤ 0.01) after the one-sided ANOVA test.Results and discussion
In this study, copper nanoparticles were prepared and synthesized in a watered environment using C. sativus leaf extract as decreasing and stabilizing agents. Also, we investigated their potential against methadone-induced cell death in PC12.
chemical characterization of copper nanoparticles.
The surface size and morphology of these nano-particles were determined using FE-SEM in Fig. 1. The pictures showed that the biosynthesized copper nanopar-ticles are homogeneous, well dispersed and homogeneous. A collection tendency is seen for synthetic nanoparti-cles. Metallic nanoparticles, such as copper nanoparticles, silver nanoparticles, gold nanoparticles, and titanium nanoparticles synthesized using environmentally friendly methods have been reported in previous studies49–51.In our analysis of the literature, various sizes have been recorded for biosynthesized copper nanoparticles utilizing herbal extracts. For instance, 10–100 nm for Punica granatum peels extract52.; 21.50–34.23 nm for the aqueous oak fruit hull extract53; 20–60 nm for extracting coffee powder54; 5–20 nm for extract Z. spina-christi55; 20–50 nm for the synthetic copper nanoparticles using Olea europaea leaf extract56; 20–40 nm for leaf extract from Solanum lycopersicum57; and 15–20 nm for extract of Punica granatum58.
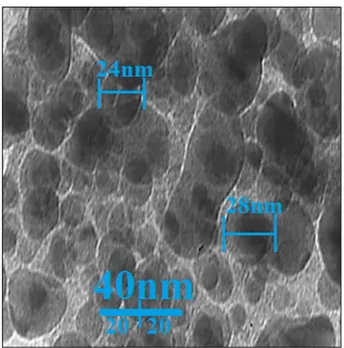
In addition, the average nanoparticles scale (27.5 nm) determined by means of TEM images and ImageJ software package (Fig. 2). In addition, the histogram graph showed in TEM image that biosynthesized copper nanoparticles are in particle size distribution in the range of 14 to 38 nm. In previous experiments in the ranges of 10–50 nm the scale of copper nanoparticles developed by aqueous extract of medicinal plants with the shape of spherical was measured45,59. The findings reported in such studies reflect the existing research outcomes.
UV–Vis. spectrums of biosynthesized copper nanoparticles utilizing the aqueous extremity of the C. sativus leaf are shown in Fig. 3. UV–Vis. spectroscopy was used for the surface plasmon resonance of copper ticles. The appearance of a band with a wavelength of 545 nm indicates that the formation of copper nanopar-ticles has taken place. Previous studies have reported that copper oxide is on a hill in the wavelength range of 540–580 nm for biosynthesis51,58.
Peaks between 450 and 700 cm−1 in the FT-IR spectrum (Fig. 4), 450 to 700 cm−1 in the region belong to
metal–oxygen vibrations. The development of copper oxide nanoparticles in this sample is confirmed by the DPPH free radical scavenging (%) = (Control − Test/Control) × 100
inclusion of a hill at 488 cm−1 Cu(II)–O belongs to the bending vibration60,61 and the bending vibration of Cu(I)–O at 618 cm−138. Simultaneous biosynthesis of CuONPs and Cu
2ONPs using herbal extracts has already
been reported previously. Copper nitrate was used using the FT-IR method to examine the existence of second-ary metabolites responsible for closure and growing its predecessor to CuONPs. In the C. sativus leaf extract was observed the existence of various IR bands involved in the existence of diversified functional groupings. For example, peaks in the range of 1,402 to 1601 cm−1 provisions with C=C and C=O yawning peaks at 3,409
and 2,924 cm−1 relate to O–H and aliphatic C–H stretching and can be attributed to the peak –C–O–C yawn
at 1,088 cm−1. These peaks can be used to confirm compounds with the help of compounds, such as previously
reported phenolic, flavonoid and carboxylic experiments obtained at the end62.
XRD is used for the phase identification and characterization of copper nanoparticles’ crystal structure (Fig. 5). In the case of Cu comprising the sample, it is possible to index the XRD peaks at 43.2°, 50.3° and 74.1° to (111), (200) and (220) Bragg ’s face-centered cubic (fcc) structure of metallic Cu, similar to the Joint Committee on Powder Diffraction Specifications (JCPDS No. 04–0,784).
Also previously the peaks are reported at different degrees37,45.
The mean crystal size of CuNPs was calculated using the Scherrer equation for the diffraction of the X-ray:
CuNPs average crystal size has been found as 28.92 nm.
D = k
βcosθ
Figure 2. TEM image of copper nanoparticles.
www.nature.com/scientificreports/
Figure 4. FT-IR spectra of copper nanoparticles which are biosynthesized.
The prepared nanoparticles’ energy-dispersive X-ray (EDX) profile shows strong elemental copper signals and confirms the presence of copper in a nano form, as shown in Fig. 6. It indicates that the CuNPs prepared comprise only metallic copper, without any other impurities. The EDX pattern obviously demonstrations that the CuNPs are crystalline. The detected signals like CuLα below 1 keV; CuKα around 8 keV; and CuKβ below 9 keV were confirmed the presence of copper in synthesized nanoparticles. Such signals match an earlier analysis of synthesized CuNPs utilizing many medicinal plants as well37,45. The other signals like Okα and CKα correspond to the organic molecules existing in C sativus extract that connected to CuNPs.
Antioxidant activities of copper nanoparticles synthesized using C. sativus leaf aqueous
extract.
