http://journals.tubitak.gov.tr/agriculture/ © TÜBİTAK
doi:10.3906/tar-1204-38
Multiple shoot regeneration of plumular apices of chickpea
Muhammad AASIM1,*, Sibel DAY2, Fereshteh REZAEI2, Mortaza HAJYZADEH2
1 Department of Biology, Kamil Özdağ Faculty of Science, Karamanoğlu Mehmetbey University,
Yunus Emre Campus, 70200 Karaman, Turkey
2 Department of Field Crops, Faculty of Agriculture, Ankara University, 06110, Dışkapı, Ankara, Turkey
1. Introduction
Chickpea (Cicer arietinum L.) is an important grain legume that plays a significant role in the nutrition of the rural and urban poor of the world. It is also an important grain legume of Turkey for both cultivation and production. It is an important source of protein, phosphorus, iron, certain water-soluble vitamins, and unsaturated fat. However, its production is limited due to many biotic stresses like disease and insects and abiotic stresses like drought, salinity, and low temperature.
Modern biotechnology has provided new opportunities to enhance the germplasm of crop plants through tissue culture, genetic engineering, and genetic transformation techniques (Sharma and Ortiz 2000). A reliable shoot regeneration protocol is a prerequisite for efficient application of genetic transformation strategies (Jayanand et al. 2003). Previous studies suggest regeneration from cotyledons and epicotyl explants (Rao and Chopra 1987). Furthermore, the effect of zeatin and gibberellic acid (GA3) on regeneration from immature cotyledons of chickpea was studied by Hita et al. (1997). Similarly, induction of multiple shoots and plant regeneration from immature
cotyledon explants of chickpea has been reported by Islam and Rizauddin (1994). Shoot regeneration by direct shoot organogenesis (Polisetty et al. 1996, 1997; Paul et al. 2000; Rizvi and Singh 2000; Chauhan et al. 2003; Jayanand et al. 2003; Chakraborti et al. 2006) or direct somatic embryogenesis and that through callus (Sagare et al. 1993; Barna and Wakhlu 1994; Kumar et al. 1994; Suhasini et al. 1994; Kar et al. 1996, 1997; Rizvi and Singh 2000; Chauhan et al. 2002; Kiran et al. 2005) have also been reported previously in chickpea with low recovery frequency, which ultimately limits the genetic transformation frequency.
The development of new repeatable and reliable tissue culture protocols is very important for the improvement of breeding and genetic transformation studies. Although successful shoot regeneration and genetic transformation protocols of chickpea have been reported, regeneration of plants from plumular apices has not. Previous studies suggest that chickpea tissue culture response is genotypically oriented and different chickpea cultivars regenerated under similar environmental conditions exhibit variable regeneration frequency. Therefore, the study aimed to develop an efficient shoot regeneration
Abstract: Chickpea (Cicer arietinum L.) is an important grain legume used almost all over the world. Development of new repeatable
and reliable tissue culture protocols is very important for improvement of breeding and genetic transformation studies. It is a highly recalcitrant plant and there is an urgent need to develop a regeneration protocol that can ensure easy multiple and qualitative superior shoots that could be rooted and yield fertile plants. Unconditioned plumular apices and plumular apices preconditioned with 10 mg L–1
benzylaminopurine (BA) for 10 days were cultured on Murashige and Skoog (MS) medium supplemented with 0.25–2.00 mg L–1 BA
with or without 0.25 mg L–1 naphthalene acetic acid (NAA). Comparing the 2 culture conditions, the preconditioned explants had 2-fold
to 5-fold more regeneration and shoots per explant compared to the unconditioned plumular apices. The presence of NAA in the culture medium positively increased the number of shoots per preconditioned explant at the lower concentrations of BA. In contrast, NAA inhibited the number of shoots per explants and the mean shoot length of the unconditioned explants at all concentrations of BA. An increased sucrose concentration of 45 and 60 g L–1 (R1) with 1.00 mg L–1 indole-3 butyric acid resulted in 50% rooting with multiple and
earlier hardening of shoots. Subculturing of multiple shoots on MS medium containing 45–60 g L–1 sucrose (R2) enhanced the rooting
frequency by 60%–100%. The rooted plantlets were successfully acclimatized.
