Cite this: RSC Advances, 2013, 3, 19582
Synthesis of an amine-functionalized
naphthalene-containing conducting polymer as a matrix for
biomolecule immobilization
Received 4th May 2013, Accepted 26th July 2013 DOI: 10.1039/c3ra42212g www.rsc.org/advances
Hacer Azak,aEmine Guler,bUmmugulsum Can,aDilek Odaci Demirkol,*bHuseyin Bekir Yildiz,*a Oktay Talazaand Suna Timurb
N-functionalized dithienopyrroles (DTP-NH2) were synthesized and electropolymerized onto a graphite electrode as a novel conducting polymer matrix for biomolecule immobilization.1H-NMR and13C-NMR were utilized to investigate the characteristics of the monomer. After that, glucose oxidase (GOx) was immobilized onto the amino-functionalised matrix by means of glutaraldehyde. The surface morphologies of both DTP-NH2 and DTP-NH2–GOx were visualised by using SEM and fluorescence microscopy. The chronoamperometric signals of the electrochemical DTP-NH2–GOx biosensors were measured by monitoring the O2 consumption during an enzymatic reaction in the presence of glucose at 20.7 V. After the optimization of the pH and scan number of the polymer deposition in batch mode, the DTP-NH2–GOx biosensor was also tested in Flow Injection Analysis (FIA) mode. The DTP-NH2–GOx biosensors had a very good linearity between 0.05 and 1.0 mM, and between 0.1 and 2.5 mM for glucose in batch and FIA modes, respectively. Finally, it was applied for glucose analysis in real samples where commercial glucose kits were used as the reference method to verify the data obtained with the proposed biosensor.
Introduction
DTP-NH2 is one of the DTP (polydithienopyrrole) type
conducting polymers which have been investigated as new units in conjugated materials because of their planar structure and fused ring systems.1 These organic materials with conjugation properties have received considerable attention, since they can be processed in simpler and more cost-effective ways than their inorganic counterparts. Another advantage of these materials is their simple and low-cost processing as well as their potential for electronic, optoelectronic and biosensing applications. The charge transport properties of p-conjugated materials play a key role in the design and fabrication of biosensors.2,3 Therefore, these types of conducting polymers as immobilization matrices can have amazing properties in the design of biosensors. Considering the advantages of its homogeneous and manageable film character, tuneable physical and optical properties, stability and biocompatibility, and its reproducible and easy production, it is thought that DTP-NH2 can be effectively used in biosensors. The
applica-tion of polymers to the fabricaapplica-tion of biosensors is useful
because it minimizes the access of interfering compounds to the sensor surface and prevents biofouling.4
Different types of polymer membranes such as cellulose acetate,5nafion,6polyethylene glycol/oxide (PEG/PEO),7 hydro-gels [usually based on polymers such as poly(vinyl alcohol) or poly(acrylic acid)],8 plasma polymers9 and conducting
poly-mers10–13have been investigated as coatings or supports for
use in biosensors. These conducting polymers which are obtained via the deposition of a variety of monomers contain a highly conjugated backbone and have been utilized as matrices to immobilize the biological components of biosen-sors. In previous studies, biomolecules were either co-deposited with conducting polymers or adsorbed to the surfaces immediately after the electropolymerization of the monomers.14–16Nowadays, functionalized unique conducting polymers, which have a variety of functional groups such as amino or carboxyl groups, have received great attention for their ability to form highly stable biodetection systems through the strong bonds between the polymeric platforms and biomolecules. Recently, 4-(2,5-di(thiophen-2-yl)-1H-pyrrol-1-yl)benzenamine (SNS-NH2) was electrodeposited onto an
electrode surface and used as an immobilization support for different biocomponents such as glucose oxidase17,18and G. oxydans cells.19 Furthermore, Ekiz Kanik et al. utilized a new synthesized conducting polymer, poly(6-(4,7-di(thiophen-2-yl)-2H-benzo[d][1,2,3]triazol-2-yl)hexan-1-amine) [poly(TBT6-NH2)], where choline oxidase was successfully immobilized aKaramanoglu Mehmetbey University, Kamil O¨zdag Science Faculty, Chemistry
Department, 70100, Karaman, Turkey. E-mail: yildizhb@kmu.edu.tr; Fax: +903382262116; Tel: + 903382263840
b
Ege University Faculty of Science, Biochemistry Department, 35100, Bornaova-Izmir. E-mail: dilek.odaci@ege.edu.tr
PAPER
Published on 30 July 2013. Downloaded by Middle East Technical University (Orta Dogu Teknik U) on 04/11/2013 09:51:16.