Previously, synthesized copper nanoparticles also demonstrated higher antioxidant activity to form free radicals within the living system. Since CuNPs possess redox properties, they take an important role in neutralizing free radicals in living systems57.In our research, the antioxidant effects of synthesized copper nanoparticles utilizing C. sativus leaf aqueous extract were tested by DPPH assay dig up concentration-addicted results i.e. an rise in copper nanoparticles con-centration induces an increase in antioxidant function. The best results were found in the tested concon-centrations at the highest concentration of 1,000 μg/mL (Fig. 7). The results of the comparative study of individual antioxidant research have identified major differences in the usage of radical scavenging effects. Between all materials tried (Cu(NO3)2, C. sativus leaf aqueous extract, and copper nanoparticles), Copper nanoparticles have demonstrated
more outstanding results on DPPH inhibitions. Conversely, standard (butylated hydroxytoluene) represented lowly antioxidant effects in proportion to the copper nanoparticles. The IC50 of C. sativus leaf aqueous extract,
butylated hydroxytoluene, and copper nanoparticles were 342, 252, and 188 µg/mL, respectively.
Recently, researchers have made studies to evaluate the antioxidant activity properties of plants and bio-mediated synthesized copper nanoparticles. The cause of green or biosynthesized copper nanoparticles with anti-oxidant activity can be caused by the presence of metabolite compounds such as phenol compounds, flavonoids, sugar and other carbohydrates63–66. In addition, many researchers have noted that phenolic and flavonoids that are bound to copper nanoparticles show antioxidant activity. The C. sativus leaf has previously been documented to be high in antioxidant compounds such as picrocrocin, crocin, and safranal. Various studies have been con-ducted in the field of nanotechnology using diversified medicinal plants, but there are yet no reports on copper nanoparticles synthesized using C. sativus leaf aqueous extract.
neuroprotective activities of copper nanoparticles.
As a result of long-term use of methadone used to treat chronic and acute pain and opioid dependence, it causes neurotoxic effects, the etiology of which is not known67,68. Organs involved in excretion and metabolism of methadone can show side effects of this drug69,70, however, direct effects on the central nervous system due to their negative effects on the central nerv-ous system71,72. Li et al. (2016) argued that methadone harms the entirety of the white matter of the methadone consumer for a long time in a dose-dependent order73. As a result of radiological studies, methadone affects thewww.nature.com/scientificreports/
structure of the brain in the deep white matter of the cerebellum, basal ganglia and cerebral hemispheres at high concentration and long-term consumption74,75.
For methadone two signal pathways were proposed: one interacting with opioid receptors, and the other functioning as an antagonist in NMDR76. This NMDR path seems to have more pronounced effects on metha-done toxicity in cortical cell cultures. In the field of immunology, one study has shown that people with opioid-dependent methadone maintenance therapy affect immune system activities thus, prolonged inflammation in the nervous system may also occur. A previous studies found that prolonged methadone maintenance treatment raises the rates of IL-1β cytokine, and large dose methadone cytokinine improves rates of IL-6 and TNF-a77.
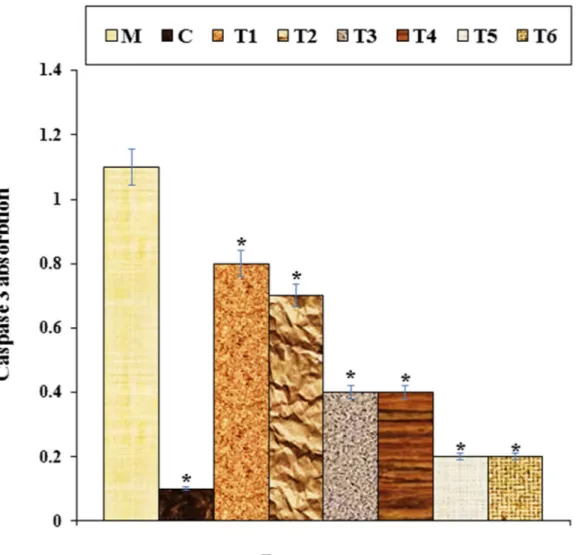
High concentrations of methadone toxicity have a reducing effect on the potential for cell proliferation between nerve cells, leading to cell death77. Friesen et al. (2008) methadone revealed that apoptosis and cell proliferation inhibition in leukemia cells causes cell death through inhibition. Many processes took part in this path. For instance, in apoptosis, caspase-9 and 3 are activated by methadone. In addition, apoptosis due to the Bcl-xl and X chromosome is arranged down and poly (ADP-ribose) polymerase decolletage78. The results of our study confirmed the findings. They showed that high concentrations of methadone had a significant lowering effect on cell viability (p ≤ 0.01) and inflammatory cytokine concentrations and caspase-3 activity. Treatment of these cells improved the capacity for cell viability and cell proliferation for both doses of copper nanoparticles owing to decreased cytotoxicity of the cells (Figs. 8, 9, 10, 11).
Ligand-receptor system is used by Methadone in the activation of mitochondrial apoptosis. It can also directly rouse up apoptosis. Members of the Bcl2 family regulate mitochondrial apoptosis79,80. This form of apoptosis causes many replace in mitochondria. For instance, free oxygen radicals are churn out that cause pore formation in the mitochondrial membrane. As noted below, cytochrome C activates Caspase 3 using caspase 2 or 9, releas-ing factors that induce apoptosis from the mitochondrial membrane to the cytoplasm79,81. Apoptosis complexes are formed of Apaf-1, Caspase 9 and Cytochrome C are effectuated for activation of Caspase-3 and suction of apoptosis80. Increased absorption of Rhodamine123 indicates enhancement mitochondrial membrane potency with enhancement activity of proton pumps. Active proton pumps control membrane function in the mitochon-dria and inhibit the activation of apoptosis80. The reduction in the potential of mitochondrial membrane causes c release and cascade activates of the caspase81. Apoptosis study conducted with TUNEL testing has shown in our study that methadone causes fragmentation of DNA and causes apoptosis in nerve-like PC12 cells. Our findings
Figure 7. The antioxidant properties of Cu(NO3)2, Crocus sativus L. leaf aqueous extract, CuNPs, and BHT
also show that copper nanoparticles significantly (p ≤ 0.01) increase the mitochondrial membrane potential and reduce the rate of DNA degradation in methadone-treated PC12 cells (Fig. 12).