Key words: Cicer, in vitro, plumular apices, preconditioning, shoot regeneration
Received: 12.05.2012 Accepted: 16.07.2012 Published Online: 15.01.2013 Printed: 15.02.2013
and rooting protocol from preconditioned plumular apice explants of chickpea cultivar Gökçe for ultimate use in transformation studies.
2. Materials and methods
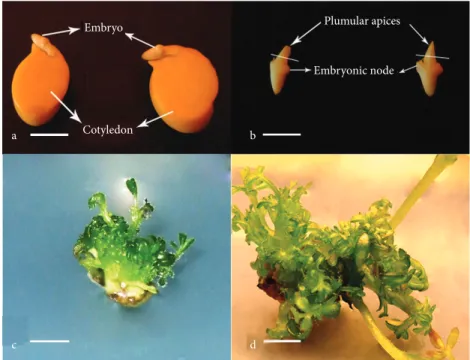
Seeds of chickpea cultivar Gökçe were obtained from the General Directorate of Agricultural Research in Ankara, Turkey. Uniform seeds free of mechanical damage were selected manually before surface sterilization. The seeds were surface sterilized with 100% commercial bleach containing 5% NaOCl for 10 min followed by 3 × 5 min rinsing with bidistilled sterilized water for 5 min. Thereafter, the seeds were submerged in bidistilled sterilized water and shaken using an orbital shaker for 24 h at 100 rpm to soften the testa (seed coat) (Figure 1a) for easy recovery of embryos under aseptic conditions. The embryos (Figure 1b) were then preconditioned with 10 mg L–1 benzylaminopurine (BA) for 10 days on agar-solidified
Murashige and Skoog (MS) basal medium (Murashige and Skoog 1962). A comparison was made by planting a control containing unconditioned mature embryos cultured on MS medium free of BA. After 10 days, the plumular apices were isolated from both the preconditioned and unconditioned mature embryos under aseptic conditions and cultured on MS medium containing 0.25, 0.50, 1.00, and 2.00 mg L–1 BA with or without 0.25 mg L–1
naphthalene acetic acid (NAA) (Table 1) supplemented with 1 mg L–1 polyvinylpyrrolidone (PVP) and 3.0%
sucrose gelled with 0.65% agar. To eradicate endogenic
bacterial contaminations, 500 mg L–1 Augmentin was
also added to the medium. Data regarding frequency of shoot regeneration, mean number of shoots per explant, and mean shoot length were recorded after 8 weeks of culture.
Rooting was induced in 2 steps. In the first step, well-developed regenerated shoots were cultured on root induction medium containing MS medium (R1) supplemented with 1 mg L–1 indole-3 butyric acid (IBA)
and 15, 30, 45, and 60 g L–1 sucrose in separate Magenta
GA7 culture vessels for 6 weeks. Thereafter, the shoots from each of the induction media were transferred to IBA-free root proliferation medium (R2) containing each of 30, 45, and 60 g L–1 sucrose (Table 2) for 4 weeks. The rooted
plantlets were carefully removed from the agar-containing media under tap water and kept submerged in water for 10–15 min before being transferred to pots containing peat and coarse-grained sand in 1:1 or 2:1 ratios, peat alone, or coarse-grained sand alone. The pots were covered with transparent polythene bags to avoid wilting and placed in a growth room at ambient conditions of temperature and humidity.
The pH values of the regeneration, root induction, and proliferation media were adjusted to 5.6–5.8 using 0.1 N KOH or 0.1 N HC1 before autoclaving at 118 kPa and 121°C for 20 min. Filter-sterilized IBA and Augmentin were added to the culture media after autoclaving at approximately 44–46 °C. All cultures were incubated in a growth chamber at 24 ± 2 °C with a 16-h light photoperiod.
Embryo Cotyledon Embryonic node Plumular apices c d a b
Figure 1. In vitro shoot regeneration from plumular apice explants of chickpea: a, b)
isolation of embryo for preconditioning with 10 mg L–1 BA, c) initiation of shoots after
The experimental design was single factorial involving 8 treatments and 6 replications containing 8 explants per replicate (8 × 6 = 48 explants). Data for frequency (%) of shoot regeneration, mean number of shoots per explant, mean shoot length, and frequency (%) of rooting were subjected to a one-way analysis of variance (ANOVA) using the F test in SPSS 17.00 for Windows. The post hoc tests were performed using Duncan’s multiple range test to compare the differences among control and treatments. Data given in percentages were subjected to arcsine square root transformation (Snedecor and Cochran 1967) before statistical analysis.