View Article Online
enzyme molecules and the support. DTP (polydithienopyr-roles) are emerging as useful structures for both molecular and polymeric materials. DTP-NH2 was polymerized
electro-chemically to be used as a support for enzyme immobilization. In general, electrochemical polymerization is favorable com-pared to the chemical polymerization technique because of its simplicity, easy control of thickness and morphology, and the purity of the prepared polymer. Considering the easy produc-tion and biocompatibility as well as the free funcproduc-tional groups on the backbone, P(DTP-NH2) was designed and used in the
immobilization process. The functionalization of DTP-NH2
with electron-releasing groups also supports the development of materials with high-energy electronic transitions, such as the preparation of OLEDs, OFEDs and biosensors. This property helps to get fast, direct electron transfer to the electrode.22 In general, enzyme-based biosensors are fabri-cated via a two-step procedure: the preparation of the biocompatible support layer and the immobilization of the biomolecule. Therefore, the most crucial step in this proce-dure is the immobilization. It is highly dependent on the properties of the supporting material. In order to achieve a stable and sensitive biosensor, it is important to use robust interactions, such as covalent binding, between the enzyme molecules and the support. In this work, a novel monomer, DTP-NH2
[5-(4H-dithieno[3,2-b:29,39-d]pyrrol-4-yl)naphthalen-1-amine], was successfully synthesized and electrochemically polymerized and used as an immobilization matrix for enzyme immobilization. In general, electrochemical polymerization is favorable because of its simplicity, easy control of thickness and morphology and purity of the prepared polymer compared to the chemical polymerization technique. Considering its easy production and biocompatibility as well as the free functional groups on the backbone, the proposed conducting polymer was designed and used in the immobilization process. The second reason is to overcome the difficulty of the direct electron transfer between GOx and the electrode. Therefore, because it has a planar structure, fused ring systems and conjugation properties, the polymer of 5-(4H-dithieno[3,2-b:29,39-d]pyrrol-4-yl)naphthalen-1-amine was used to increase the electron transfer rate in these biosensors.
1H-NMR and13C-NMR techniques were utilized to investigate
the characteristics of the monomer. Then the GOx-immobi-lized poly(DTP) matrix was optimized and characterized as an
real samples.
Results and discussion
Characterization of the monomer
The monomer was synthesized by traditional Ullmann reac-tions. The Ullmann reaction is one of the fundamental reactions in organic chemistry. It has been shown that these reactions can be carried out under mild conditions, in the presence of copper(I) iodide and cesium carbonate.23We used copper(I) iodide, L-proline and DMSO as the reaction condi-tions that gave high yields of 5-(4H-dithieno [3,2-b:29,39-d]pyrrol-4-yl)naphthalen-1-amine (Scheme 1). Chemical shifts were reported in ppm. Data were reported as follows: chemical shift, multiplicity (bs = broad singlet, d = doublet, t = triplet, m = multiplet), coupling constants (Hz), integration. The 1 H-NMR and 13C-NMR spectra of 5-(4H-dithieno [3,2-b:29,39-d]pyrrol-4-yl)naphthalen-1-amine strongly support the struc-ture of the monomer. The1H-NMR spectrum of 5-(4H-dithieno [3,2-b:29,39-d]pyrrol-4-yl)naphthalen-1-amine contained two doublets at 7.08 and 7.01 ppm (J = 5.2 Hz), which correspond to the thiophene protons, the NH2protons were observed as a
board singlet at 4.18–4.01 ppm, and the unsymmetrical napthyl protons are visible in the spectrum. 14 carbon peaks were observed in the13C-NMR spectrum of 5-(4H-dithieno [3,2-b:29,39-d]pyrrol-4-yl)naphthalen-1-amine (Fig. 1 and 2).
Fig. 11H-NMR spectrum of 5-(4H-dithieno[3,2-b:29,39-d]pyrrol-4-yl)naphthalen-1-amine (3).
Fluorescence characteristics of the monomer
Fluorescence measurements of 5-(4H-dithieno[3,2-b:29,39-d]pyrrol-4-yl)naphthalen-1-amine were carried out in THF, DMF, DCM, CHCl3and MeOH (Fig. 3). The emission spectra
show the solvent-fluorescence intensity relationships of the monomer. These results clearly indicate that the 5-(4H-dithieno[3,2-b:29,39-d]pyrrol-4-yl)naphthalen-1-amine monomer is fluorescent. It has a higher emission intensity in MeOH, CHCl3and DCM than in THF and DMF.