Treatment with C. sativus extract (5 and 25 mg/ml) may reduce the neurotoxic impact of biochemical param-eters in PC12 cells, in accordance with our research. The results showed that glucose (13.5 and 27 mg/ml) decreased the viability of PC12 cells whereas cell death decreased with pretreatment of C. sativus82. Another study revealed that for 45 days administration of C. sativus extract (200 mg/kg) and honey syrup (500 mg/kg) decreased neurotoxicity in mice to aluminum chloride-induced83. Certain experiments have shown that C. sativus has a certain beneficial impact on multiple oxidative injury factors in ischemic rat hippocampal tissue and in hippocampal tissue following administration of quinolinic acid (QA)84. C. sativus also decreased extracellular glutamate and aspartate concentrations (excitatory amino acids) in anesthetized rat hippocampus following administration of kainic acid85.
With respect to the impact of C. sativus on neuronal damage and apoptosis, it was proposed that the neu-roprotective impact of C. sativus on brain injury in animal experiments are attributed to its ability to suppress apoptosis at the early phases of the injury and its ability to induce angiogenesis at the sub-acute level as motivated by greater endothelial vascular growth factor receptor-2 levels (VEGFR) and serum response factor (SRF)86. Recent research showed that C. sativus (50 mg/kg) blocked apoptosis of retinal ganglion cells (RGCs) follow-ing retinal ischemia/reperfusion injury through the signalfollow-ing pathway for phosphatidylinositol 3-kinase/AKT (PI3K/AKT). Additionally, the Bcl-2/BAX ratio improved with C. sativus87. C. sativus (10 μM) can suppress proapoptotic mRNA expression induced by tumor necrosis factor-alpha (TNF-α) that releases cytochrome c from mitochondria, and it has been proposed that crocin prevents neuronal cell death triggered by both internal and external apoptotic stimulus88. In addition, C. sativus can inhibit RGC-5 cell death caused by H
2O2 and
sup-press caspase-3 and caspase-9 development89. The lipid peroxidation may increase in serum/glucose-deprived
Figure 8. The caspase 3 absorption in different treatments after 48 h. M: Methadone, C: Control, T1: 100 μM
methadone and 2 μg of Cu(NO3)2, T2: 100 μM methadone and 4 μg of Cu(NO3)2, T3: 100 μM methadone and
2 μg of Crocus sativus L. leaf aqueous extract, T4: 100 μM methadone and 4 μg of Crocus sativus L. leaf aqueous extract, T5: 100 μM methadone and 2 μg of CuNPs, T6: 100 μM methadone and 4 μg of CuNPs. *It indicates the substantial gap (p ≤ 0.01) for methadone treatment trial studies.
www.nature.com/scientificreports/
cells, which may be blocked by C. sativus. This plant may have been able to suppress caspase-8 activation and its antioxidant properties are more pronounced at the same concentration than α-tocopherol90. Furthermore, C sativus blocked caspase-8 activation triggered by lack of serum /glucose91. In serum-deprived and hypoxic PC12 cells, C. sativus actively prevented membrane lipid peroxidation, caspase-3 activation, and cell death, which was more differentiated than tricrocin. It has been reported that C. sativus has several related glucose esters92.
C. sativus suppressed syncytin-1 and nitric oxide (NO)-induced cytotoxicity of astrocytes which oligoden-drocytes and decreased neuropathology with slightly less neurological impairment in experimental autoimmune encephalomyelitis (EAE). Syncytin-1 has led to mortality and neuroinflammation of the oligodendrocytes93. Syncytin-1 is strongly expressed in lesions with multiple sclerosis in astrocytes, microglia, and the glial cells94. The stimulation of endoplasmic reticulum (ER) is directly related to the inflammatory pathways. It has been shown that the transcript rates of the ER tension genes XBP-1/s are enhanced by EAE95. C. sativus administration decreased the repression of ER stress and inflammatory gene expression in the spinal cord on day 7 after EAE induction, as well as the release of stress genes from ER XBP-1/s96.
In accordance with our work, extracts from C sativus aqueous (80–320 mg/kg) and ethanolic (400–800 mg/ kg) have minimized symptoms of opioid withdrawal induced by naloxone in mice97. Crocin (200 and 600 mg/ kg) can even decrease the withdrawal signal without reducing locomotor activity98.