3. Results
3.1. Shoot regeneration
Axillary shoot regeneration was recorded on all culture media irrespective of preconditioning or lack of conditioning (Table 1). All explants induced callus followed by shoot regeneration. Shoot regeneration started 4–5 days earlier on all preconditioned plumular apice explants compared to the unconditioned explants and well-developed shoots were observed after 10–11 days (Figure 1c) and 15–18 days, respectively. Moreover, conspicuously, the presence of NAA in the culture medium promoted induction of callus at the proximal ends of the plumular apices and delayed the development of shoot regeneration on all preconditioned explants when compared to the MS medium devoid of NAA (data not shown).
ANOVA showed insignificant effects of BA–NAA on frequency (%) of callus induction and shoot regeneration. The results showed significant differences (P < 0.05) for shoots per explant and shoot length on preconditioned
and unconditioned explants subjected to variations in BA–NAA in MS medium. In general, preconditioning promoted a high number of shoots per explant (Figure 1d) compared to shoot regeneration from unconditioned explants. Moreover, the presence or absence of NAA in the culture medium also had a noticeable effect on the mean number of shoots per explants for both preconditioned and unconditioned explants.
The number of shoots per explant of preconditioned plumular apices increased with each increase in BA concentration without NAA and ranged from 7.25 to 16.83 in ascending order (Table 1). In contrast, an increase in BA concentration in the presence of NAA decreased the mean number of shoots per explants, which ranged from 22.50 to 12.50 in descending order. The maximum number of shoots per explant from the preconditioned explants was 22.50 and was recorded on MS medium containing 0.25 mg L–1 BA and 0.25 mg L–1 NAA. This was followed by
19.08 shoots per explant on MS medium containing 0.50 mg L–1 BA and 0.25 mg L–1 NAA. On the other hand, the
unconditioned plumular apices were less regenerative and showed variable responses to BA concentrations with or without NAA. The variants of BA without NAA promoted higher shoot regeneration in the range of 6.66 to 13.17 compared to variants of BA with 0.25 mg L–1 NAA, which
had lower shoot regeneration in the range of 3.33 to 5.33. A maximum of 13.17 shoots per explant from unconditioned plumular apices were recorded on MS medium containing 0.50 mg L–1 BA without NAA.
Shoot length decreased with each increase in BA concentration for both preconditioned and unconditioned explants in the culture medium. The presence of NAA in
Table 1. The effects of preconditioning with BA on shoot regeneration in chickpea.
BA
(mg L–1) (mg LNAA–1)
Frequency of shoot
regeneration (%) Number of shoots per explant Shoot length (cm)
CPA* UCPA** CPA UCPA CPA UCPA
0.25 0.00 100ns 100ns 7.25e 9.00b 1.77a 1.95a
0.50 0.00 100 100 14.83cd 13.17a 1.72a 1.66b 1.00 0.00 100 100 15.50bc 7.83c 1.54a 1.46c 2.00 0.00 100 100 16.83abc 6.67d 1.25bc 1.21d 0.25 0.25 100 100 22.50a 4.60f 1.30b 1.10d 0.50 0.25 100 100 19.08ab 3.40h 1.17bc 0.83e 1.00 0.25 100 100 13.50cd 3.83g 1.13bc 0.78e
2.00 0.25 100 100 12.50de 5.33e 1.01c 0.80e
*CPA = conditioned plumular apice explant, **UCPA = unconditioned plumular apice explant. Values within a column followed by different letters are significantly different at the P = 0.05 level according to Duncan’s test.
the culture media had a negative effect on the shoot length of both preconditioned and unconditioned explants. However, the negative effect of NAA on shoot length was more conspicuous in unconditioned explants than in preconditioned explants. Mean shoot length ranged from 1.01 to 1.77 cm and from 0.78 to 1.95 cm on preconditioned and unconditioned explants, respectively (Table 1). The maximum shoot length for both preconditioned and unconditioned explants was recorded on MS medium containing 0.25 mg L–1 BA.