These results clearly indicate that the conjugated organic materials are good candidates for use in light-emitting diodes, electrochromic devices, and analytical sensors.24–27Currently, these compounds are becoming some of the major tools for nanobiotechnological applications.28
Application of DTP-NH2in biosensor constructions
Conducting polymers are considered to be suitable supports for the immobilization of a variety of biomolecules. Among other strategies, covalent binding of biomolecules to func-tional groups after deposition of conducting polymers on the electrode surface has some advantages. One of them is the necessity of small amounts of biomolecules; for the electro-deposition of the conducting polymer together with the biomolecule via the entrapment method, large amounts of
biomolecules are required in solution. Secondly, in this situation, the formation of covalent bonds improves the stability of biosensors. Herein, the electropolymerization of DTP-NH2under potentiostatic conditions was performed on a
graphite electrode support via cyclic voltammetry (Fig. 4), and then, the surface of the electrode was rinsed with distilled water to eliminate impurities. Finally, the crosslinking between DTP-NH2 and GOx was realized via glutaraldehyde.
After the fabrication of the DTP-NH2–GOx biosensors, the
morphology of the surface was characterized using SEM (Fig. 5). Additionally, fluorescence microscopy images of the monomer and DTP-NH2–GOx biosensors were obtained
(Fig. 6). The polymer is fluorescent, and GOx also exhibits fluorescent properties because of the FAD centers. Therefore, it is possible to display biomolecule immobilization via fluorescent microscopy. According to the fluorescence micro-scopy and SEM images, it can be said that the surface morphologies are coherent with each other.
As seen from the Fig. 5 and 6, DTP-NH2provided an efficient
immobilization platform with a compact structure for the biomolecule immobilization. Hence, enzymes could be kept on to the surface where higher sensor responses with high operational stabilities are obtained. The presence of amino groups in the structure may also contribute to the attachment of the enzymes on the matrix due to the covalent bond between the enzyme and this functional group. After immo-bilization of enzymes, the compact structure of the polymer has been lost because of participation of functional groups in the crosslinking process and the obtained holes facilitate the diffusion of the substrate and oxygen.
Fig. 213C-NMR spectrum of 5-(4H-dithieno[3,2-b:29,39-d]pyrrol-4-yl)naphtha-len-1-amine (3).
Fig. 3 Emission spectra of 5-(4H-dithieno[3,2-b:29,39-d]pyrrol-4-yl)naphthalen-1-amine. Slit width: lEm= 3 nm.
Fig. 4 (A) Electropolymerization of DTP-NH2. (B) Repeated potential-scan electro-polymerization of the monomer in dichloromethane–TBAPF6(0.1 M) solvent– electrolyte system at a scan rate of 0.1 V s21on graphite (up to ten cycles).
Effect of the pH on the biosensor response
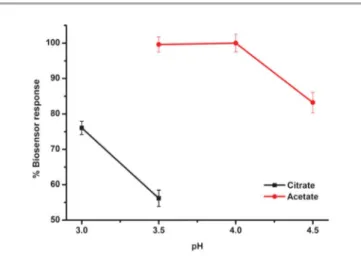
The influence of the pH on the electrochemical behavior and the stability of the DTP-NH2–GOx biosensors were tested using
buffers varied from pH 3.0 to 5.5. The biosensor response of DTP-NH2–GOx was increased from pH 3.0 to 4.0 (Fig. 7).
Hence, in all the following assays, the pH of the reaction medium was maintained at pH 4 using 50 mM acetate buffer. Influence of the scan number for the electropolymerization on the biosensor response
The performance of the DTP-NH2–GOx biosensor for the
detection of glucose was evaluated at different scan numbers of electropolymerization; from 10 to 30.
The biosensor response was highest at 20 cycles (Fig. 8). Hence, in the entire assay, the electropolymerization of DTP-NH2was carried out using 20 scans. The electropolymerization
time (or scan number) affects both the rate of growth and the quality of the conducting polymer films produced.11It is clear that the best film structure with respect to the thickness was obtained at 20 cycles of electropolymerization as indicated by CV for the enzyme immobilization. Longer deposition times might cause degradation and an incompact microstructure, as
reported in previous work.19 Additionally, the shorter
deposi-tion time causes the ineffective funcdeposi-tional groups of the polymer to immobilize enzymes.
Fig. 5 SEM images of poly(DTP-NH2) (A) and after immobilization of the enzyme (DTP-NH2–GOx) (B) under optimized conditions on ITO glass (with 406 magnification).