It seems that copper nanoparticles, due to its antioxidant potential, dramatically (p ≤ 0.01) enhancement cell survival and mitochondrial membrane potential, and decreased inflammatory cytokines concentrations, cas-pase-3 activity, and DNA fragmentation in the high concentration of methadone-treated PC12 cells. Antioxidants
Figure 9. The cytokine concentration in different treatments after 48 h. M: Methadone, C: Control, T1: 100 μM
methadone and 2 μg of Cu(NO3)2, T2: 100 μM methadone and 4 μg of Cu(NO3)2, T3: 100 μM methadone and
2 μg of Crocus sativus L. leaf aqueous extract, T4: 100 μM methadone and 4 μg of Crocus sativus L. leaf aqueous extract, T5: 100 μM methadone and 2 μg of CuNPs, T6: 100 μM methadone and 4 μg of CuNPs. *It indicates the substantial gap (p ≤ 0.01) for methadone treatment trial studies.
cell cytotoxicity reducing effect, which is the reason for blocking the production of reactive oxygen species and oxidative stress in cells23.
conclusions
The recent research determined the chemical characterization, neuroprotective, cytotoxicity, and antioxidant properties of copper nanoparticles using Crocus sativus L. leaf aqueous extract. This was done using TEM, FT-IR, FE-SEM and UV–Vis tests to make chemical characterization of nanoparticles. The resulting data showed that CuNPs were synthesized in the best probable state. In the FT-IR analysis, the inclusion in copper nanoparticles of many antioxidant compounds with related bonds induced excellent copper-reduction state. In the images of TEM and FE-SEM the copper nanoparticles had a spherical shape at an average size of 27.5 nm. Also, UV–Vis revealed the copper nanoparticles formation by a clear peak in the wavelength of 569 nm.
Assessment of the antioxidant properties of copper nanoparticles was done with the common free radical scavenging test i.e., DPPH in the existence of butylated hydroxytoluene as the positive control. The copper nanoparticles inhibited half the concentration of 188 μg/ml of DPPH molecules.
Copper nanoparticles increased cell survival and Rh123 absorption in methadone-treated PC12 cells. These nanoparticles reduced the caspase 3 absorption, IL-1β, IL-6, and TNF-α inflammatory cytokines concentration, and apoptosis index. These events show that copper nanoparticles are suppressed in a dose-dependent manner by methadone-induced cell death in PC12 cells. In clinical studies, after confirmation of the above-mentioned results, copper nanoparticles can be applied as neuroprotective support in the treatment of human neurotoxicity.
Figure 10. The apoptosis index of different treatments after 48 h. M: Methadone, C: Control, T1: 100 μM
methadone and 2 μg of Cu(NO3)2, T2: 100 μM methadone and 4 μg of Cu(NO3)2, T3: 100 μM methadone and
2 μg of Crocus sativus L. leaf aqueous extract, T4: 100 μM methadone and 4 μg of Crocus sativus L. leaf aqueous extract, T5: 100 μM methadone and 2 μg of CuNPs, T6: 100 μM methadone and 4 μg of CuNPs. *It indicates the substantial gap (p ≤ 0.01) for methadone treatment trial studies.
www.nature.com/scientificreports/
Figure 11. After 48 h the cell viability for multiple therapies. M: Methadone, C: Control, T1: 100 μM
methadone and 2 μg of Cu(NO3)2, T2: 100 μM methadone and 4 μg of Cu(NO3)2, T3: 100 μM methadone and
2 μg of Crocus sativus L. leaf aqueous extract, T4: 100 μM methadone and 4 μg of Crocus sativus L. leaf aqueous extract, T5: 100 μM methadone and 2 μg of CuNPs, T6: 100 μM methadone and 4 μg of CuNPs. *It indicates the substantial gap (p ≤ 0.01) for methadone treatment trial studies.
Figure 12. The mitochondrial membrane potential of different treatments after 48 h. M: Methadone, C:
Control, T1: 100 μM methadone and 2 μg of Cu(NO3)2, T2: 100 μM methadone and 4 μg of Cu(NO3)2, T3:
100 μM methadone and 2 μg of Crocus sativus L. leaf aqueous extract, T4: 100 μM methadone and 4 μg of Crocus sativus L. leaf aqueous extract, T5: 100 μM methadone and 2 μg of CuNPs, T6: 100 μM methadone and 4 μg of CuNPs. *It indicates the significant difference (p ≤ 0.01) for methadone treatment trial studies.
Received: 2 April 2020; Accepted: 18 June 2020
References
1. Karimi-Maleh, H. vd. Palladium–Nickel nanoparticles decorated on functionalized-MWCNT for high precision non-enzymatic glucose sensing. Mater. Chem. Phys. 250, 123042 (2020).
2. Karimi-Maleh, H. vd. The role of magnetite/graphene oxide nano-composite as a high-efficiency adsorbent for removal of phena-zopyridine residues from water samples, an experimental/theoretical investigation. J. Mol. Liq. 298, 112040 (2020).
3. Karimi-Maleh, H., Karimi, F., Alizadeh, M. & Sanati, A. L. Electrochemical sensors, a bright future in the fabrication of portable kits in analytical systems. Chem. Rec. https ://doi.org/10.1002/tcr.20190 0092 (2019).
4. Karimi-Maleh, H., Fakude, C. T., Mabuba, N., Peleyeju, G. M. & Arotiba, O. A. The determination of 2-phenylphenol in the pres-ence of 4-chlorophenol using nano-Fe3O4/ionic liquid paste electrode as an electrochemical sensor. J. Colloid Interface Sci. 554, 603–610 (2019).
5. Shamsadin-Azad, Z., Taher, M. A., Cheraghi, S. & Karimi-Maleh, H. A nanostructure voltammetric platform amplified with ionic liquid for determination of tert-butylhydroxyanisole in the presence kojic acid. J. Food Meas. Character. 13, 1781–1787 (2019). 6. Tahernejad-Javazmi, F., Shabani-Nooshabadi, M. & Karimi-Maleh, H. 3D reduced graphene oxide/FeNi3–ionic liquid
nanocom-posite modified sensor: an electrical synergic effect for development of tert-butylhydroquinone and folic acid sensor. Compos. B
Eng. 172, 666–670 (2019).