3.2. Rooting
No root induction, and consequently no rooting, was noted for the R1 medium containing IBA supplemented with 15 g L–1 sucrose followed by transfer to the R2
medium containing 30, 45, and 60 g L–1 sucrose (Table 2).
Shoots taken from the medium containing 30 g L–1 sucrose
induced 12.50% root initials. However, on transfer to R2 media containing 30, 45, and 60 g L–1 sucrose, the rooted
shoots failed to show any development and withered. At a maximum frequency of 50% for each, root initial induction was recorded from the R1 rooting medium containing 4.5% and 6.0% sucrose.
The root initials began to proliferate after 3 weeks of culture (Figure 2a) in both root initiation media. After transfer to the root development media, it was conspicuous that rooting was observed only on hardened and semihardened shoots in the rooting media, which also induced multiple secondary shoots (Figure 2b). The application of different sucrose concentrations was employed to bring about hardening and rooting and
showed positive effects on the hardening of shoots in the R1 rooting medium. All R2 rooting media containing 30, 45, and 60 g L–1 sucrose and devoid of IBA further
enhanced the hardening of shoots at a variable frequency. The best rooting and hardening of shoots, which ranged from 60% to 100% (Figure 2c), was observed on shoots taken from R1 root initials induction media containing 45 g L–1 sucrose followed by culturing on R2 rooting medium
containing 30, 45, and 60 g L–1 sucrose. However, the
rooting on shoots taken from R1 root initials induction media containing 60 g L–1 sucrose and moved to R2 rooting
medium containing 30, 45, and 60 g L–1 sucrose ranged
from 0% to 100%. Shoots with 50% root initials from R1 root initials induction media containing 60 g L–1 sucrose
with 1 mg L–1 IBA withered on R2 root development
medium containing 30 g L–1 sucrose.
The results further showed that secondary shoots on the rooting media could be best rooted on IBA-free rooting media containing 45 g L–1 sucrose, whereas shoots from
the media with 15 and 30 g L–1 sucrose failed to regenerate
roots (Figure 2c). Rooted shoots failed to acclimatize in substrate containing peat and coarse-grained sand in a 1:1 ratio, peat singly, or coarse-grained sand singly within 1–2 weeks after transfer to the pots. Excessive moisture of the soil also promoted fungus growth, which damaged the stems of the tissue-cultured plantlets. However, soil with a 2:1 ratio of peat and coarse-grained sand was found to be more favorable for prolonged plant growth (Figure 2d). The acclimatized plantlets were transferred to a greenhouse for seed setting.
Table 2. The effect of sucrose concentration on rooting in chickpea.
Root initials induction (R1) media
containing 1 mg L–1 IBA and sucrose (g L–1) Root initials induction percentage (%) Root development (R2) media with sucrose (g L–1) and no IBA percentage (%)Rooting
15 0c 30 0d 45 0d 60 0d 30 12.50b 30 0d 45 0d 60 0d 45 50.00a 30 80.0b 45 100.0a 60 60.0c 60 50.00a 30 0d 45 100.0a 60 80.0b
4. Discussion
A plumule (plumular apice) is a powerful explant with a high regeneration frequency and has been reported in legumes like pigeonpea (Surekha et al. 2007), cowpea (Aasim et al. 2009a), peanut (Singh and Hazra 2009), and lentil (Aasim 2012). This experiment reports preconditioning of explants with cytokinin for enhancing cell division, which ultimately increased the shoot regeneration. Brar et al. (1999) used pretreated cotyledons of cowpea with 25 mg L–1 BA for 15 days followed by culturing on MS
medium containing 1 mg L–1 BA for shoot regeneration.
Aasim et al. (2009a, 2010) reported successful in vitro shoot regeneration in a short time from preconditioned plumular apices and embryonic axis of cowpea using 10 mg L–1 BA for 5 days. Aasim et al. (2011) reported
successful shoot regeneration from preconditioned mature embryo and embryonic axis explants of chickpea. Similarly, Aasim (2012) also reported shoot regeneration from preconditioned immature plumular apices of lentil. Likewise, this study also confirms that preconditioning of the plumular apice explants of chickpea improves shoot regeneration compared to unconditioned explants.