Fig. 6 Fluorescence images of poly(DTP-NH2) (A) and after immobilization of the enzyme (DTP-NH2–GOx) (B) under optimized conditions on ITO glass (with 406 magnification)
Fig. 7 The effect of the pH on the biosensor response of the DTP-NH2–GOx biosensor in the batch mode (in sodium acetate buffers, 50 mM, 20.7 V). Error bars show the S.D. of three measurements.
Analytical characteristics
The biosensor response was measured in terms of the current (in mA) at various glucose concentrations in batch and FIA modes from 0.05 to 1.0 mM and 0.1 to 2.5 mM, respectively (Fig. 9). Because of the saturation of the active sites of the enzyme units with glucose, the concentration–current graph shows a deviation from linearity at a high concentration of the substrate. The detection limit (LOD) of the prepared DTP-NH2–
GOx biosensors in batch mode was 0.005 mM.
To detect the glucose concentration of real samples, the reproducibility of the DTP-NH2–GOx biosensor is an important
parameter. For this aim, in batch mode, 7 successive measurements of glucose had a standard deviation and variation coefficient (%) of ¡0.006 mM and 3.4%, respectively. When the biosensors were used in the flow injection mode due to the restricted contact time of the substrate with the bioactive layer and the dilution of the sample concentration in the flow system before reaching the electrode surface, a lower response was observed compared to the ones obtained via batch measurements. Also, in FIA mode, the dilution of the substrate with a carrier buffer allowed for the detection of higher concentrations of glucose.29
A comparison of the analytical performance of the conduct-ing polymer-based glucose biosensors is shown in Table 1. In this work, the adaptation of a conducting polymer-based glucose biosensor to FIA is the main advantage of the system. In FIA mode, much more analysis can be carried out by the on-line monitoring of compounds with computer-based measure-ments.
Interference study
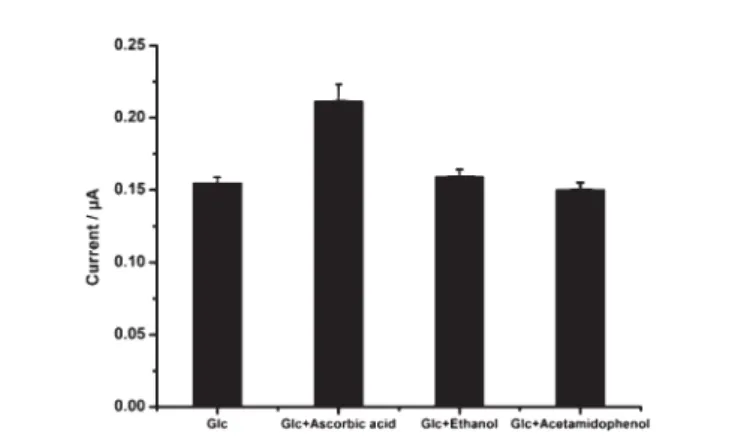
By exposing the DTP-NH2–GOx biosensor to interferents, the
interference study was carried out using ascorbic acid, ethanol, and acetamidophenol solutions containing a glucose standard solution. Fig. 10 summarizes the results of the obtained current values.
The DTP-NH2–GOx biosensor response to glucose was not
affected by some compounds including ethanol and
acetami-dophenol, while ascorbic acid affected the response to glucose (1.0 mM) when it was used a 0.1 mM concentration.
It is known that at higher potentials, most common metabolites such as acetamidophenol and ascorbic acid are oxidized and interfere with the electrochemical signal at lower concentrations. It is therefore essential to apply the lowest possible electrode potential. According to the results given in Fig. 10, the use of 20.7 V as the working potential is favorable because here, none of the compounds tested interfered with the biosensor signal below concentrations of 0.1 mM. However, the addition of 0.1 mM ascorbic acid to the reaction medium affected the biosensor response.
Sample application
The DTP-NH2–GOx biosensor was applied to the
determina-tion of glucose in fruit juice and an orange fizzy drink, and the obtained results were compared by a spectrophotometric method as a reference approach. Table 2 summarizes the results of the recoveries of the two different methods. As a result of the good correlation between them, it can be said that the DTP-NH2–GOx biosensor is suitable for the determination
of glucose in real samples.
Fig. 8 The effect of the scan number on the biosensor response of the DTP-NH2–GOx biosensor in the batch mode (in a pH 4.0 sodium acetate buffer, 50 mM, 20.7 V). Error bars show the S.D. of three measurements.