7. Experimental and docking theoretical investigations. Khodadadi, A. vd. A new epirubicin biosensor based on amplifying DNA interactions with polypyrrole and nitrogen-doped reduced graphene. Sens. Actuators B Chem. 284, 568–574 (2019).
8. Miraki, M. vd. Voltammetric amplified platform based on ionic liquid/NiO nanocomposite for determination of benserazide and levodopa. J. Mol. Liq. 278, 672–676 (2019).
9. Alavi-Tabari, S. A. R., Khalilzadeh, M. A. & Karimi-Maleh, H. Simultaneous determination of doxorubicin and dasatinib as two breast anticancer drugs uses an amplified sensor with ionic liquid and ZnO nanoparticle. J. Electroanal. Chem. 811, 84–88 (2018). 10. Baghizadeh, A., Karimi-Maleh, H., Khoshnama, Z., Hassankhani, A. & Abbasghorbani, M. A voltammetric sensor for simultaneous
determination of vitamin C and vitamin B6 in food samples using ZrO2 nanoparticle/ionic liquids carbon paste electrode. Food
Anal. Methods 8, 549–557 (2015).
11. Jamali, T., Karimi-Maleh, H. & Khalilzadeh, M. A. A novel nanosensor based on Pt:Co nanoalloy ionic liquid carbon paste electrode for voltammetric determination of vitamin B9 in food samples. LWT Food Sci. Technol. 57, 679–685 (2014).
12. Bijad, M., Karimi-Maleh, H. & Khalilzadeh, M. A. Application of ZnO/CNTs nanocomposite ionic liquid paste electrode as a sensitive voltammetric sensor for determination of ascorbic acid in food samples. Food Anal. Methods 6, 1639–1647 (2013). 13. Bijad, M., Karimi-Maleh, H., Farsi, M. & Shahidi, S. A. An electrochemical-amplified-platform based on the nanostructure
vol-tammetric sensor for the determination of carmoisine in the presence of tartrazine in dried fruit and soft drink samples. J. Food
Meas. Character. 12, 634–640 (2018).
14. Eren, T., Atar, N., Yola, M. L. & Karimi-Maleh, H. A sensitive molecularly imprinted polymer based quartz crystal microbalance nanosensor for selective determination of lovastatin in red yeast rice. Food Chem. 185, 430–436 (2015).
15. Tahernejad-Javazmi, F., Shabani-Nooshabadi, M. & Karimi-Maleh, H. Analysis of glutathione in the presence of acetaminophen and tyrosine via an amplified electrode with MgO/SWCNTs as a sensor in the hemolyzed erythrocyte. Talanta 176, 208–213 (2018). 16. Hagh-Nazari, L. vd. Stereological study of kidney in streptozotocin-induced diabetic mice treated with ethanolic extract of Stevia
rebaudiana (bitter fraction). Comp. Clin. Pathol. 26, 455–463 (2017).
17. Zangeneh, M. M., Goodarzi, N., Zangeneh, A., Tahvilian, R. & Najafi, F. Amelioration of renal structural changes in STZ-induced diabetic mice with ethanolic extract of Allium saralicum R.M. Fritsch. Comp. Clin. Pathol. 27, 861–867 (2018).
18. Sherkatolabbasieh, H. vd. Ameliorative effects of the ethanolic extract of Allium saralicum RM Fritsch on CCl4-induced nephro-toxicity in mice: A stereological examination. Arch. Biol. Sci. 69, 535–543 (2017).
19. Tahvilian, R. vd. Green synthesis and chemical characterization of copper nanoparticles using Allium saralicum leaves and assess-ment of their cytotoxicity, antioxidant, antimicrobial, and cutaneous wound healing properties. Appl. Organomet. Chem. 33 (2019). 20. Goorani, S. The aqueous extract of Glycyrrhiza glabra effectively prevents induced gastroduodenal ulcers: Experimental study on
Wistar rats. Comp. Clin. Pathol. 28, 339–347 (2019).
21. Goorani, S. vd. Hepatoprotective and cytotoxicity properties of aqueous extract of Glycyrrhiza glabra in Wistar rats fed with high-fat diet. Comp. Clin. Pathol. 28, 1305–1312 (2019).
22. Zangeneh, A. vd. Therapeutic effects of Glycyrrhiza glabra aqueous extract ointment on cutaneous wound healing in Sprague Dawley male rats. Comp. Clin. Pathol. 28, 1507–1514 (2019).
23. Srivastava, R., Ahmed, H., Dixit, R., Dharamveer, A. & Saraf, S. Crocus sativus L.: A comprehensive review. Pharmacogn. Rev. 4, 200–208 (2010).
24. McLaughlin, P. J., Levin, R. J. & Zagon, I. S. Regulation of human head and neck squamous cell carcinoma growth in tissue culture by opioid growth factor. Int. J. Oncol. 14, 991–998 (1999).
25. Papandreou, M. A. vd. Memory enhancing effects of saffron in aged mice are correlated with antioxidant protection. Behav. Brain
Res. 219, 197–204 (2011).
26. Hosseinzadeh, H. & Talebzadeh, F. Anticonvulsant evaluation of safranal and crocin from Crocus sativus in mice. Fitoterapia 76, 722–724 (2005).
27. Ochiai, T. vd. Crocin prevents the death of PC-12 cells through sphingomyelinase-ceramide signaling by increasing glutathione synthesis. Neurochem. Int. 44, 321–330 (2004).
28. Leppert, W. The role of methadone in cancer pain treatment—a review. Int. J. Clin. Pract. 63, 1095–1109 (2009).
29. Perez-Alvarez, S. vd. Methadone induces necrotic-like cell death in SH-SY5Y cells by an impairment of mitochondrial ATP syn-thesis. Biochim. Biophys. Acta Mol. Basis Dis. 1802, 1036–1047 (2010).