Callus induction was observed at the proximal ends of the plumular apice explants on MS medium containing variants of BA with or without 0.25 mg L–1 NAA on
both preconditioned and unconditioned explants. Aasim et al. (2009a) also reported callus induction on preconditioned plumular apices of cowpea cultured on different concentrations of BA with or without NAA. Similarly, Aasim et al. (2011) reported callus induction on preconditioned mature embryo and embryonic axes of chickpea.
It was observed that initial preconditioning with BA not only enhanced callogenesis but also promoted earlier shoot initiation compared to unconditioned explants. However, the presence of NAA in the culture medium favored more callusing compared to callusing in the culture medium devoid of NAA, which is in agreement with the findings of Aasim et al. (2009b), who also reported a positive effect of the presence of NAA in the culture medium on callus induction with increased diameter in cowpea.
A comparison of preconditioned and unconditioned explants suggests that plumular apice explants have a high
shoot regeneration potential that improves even further when the explants are preconditioned. Preconditioning of plumular apices exploited the potential of the explant in positive way and resulted in a 2-fold to 5-fold increase in the number of shoots per explant compared to unconditioned explants. These results are in agreement with those of Aasim et al. (2009a), who also reported 100% shoot regeneration from plumular apice explants of cowpea.
The addition of NAA in the culture medium resulted in decreased shoot regeneration. This is in agreement with Brar et al. (1997) and Aasim et al. (2009a, 2009b), who also reported a negative effect of NAA along with BA on shoot regeneration in cowpea. In another study, Aasim et al. (2011) reported variable effects of concentrations of NAA+BA in the culture medium on the mean number of shoots per explant of preconditioned embryo and embryonic axis explants. It was also noticed in this study that a BA concentration used singly had a variable effect on shoot regeneration from preconditioned and unconditioned explants. Comparing the effects of preconditioning with lack of conditioning, the mean number of shoots per explant was higher for preconditioned explants in culture media containing variants of BA with or without NAA.
If we compare the effects of preconditioning or lack of conditioning of plumular apices on regenerated shoot length, the results of this study show that a very similar shoot length was recorded for preconditioned and unconditioned explants with variants of BA used singly in the culture medium. However, variants of BA with 0.25 mg L–1 NAA led to the development of stunted shoot length
on unconditioned explants compared to the shoot length on preconditioned explants. The occurrence of stunted shoots on unconditioned explants cultured on medium containing BA with NAA might be due to inhibition and less active cell division in these explants. These results confirm previous studies by Chen et al. (1995) in mung bean and Aasim et al. (2009a, 2009b) in cowpea.
The application of phytohormones, especially auxins, is used to induce rooting in legumes. However, the recalcitrant nature of legumes towards rooting has slowed down the application of biotechnological tools in legume crops (Fratini and Ruiz 2003). Rooting and acclimatization of in
a b c d
(ii) (i)
Figure 2. Root induction and acclimatization of chickpea: a) root initiation in the R1
rooting media, b) multiple shoot induction in the R1 rooting media, c) 100% rooting in the R2 rooting media, and d) acclimatization of chickpea plants in the green house.
vitro regenerated chickpea shoots are the major problems in successful regeneration of plantlets. The results of this study show that regenerated shoots transferred to rooting media containing IBA induced multiple shoots. Multiple shoot induction in rooting media containing IBA or any auxins in chickpea is an unknown phenomenon. However, the results are supported by previous findings of Aasim et al. (2009a), who reported multiple secondary shoots on root induction media containing IBA in cowpea. Similarly, Aasim (2012) also reported the proliferation of 4–6 secondary shoots along with rhizogenesis arising from the same points on shoots in lentil, although several protocols with variable success had been reported previously. Some studies suggested that sucrose concentration has effects on the rooting of plant shoots. The results of this study showed that rooting was affected by the concentration of sucrose along with IBA. IBA with 45 g L–1 sucrose
followed by transfer to MS medium containing 45 g L–1
sucrose was the most favorable rooting medium. Polisetty et al. (1996, 1997) and Sanyal et al. (2005) reported a high percentage of root induction from excised shoots in a medium containing reduced nitrogen and sucrose. To overcome the problem of rooting in chickpea, Chakraborti et al. (2006) grafted shoots onto rootstocks and obtained 90%–95% survival in soil after hardening, whereas Khawar and Ozcan (2004) used Agrobacterium rhizogenes for root formation in chickpea.