Fig. 9 Calibration curves for the detection of glucose in batch (A) and FIA (B) modes (in a pH 4.0 sodium acetate buffer, 50 mM, 20.7 V). Error bars show the S.D. of three measurements each. Inset: time-dependent current response with the addition of glucose for batch and FIA mode (batch mode [Glc]: 0.1 mM and FIA mode [Glc]: 2.5 mM).
GE SNS-COOH Chronoamperometry (20.7 V) 0.01–1.2 mM 4 mM 31 GE SNS-COOH–Lys Chronoamperometry (20.7 V) 0.01–2.40 mM 19.0 mM 21 GE SNS-COOH/PAMAM G2 Chronoamperometry (20.7 V) 0.02–1.20 mM 3.47 mM 21 GE Chronoamperometry (20.7 V) 0.02–1.20 mM 2.92 mM 21 GE PBDT Chronoamperometry (20.7 V) 0.05–2.0 mM 50 mM 32 GE PESeE Chronoamperometry (20.7 V) 0.01–2.0 mM 10 mM 32
GE DTP-NH2 Chronoamperometry (20.7 V) 0.05–1.0 mM 5 mM This work (batch)
Materials and methods
Materials
Glucose oxidase (GOx; EC 1.1.3.4, 50 units mg21), D-glucose,
ethanol, ascorbic acid, and acetamidophenol were purchased from Sigma Chem. Co., and tetrabutylammoniumhexafluor-ophosphate (TBAPF6) was purchased from Aldrich.
Dichloromethane (DCM) was obtained from Merck. Glutaraldehyde solution (25%, v/v) was purchased from Sigma Aldrich. The commercial enzyme assay kit (Glucose MR, Cat. No. 1129010) was obtained from Cromatest. Cul, DMSO, Cs2CO3, 1,5-diamine naphthalene and
3,39-dibromo-2,29-bithiophene were obtained from Aldrich. Dimethyl sulf-oxide (DMSO) was purchased from Sigma–Aldrich. L-Proline was obtained from Alfa Aesar. Dichloromethane was dried (CaCl2), distilled from CaH2and stored over molecular sieves.
Other commercial grade solvents were distilled, and then stored over molecular sieves. The drying agent employed was anhydrous MgSO4. All other chemicals were of analytical
grade. Apparatus
1H-NMR and 13C-NMR spectra were recorded at room
temperature on a Varian 400 MHz spectrometer in CDCl3.
Analytical TLC was performed using Merck prepared plates
(silica gel 60 F254 on aluminum). Melting points were determined on an Stuart SMP30 apparatus in a sealed capillary. All reactions, unless otherwise noted, were con-ducted under a nitrogen atmosphere. SEM images were registered by using SEM (JEOL5600-LU). A Shimadzu RF-5301PC spectrofluorophotometer was used for the fluores-cence measurements. Cyclic voltammetric and chronoampero-metric measurements in the batch mode were carried out by the Radiometer Voltalab PGP201 and PalmSens Electrochemical Measurement Units, respectively. All the experiments were performed in a reaction cell (10 mL) at room temperature using a three electrode configuration, consisting of a graphite working electrode, a Ag/AgCl reference electrode (3.0 M KCl, Radiometer) and a platinum counter electrode (Metrohm, Switzerland). For the flow injection measurements, the electrodes were mounted into an electro-chemical flow-through cell of the cross-flow type with glassy carbon working, Ag/AgCl reference, and platinum wire counter electrodes (CHI130, Austin, www.chinstruments.com). The FIA system contained a peristaltic pump (FIAtron, Oconomovoc, WI, USA), an eight-port injection valve (FIAtron, Oconomovoc, WI) and a cross-flow cell with three electrodes. The peristaltic pump equipped with Teflon tubing (0.50 mm inner diameter) carried 1.6 mL buffer solution per minute. Samples were injected with an eight-port injection valve with a 100 mL sample injection loop. The FIA system was connected to a PalmSens potentiostat for the electrochemical measurements. Spectrophotometric experiments were performed with a Pharmacia LKB Novaspec II spectrophotometer (LKB Biochrom, England). An Olympus BX53F Fluorescence micro-scope, an Olympus DP72 camera and an Uplanapo 1006
Table 1 (Continued)
Electrode Conducting polymer Principle of detection (working potential) Linearity for glucose LOD Reference
GE DTP-NH2 Chronoamperometry (20.7 V) 0.1–2.5 mM 64 mM This work (FIA)
GE: graphite electrode; TBT: 2-dodecyl-4,7-di(thiophen-2-yl)-2H-benzo[d][1,2,3]triazole; SNS-NH2: 4-(2,5-di(thiophen-2-yl)-1H-pyrrol-1-yl)benzenamine; AuNP: gold nanoparticles; SNS-COOH: 2-(2,5-di(thiophen-2-yl)-1H-pyrrol-1-yl)acetic acid; Lys: lysine; PAMAM:
poly(amidoamine) dendrimer (G: generation); PBDT: poly(4,7-di(2,3)-dihydrothienol[3,4-b][1,4]dioxin-5-yl-benzo[1,2,5]thiadiazole); PESeE: poly(4,7-di(2,3)-dihydrothienol[3,4-b][1,4]dioxin-5-yl-2,1,3-benzoselenadiazole); DTP-NH2; N-functionalized dithienopyrroles.