30. De Vos, J. W. vd. l-methadone and d,l-methadone in methadone maintenance treatment: A comparison of therapeutic effective-ness and plasma concentrations. Eur. Addic. Res. 4, 134–141 (1998).
31. Quillinan, N., Lau, E. K., Virk, M., Von Zastrow, M. & Williams, J. T. Recovery from μ-opioid receptor desensitization after chronic treatment with morphine and methadone. J. Neurosci. 31, 4434–4443 (2011).
32. Gorman, A. L., Elliott, K. J. & Inturrisi, C. E. The d- and l-isomers of methadone bind to the non-competitive site on the N-methyl-d-aspartate (NMDA) receptor in rat forebrain and spinal cord. Neurosci. Lett. 223, 5–8 (1997).
33. Reig, F., Busquets, M. A., Haro, I., Rabanal, F. & Alsina, M. A. Interaction of opiate molecules with lipid monolayers and liposomes.
J. Pharm. Sci. 81, 546–550 (1992).
34. Mintzer, M. Z. & Stitzer, M. L. Cognitive impairment in methadone maintenance patients. Drug Alcohol Depend. 67, 41–51 (2002). 35. Rass, O. vd. Cognitive performance in methadone maintenance patients: Effects of time relative to dosing and maintenance dose
level. Exp. Clin. Psychopharmacol. 22, 248–256 (2014).
36. Vergun, O. vd. Exploration of the role of reactive oxygen species in glutamate neurotoxicity in rat hippocampal neurones in culture.
J. Physiol. 531, 147–163 (2001).
www.nature.com/scientificreports/
38. Mohebbati, R., Khazdair, M. R. & Hedayati, M. Neuroprotective effects of medicinal plants and their constituents neuroprotective
effects of medicinal plants and their constituents on different induced neurotoxicity methods: A review. Med. Sci. J. Rep. Pharm. Sci.
6, (2017).
39. Karimi-Maleh, H. & Arotiba, O. A. Simultaneous determination of cholesterol, ascorbic acid and uric acid as three essential biological compounds at a carbon paste electrode modified with copper oxide decorated reduced graphene oxide nanocomposite and ionic liquid. J. Colloid Interface Sci. 560, 208–212 (2020).
40. Karimi-Maleh, H. vd. A novel electrochemical epinine sensor using amplified CuO nanoparticles and a: N-hexyl-3-methylimida-zolium hexafluorophosphate electrode. New J. Chem. 43, 2362–2367 (2019).
41. Cheraghi, S., Taher, M. A. & Karimi-Maleh, H. Highly sensitive square wave voltammetric sensor employing CdO/SWCNTs and room temperature ionic liquid for analysis of vanillin and folic acid in food samples. J. Food Compos. Anal. 62, 254–259 (2017). 42. Karimi-Maleh, H. vd. Simultaneous determination of 6-mercaptopruine, 6-thioguanine and dasatinib as three important anticancer
drugs using nanostructure voltammetric sensor employing Pt/MWCNTs and 1-butyl-3-methylimidazolium hexafluoro phosphate.
Biosens. Bioelectron. 86, 879–884 (2016).
43. Wang, G. vd Chemical characterization and therapeutic properties of Achillea biebersteinii leaf aqueous extract synthesized copper nanoparticles against methamphetamine-induced cell death in PC12: A study in the nanotechnology and neurology fields. Appl.
Organomet. Chem. 34 (2020).
44. Tahvilian, R. vd. Green synthesis and chemical characterization of copper nanoparticles using Allium saralicum leaves and assess-ment of their cytotoxicity, antioxidant, antimicrobial, and cutaneous wound healing properties (2019).
45. Zangeneh, M. M. vd. Novel synthesis of Falcaria vulgaris leaf extract conjugated copper nanoparticles with potent cytotoxicity, antioxidant, antifungal, antibacterial, and cutaneous wound healing activities under in vitro and in vivo condition. J. Photochem.
Photobiol. B: Biol. 197, 111556 (2019).
46. Gurtu, V., Kain, S. R. & Zhang, G. Fluorometric and colorimetric detection of caspase activity associated with apoptosis. Anal.
Biochem. 251, 98–102 (1997).
47. Strober, W. Trypan blue exclusion test of cell viability. Curr. Protoc. Immunol. 111, A3.B.1-A3.B.3 (2015).
48. Baracca, A., Sgarbi, G., Solaini, G. & Lenaz, G. Rhodamine 123 as a probe of mitochondrial membrane potential: evaluation of proton flux through F(0) during ATP synthesis. Biochem. Biophys. Acta 1606, 137–146 (2003).
49. Zhaleh, M. vd. In vitro and in vivo evaluation of cytotoxicity, antioxidant, antibacterial, antifungal, and cutaneous wound healing properties of gold nanoparticles produced via a green chemistry synthesis using Gundelia tournefortii L. as a capping and reducing agent. Appl. Organomet. Chem. 33, e5015 (2019).
50. Hemmati, S. vd. Green synthesis and characterization of silver nanoparticles using Fritillaria flower extract and their antibacterial activity against some human pathogens. Polyhedron 158, 8–14 (2019).
51. Cheirmadurai, K., Biswas, S., Murali, R. & Thanikaivelan, P. Green synthesis of copper nanoparticles and conducting nanobio-composites using plant and animal sources. RSC Adv. 4, 19507–19511 (2014).