Fig. 10 The effects of various interferents on the electrode response in the presence of glucose in FIA mode (in a sodium acetate buffer, 50 mM, pH 4.0, 20.7 V).
Table 2 Results of the determination of total glucose using the DTP-NH2–GOx biosensor and a spectrophotometric method in beverages
Sample
Glucosea[M]
DTP-NH2–GOx Spectrophotometric Recovery (%)
Fruit juice 0.064 ¡ 0.006 0.065 ¡ 0.001 98.5
Orange fizzy drink 0.051 ¡ 0.008 0.050 ¡ 0.001 102 aResults were given as the mean ¡ the standard deviation (n = 3).
NaCl solution was added to the reaction mixture, which was then extracted with EtOAc (3 6 30 ml). The organic phase was dried over anhydrous MgSO4. The solvent was removed via
rotary evaporation, and the residue recrystallized from dichloromethane–n-hexane. The upper phase was separated and the solvent was removed via rotary evaporation. The resulting solid was obtained in 30% yield (118 mg, mp 187uC).
1H-NMR (400 MHz, CDCl 3): 7.41(t, J = 5.2 Hz, CH, 2H), 7.34 (d, J = 5.2 Hz, CH, 1H),7.22–7.18 (m, CH, 3H), 7.08 (d, J = 5.2 Hz, CH, 2H) 7.01(d, J = 5.2 Hz, CH, 2H), 4.18–4.01(bs, NH2, 2H). 13C-NMR (100 MHz, CDCl 3): 132.66, 129.58, 127.52, 127.07, 126.05, 124.13, 122.99, 122.74, 122.48, 122.02, 121.35, 119.63, 119.58, 118.99.
Fabrication of the DTP-NH2–GOx biosensor
Before each experiment, the graphite rods (Ringsdorff Werke GmbH, Bonn, Germany, 3.05 mm diameter and 13% porosity) were polished with wet emery paper and rinsed thoroughly with distilled water for use in batch operations. The glassy carbon electrodes (GCE) were cleaned by polishing them with 0.05 mm alumina slurry followed by ultrasonication in ethanol and distilled water for 5 min for use in the flow injection mode of analysis (FIA).
A DTP-NH2layer covered graphite electrode (GCE in the FIA
system) was used to immobilize GOx. The polymer coatings were formed on the working electrode by using twenty voltammetric cycles between 1.0 V and 21.0 V at a scan rate of 0.1 V s21 in a TBAPF
6(0.1 M)–dichloromethane medium.
For the immobilization of the enzyme, proper amounts of a GOx solution (1.0 mg in 5.0 mL, 50 mM sodium phosphate buffer, pH 7.0) and glutaraldehyde solution (5.0 mL, 1.0% in sodium phosphate buffer, pH 7.0) were spread over the polymer-coated graphite electrodes. Then, the electrodes were allowed to stand at ambient conditions for 90 min. The daily prepared DTP-NH2–GOx biosensors were used in all
experi-ments. A schematic representation of the DTP-NH2–GOx
biosensors is shown in Scheme 2. Measurements
Emission spectra of the synthesized monomer were obtained in solution in THF, DMF, DCM, CHCl3 and MeOH. The
measurements were done in a wide concentration range between 1.6 6 1021and 5 6 1023mg L21to determine the optimal fluorescence concentrations. The slit width in all measurements was 3 nm.
The basic principle of the chronoamperometric measure-ments was based on the monitoring of the oxygen consump-tion due to the catalytic activity of the enzyme in the presence of glucose as a substrate. The decrease in the amount of oxygen was monitored at 20.7 V versus the Ag/AgCl reference electrode. All the measurements in batch mode were performed under constant magnetic stirring of the solution in the reaction cell containing 10 mL sodium acetate buffer (50 mM, pH 3.5). The three electrodes were immersed into the cell and kept in a working buffer solution for 1 min, and the working buffer solution was replaced after each measurement. After the current became constant, glucose was added to the reaction cell. The current value was recorded after a steady state current had been achieved. The biosensor response was obtained as the difference between the first and the second steady state currents. The electrodes were washed with distilled water after each measurement. The DTP-NH2–GOx
biosensors were optimized for the batch system, and char-acterization studies were performed in the FIA configuration. In the FIA system, the three electrodes were integrated into a flow through the cell and the measurements were carried out at room temperature in working buffer. Glucose standard solutions and samples were applied as substrates through a computer-controlled injection valve. The obtained current signals were plotted as a calibration curve and the glucose concentrations in the samples were determined using the calibration curve.
Sample application
The DTP-NH2–GOx biosensors were tested on real samples
(fruit juice and an orange fizzy drink). The samples were degassed, diluted with working buffer and then injected into the carrier buffer in the FIA system instead of glucose as the substrate. The calibration curves for glucose were used to determine the glucose contents in the measured samples. The glucose detection in the real samples was also calculated by using a commercial enzyme assay kit based on the spectro-photometric Trinder reaction (Cromatest, Glucose MR, Cat. No. 1129010) as the reference method, and the results were compared with those obtained with the constructed
biosen-Scheme 2 Schematic representation of the DTP-NH2–GOx biosensor prepara-tion and glucose detecprepara-tion in the batch and the FIA mode.
sors. In the Trinder reaction, the glucose is oxidized to D -gluconate by glucose oxidase (GOx) with the formation of hydrogen peroxide. In the presence of peroxidase (POD), a mixture of phenol and 4-aminoantipyrine (4-AAP) is oxidized by hydrogen peroxide to form a red quinoneimine dye proportional to the glucose concentration in the sample.33
Conclusions
In this study, the stnthesis of 5-(4H-dithieno[3,2-b:29,39-d]pyrrol-4-yl)naphthalen-1-amine monomer was successfully achieved. The monomer was characterized by 13C-NMR and
1H-NMR. The 13C-NMR, 1H-NMR spectra of the monomer
clearly indicate that it was successfully synthesized. The DTP-NH2–GOx biosensor was easy to prepare and it is useful for
glucose detection in both batch and FIA modes.
Acknowledgements
The authors would like to thank the European Union through the COST Action CM1104 ‘‘Reducible Oxide Chemistry, Structure and Functions’’ and the Scientific and Technological Research Council of Turkey (TUBITAK Grant Numbers 112T622 and 111T135) for the financial support of this research.
References
1 M. Parameswaran, G. Balaji, T. M. Jin, C. Vijil, S. Vadukumpully, Z. Furong and S. Valiyaveettil, Org. Electron., 2009, 10, 1534–1540. 2 W. Zhang, J. Li, L. Zou, B. Zhang, J. Qin, Z. Lu, Y. F. Poon, M. B. Chan-Park and C. M. Li, Macromolecules, 2008, 41, 8953–8955.
3 H. A. Huitema, G. H. Gelinck, J. B. P. H. van der Putten, K. E. Kuijk, C. M. Hart, E. Cantatore, P. T. Herwing, A. J. J. M. van Breemen and D. M. de Leeuw, Nature, 2001, 414, 599–599.
4 F. Davis and S. P. J. Higson, Polymers in Biosensors, ed. M. Jenkins, Biomedical Polymers, Woodhead Publishing, 2007, ISBN 1845690702.
5 J. Tkac, I. Vostiar, L. Gorton, P. Gemeiner and E. Sturdik, Biosens. Bioelectron., 2003, 18, 1125–1134.
6 N. Marzuki, F. Abu Bakar, A. B. Salleh, H. L. Yook, N. A. Yusof and S. Siddiquee, Curr. Anal. Chem., 2012, 8, 534–542.
7 P. Kingschott and H. J. Grieser, Curr. Opin. Solid State Mater. Sci., 1999, 4, 403–412.
8 A. Mateescu, Y. Wang, J. Dostalek and U. Jonas, Membranes, 2012, 2, 40–69.
9 A. B. Turhan, D. Ataman, Y. Sen, M. Mutlu and E. O¨zbay, J. Nanophotonics, 2012, 6, 061602/1–061602/12.
10 D. Odaci, S. Kiralp Kayahan, S. Timur and L. Toppare, Electrochim. Acta, 2008, 53, 4104–4108.
11 S. Tuncag|l, D. Odac| Demirkol, S. Var|s, S. Timur and L. Toppare, Bioelectrochemistry, 2009, 76, 169–174. 12 E. Baskurt, F. Ekiz, D. Odac| Demirkol, S. Timur and
L. Toppare, Colloids Surf., B, 2012, 97, 13–18.
13 B. D. Malhotra, A. Chaubey and S. P. Singh, Anal. Chim. Acta, 2006, 578, 59–74.
14 H. B. Yildiz and L. Toppare, Biosens. Bioelectron., 2006, 21, 2306–2310.
15 H. B. Yildiz, L. Toppare, Y. Hepuzer Gursel and Y. Yagci, Enzyme Microb. Technol., 2006, 39, 945–948.
16 H. B. Yildiz, E. Sahmetlioglu, A. E. Boyukbayram, L. Toppare and Y. Yagci, Int. J. Biol. Macromol., 2007, 41, 332–337.