52. Ghidan, A. Y., Al-Antary, T. M. & Awwad, A. M. Green synthesis of copper oxide nanoparticles using Punica granatum peels extract: Effect on green peach Aphid. Environ. Nanotechnol. Monit. Manag. 6, 95–98 (2016).
53. Sorbiun, M., Shayegan Mehr, E., Ramazani, A. & Taghavi Fardood, S. Green Synthesis of zinc oxide and copper oxide nanoparticles using aqueous extract of oak Fruit Hull (Jaft) and comparing their photocatalytic degradation of basic violet 3. Int. J. Environ. Res.
12, 29–37 (2018).
54. Taghavi Fardood, S. & Ramazani, A. Green Synthesis and Characterization of Copper Oxide Nanoparticles Using Coffee Powder Extract. J. Nanostruct. 6, 167–171 (2016).
55. Khani, R., Roostaei, B., Bagherzade, G. & Moudi, M. Green synthesis of copper nanoparticles by fruit extract of Ziziphus
spina-christi (L.) Willd.: Application for adsorption of triphenylmethane dye and antibacterial assay. J. Mol. Liq. 255, 541–549 (2018).
56. Sulaiman, G. M., Tawfeeq, A. T. & Jaaffer, M. D. Biogenic synthesis of copper oxide nanoparticles using olea europaea leaf extract and evaluation of their toxicity activities: An in vivo and in vitro study. Biotechnol. Prog. 34, 218–230 (2018).
57. Vaidehi, D., Bhuvaneshwari, V., Bharathi, D. & Sheetal, B. P. Antibacterial and photocatalytic activity of copper oxide nanoparticles synthesized using Solanum lycopersicum leaf extract. Mater. Res. Express 5, 085403 (2018).
58. Kaur, P., Thakur, R. & Chaudhury, A. Biogenesis of copper nanoparticles using peel extract of Punica granatum and their antimi-crobial activity against opportunistic pathogens. Green Chem. Lett. Rev. 9, 33–38 (2016).
59. Tahvilian, R. vd. Green synthesis and chemical characterization of copper nanoparticles using Allium saralicum leaves and assess-ment of their cytotoxicity, antioxidant, antimicrobial, and cutaneous wound healing properties. Appl. Organomet. Chem. 33 (2019). 60. Mukhopadhyay, R., Kazi, J. & Debnath, M. C. Synthesis and characterization of copper nanoparticles stabilized with Quisqualis
indica extract: Evaluation of its cytotoxicity and apoptosis in B16F10 melanoma cells. Biomed. Pharmacother. 97, 1373–1385 (2018). 61. Beg, S. vd. Metal–organic frameworks as expanding hybrid carriers with diverse therapeutic applications. Organ. Mater. Smart
Nanocarriers Drug Deliv. https ://doi.org/10.1016/B978-0-12-81366 3-8.00001 -4 (2018).
62. Manuscript, A. Oxidative stress, inflammation, and cancer. Free Radic. Biol. Med. 49, 1603–1616 (2011).
63. Rehana, D., Mahendiran, D., Kumar, R. S. & Rahiman, A. K. Evaluation of antioxidant and anticancer activity of copper oxide nanoparticles synthesized using medicinally important plant extracts. Biomed. Pharmacother. 89, 1067–1077 (2017).
64. Delgado-Povedano, M. del M., Sánchez de Medina, V., Bautista, J., Priego-Capote, F. & Luque de Castro, M. D. Tentative identifica-tion of the composiidentifica-tion of Agaricus bisporus aqueous enzymatic extracts with antiviral activity against HCV: A study by liquid chromatography-tandem mass spectrometry in high resolution mode. J. Funct. Foods 24, 403–419 (2016).
65. Jeong, S. C., Koyyalamudi, S. R., Jeong, Y. T., Song, C. H. & Pang, G. Macrophage immunomodulating and antitumor activities of polysaccharides isolated from Agaricus bisporus white button mushrooms. J. Med. Food 15, 58–65 (2012).
66. Sankar, R., Maheswari, R., Karthik, S., Shivashangari, K. S. & Ravikumar, V. Anticancer activity of Ficus religiosa engineered copper oxide nanoparticles. Mater. Sci. Eng. C 44, 234–239 (2014).
67. Wildgoose, G. G., Banks, C. E. & Compton, R. G. Metal nanoparticles and related materials supported on Carbon nanotubes: Methods and applications. Small 2, 182–193 (2006).
68. Beheshtkhoo, N., Kouhbanani, M. A. J., Savardashtaki, A., Amani, A. M. & Taghizadeh, S. Green synthesis of iron oxide nanopar-ticles by aqueous leaf extract of Daphne mezereum as a novel dye removing material. Appl. Phys. A Mater. Sci. Process. 124, 1–7 (2018).
69. Smith, H. S. Opioid metabolism. Mayo Clin. Proc. 84, 613–624 (2009).
70. an experimental long term treatment model. Atici, S. vd. Liver and kidney toxicity in chronic use of opioids. J. Biosci. 30, 245–252 (2005).
71. Inturrisi, C. E. Pharmacology of methadone and its isomers. Minerva anestesiologica 71, 435–7
72. Zanin, A. vd. A delayed methadone encephalopathy: Clinical and neuroradiological findings. J. Child Neurol. 25, 748–751 (2010). 73. Li, W. vd. Methadone-induced damage to white matter integrity in methadone maintenance patients: A longitudinal self-control
DTI study. Sci. Rep. 6, 19662 (2016).
74. Cerase, A., Leonini, S., Bellini, M., Chianese, G. & Venturi, C. Methadone-induced toxic leukoencephalopathy: Diagnosis and follow-up by magnetic resonance imaging including diffusion-weighted imaging and apparent diffusion coefficient maps. J.