17 C. Ozdemir, S. Tuncagil, D. Odaci Demirkol, S. Timur and L. Toppare, J. Macromol. Sci., Part A: Pure Appl. Chem., 2011, 48, 503–508.
18 S. Tuncagil, C. Ozdemir, D. Odaci Demirkol, S. Timur and L. Toppare, Food Chem., 2011, 127, 1317–1322.
19 S. Tuncagil, D. Odaci, E. Yildiz, S. Timur and L. Toppare, Sens. Actuators, B, 2009, 137, 42–47.
20 F. Ekiz Kanik, E. Rende, S. Timur and L. Toppare, J. Mater. Chem., 2012, 22, 22517–22525.
21 S. Demirci, F. Bilge Emre, F. Ekiz, F. Oguzkaya, S. Timur, C. Tanyeli and L. Toppare, Analyst, 2012, 137, 4254–4261. 22 T. M. Pappenfus, B. J. Hermanson, T. J. Helland, G. W. Lee,
S. M. Drew, K. R. Mann, K. A. McGee and S. C. Rasmussen, Org. Lett., 2008, 10, 1553–1556.
23 L. Jiang, X. Lu, H. Zhang, Y. Jiang and D. Ma, J. Org. Chem., 2009, 74, 4542–4546.
24 S. J. Evenson, M. J. Mimm, K. I. Pohodya and S. C. Ramussen, Macromolecules, 2011, 44, 835–841.
25 Conjugated Conducting Polymers, ed. H. Kiess, Springer Series in Solid-State Sciences, Springer-Verlag Berlin Heidelberg, New York, 1992, vol. 102.
26 K. Ogawa and S. C. Rasmussen, J. Org. Chem., 2003, 68, 2921–2928.
27 M. Fujitsuka, T. Sato, F. Sezaki, K. Tanaka, A. Watanabe and O. Ito, J. Chem. Soc., Faraday Trans., 1998, 94, 3331–3337.
28 U. Lange, N. V. Roznyatovskaya and V. M. Mirsky, Anal. Chim. Acta, 2008, 614, 1–26.
29 M. Akin, M. Yuksel, C. Geyik, D. Odaci, A. Bluma, T. Hopfner, S. Beutel, T. Scheper and S. Timur, Biotechnol. Prog., 2010, 26, 896–906.
30 F. Ekiz, M. Yuksel, A. Balan, S. Timur and L. Toppare, Macromol. Biosci., 2010, 10, 1557–1565.
31 F. Ekiz, F. Oguzkaya, M. Akin, S. Timur, C. Tanyeli and L. Toppare, J. Mater. Chem., 2011, 21, 12337–12343. 32 F. B. Emre, F. Ekiz, A. Balan, S. Emre, S. Timur and
L. Toppare, Sens. Actuators, B, 2011, 158, 117–123. 33 D. Barham and P. Trinder, Analyst, 1972, 97, 142–145.
![Fig. 1 1 H-NMR spectrum of 5-(4H-dithieno[3,2-b:29,39-d]pyrrol-4-yl)naphthalen- 5-(4H-dithieno[3,2-b:29,39-d]pyrrol-4-yl)naphthalen-1-amine (3).](https://thumb-eu.123doks.com/thumbv2/9libnet/4555710.83008/2.892.472.814.76.198/spectrum-dithieno-pyrrol-naphthalen-dithieno-pyrrol-naphthalen-amine.webp)
![Fig. 3 Emission spectra of 5-(4H-dithieno[3,2-b:29,39-d]pyrrol-4-yl)naphthalen- 5-(4H-dithieno[3,2-b:29,39-d]pyrrol-4-yl)naphthalen-1-amine](https://thumb-eu.123doks.com/thumbv2/9libnet/4555710.83008/3.892.468.812.76.437/emission-spectra-dithieno-pyrrol-naphthalen-dithieno-pyrrol-naphthalen.webp)