Neu-roimaging 21, 283–286 (2011).
76. Sotgiu, M. L., Valente, M., Storchi, R., Caramenti, G. & Biella, G. E. M. Cooperative N-methyl-d-aspartate (NMDA) receptor antagonism and μ-opioid receptor agonism mediate the methadone inhibition of the spinal neuron pain-related hyperactivity in a rat model of neuropathic pain. Pharmacol. Res. 60, 284–290 (2009).
77. Chan, Y. Y. vd. Inflammatory response in heroin addicts undergoing methadone maintenance treatment. Psychiatry Res. 226, 230–234 (2015).
78. Friesen, C., Roscher, M., Alt, A. & Miltner, E. Methadone, commonly used as maintenance medication for outpatient treatment of opioid dependence, kills leukemia cells and overcomes chemoresistance. Can. Res. 68, 6059–6064 (2008).
79. Kroemer, G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat. Med. 3, 614–620 (1997).
80. Kaufmann, S. H. & Earnshaw, W. C. Induction of apoptosis by cancer chemotherapy. Exp. Cell Res. 256, 42–49 (2000). 81. Hengartner, M. O. The biochemistry of apoptosis. Nature 407, 770–776 (2000).
82. Mousavi, S. H., Tayarani, N. Z. & Parsaee, H. Protective effect of saffron extract and crocin on reactive oxygen species-mediated high glucose-induced toxicity in pc12 cells. Cell. Mol. Neurobiol. 30, 185–191 (2010).
83. Shati, A. A., Elsaid, F. G. & Hafez, E. E. Biochemical and molecular aspects of aluminium chloride-induced neurotoxicity in mice and the protective role of Crocus sativus L. extraction and honey syrup. Neuroscience 175, 66–74 (2011).
84. Sadeghnia, H. R., Kamkar, M., Assadpour, E., Boroushaki, M. T. & Ghorbani, A. Protective effect of safranal, a constituent of Crocus
sativus, on quinolinic acid-induced oxidative damage in rat hippocampus. Iran. J. Basic Med. Sci. 16, 73–82 (2013).
85. Hosseinzadeh, H., Sadeghnia, H. R. & Rahimi, A. Effect of safranal on extracellular hippocampal levels of glutamate and aspartate during kainic acid treatment in anesthetized rats. Planta Med. 74, 1441–1445 (2008).
86. Bie, X., Chen, Y., Zheng, X. & Dai, H. The role of crocetin in protection following cerebral contusion and in the enhancement of angiogenesis in rats. Fitoterapia 82, 997–1002 (2011).
87. Qi, Y. vd. Crocin prevents retinal ischaemia/reperfusion injury-induced apoptosis in retinal ganglion cells through the PI3K/AKT signalling pathway. Exp. Eye Res. 107, 44–51 (2013).
88. Soeda, S. vd. Crocin suppresses tumor necrosis factor-α-induced cell death of neuronally differentiated PC-12 cells. Life Sci. 69, 2887–2898 (2001).
89. Yamauchi, M. vd. Crocetin prevents retinal degeneration induced by oxidative and endoplasmic reticulum stresses via inhibition of caspase activity. Eur. J. Pharmacol. 650, 110–119 (2011).
90. Ochiai, T. vd. Crocin prevents the death of PC-12 cells through sphingomyelinase-ceramide signaling by increasing glutathione synthesis. Neurochem. Int. 44, 321–330 (2004).
91. Ochiai, T. vd. Crocin prevents the death of rat pheochromyctoma (PC-12) cells by its antioxidant effects stronger than those of α-tocopherol. Neurosci. Lett. 362, 61–64 (2004).
92. Ochiai, T. vd. Protective effects of carotenoids from saffron on neuronal injury in vitro and in vivo. Biochim. Biophys. Acta Gen.
Subj. 1770, 578–584 (2007).
93. Christensen, T. Association of human endogenous retroviruses with multiple sclerosis and possible interactions with herpes viruses.
Rev. Med. Virol. 15, 179–211 (2005).
94. Barnett, M. H. & Prineas, J. W. Relapsing and remitting multiple sclerosis: Pathology of the newly forming lesion. Ann. Neurol.
55, 458–468 (2004).
95. Marciniak, S. J. vd. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum.
Genes Dev. 18, 3066–3077 (2004).
96. Deslauriers, A. M. vd. Neuroinflammation and endoplasmic reticulum stress are coregulated by crocin to prevent demyelination and neurodegeneration. J. Immunol. 187, 4788–4799 (2011).
97. Hosseinzadeh, H. & Jahanian, Z. Effect of Crocus sativus L. (saffron) stigma and its constituents, crocin and safranal, on morphine withdrawal syndrome in mice. Phytother. Res. 24, 726–730 (2010).
98. Amin, B. & Hosseinzadeh, H. Evaluation of aqueous and ethanolic extracts of saffron, Crocus sativus L., and its constituents, safranal and crocin in allodynia and hyperalgesia induced by chronic constriction injury model of neuropathic pain in rats. Fitoterapia 83, 888–895 (2012).
Author contributions
J.C., and F.S. organized all experiments and wrote the manuscript.; P.Z., S.M., A.S.K., A.Z., M.M.Z., N.S., P.T., and R.B., performed all experiments. They have also drawn the figures.
competing interests
The authors declare no competing interests.
Additional information
Correspondence and requests for materials should be addressed to J.C.
Reprints and permissions information is available at www.nature.com/reprints.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International
License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creat iveco mmons .org/licen ses/by/4.0/.