The application of different sucrose concentrations employed to bring about hardening and rooting showed that 45 g L–1 sucrose with IBA was the most promising
sucrose concentration in the culture media to induce rooting in the R1 medium. Chakraborti et al. (2006) cultured shoots with bright green properly opened leaves as well as distinct nodes and internodes on elongation media for rooting. Similar results were also reported by Jayanand et al. (2003) in chickpea. The results of this study showed that 45 g L–1 sucrose concentration increased the
hardening frequency and rooting (50%) on MS medium (R1) containing 1 mg L–1 IBA. Aasim et al. (2011) reported
a low rooting frequency at 3.0% sucrose in chickpea. Those results clearly showed the influence of sucrose concentration on rooting, in agreement with Staikidou et al. (2006), who also used increased sucrose concentrations for rooting in Galanthus species.
Our results show the importance of the substrate used during acclimatization. The rooted plantlets were best acclimatized in the substrate containing peat and coarse-grained sand in a 2:1 ratio. All the other substrates failed in the acclimatization of the plantlets. The plants failed to acclimatize in pots containing compost or coarse sand singly or in a 1:1 combination, mainly due to the high or low water-retaining capacity of the substrates. Anwar et al. (2008) suggested having a substrate with good aeration and a good water-holding capacity to achieve successful establishment of chickpea plantlets.
A successful plant regeneration and acclimatization protocol from previously unreported plumular apice explants establishes a new vista in chickpea biotechnology, which will hopefully facilitate easy genetic transformation of this important grain legume in the future.
References
Aasim M (2012) Micropropagation of lentil (Lens culinaris Medik.) using pulse treatment of immature plumular apices. Pak J Agric Sci 49: 149–154.
Aasim M, Day S, Rezai F, HajyzadehM, Mahmud ST, Ozcan S (2011) In vitro shoot regeneration from pre-conditioned explants of chickpea (Cicer arietinum L.) cv. Gokce. African J Biotech 10: 2020–2023.
Aasim M, Khawar KM, Özcan S (2009a) In vitro micropropagation from plumular apices of Turkish cowpea (Vigna unguiculata L.) cultivar Akkiz. Sci Horti 122: 468–471.
Aasim M, Khawar KM, Özcan S (2009b) Comparison of shoot regeneration on different concentrations of TDZ from shoot tip explant of cowpea on gelrite and agar containing medium. Not Bot Horti Agrobot Cluj 37: 89–93.
Aasim M, Khawar KM, Özcan S (2010) Efficient in vitro propagation from pre-conditioned embryonic axes of Turkish cowpea (Vigna unguiculata L.) cultivar Akkiz. Arch Biol Sci 62: 1047– 1052.
Anwar F, Sharmila P, Pardha Saradhi P (2008) An optimal protocol for in vitro regeneration, efficient rooting and stable transplantation of chickpea. Physiol Mol Biol Plants 14: 329– 335.
Barna KS, Wakhlu AK (1994) Whole plant regeneration of Cicer arietinum from callus cultures via organogenesis. Plant Cell Rep 13: 510–513.
Brar MS, Al-Khayri JM, Morelock TE, Anderson EJ (1999) Genotypic response of cowpea Vigna unguiculata (L.) to in vitro regeneration from cotyledon explants. In Vitro Cell Dev Biol 35: 8–12.
Brar MS, Al-Khayri JM, Shamblin CE, McNew RW, Morelock TE, Anderson EJ (1997) In vitro shoot tip multiplication of cowpea Vigna unguiculata (L.) Walp. In Vitro Cell Dev Biol 33: 111– 118.
Chakraborti D, Sarkar A, Das S (2006) Efficient and rapid in vitro plant regeneration system for Indian cultivars of chickpea (Cicer arietinum L.). Plant Cell Tiss Org Cult 86: 117–123.
Chauhan R, Singh NP (2002) Plant regeneration via somatic embryogenesis in chickpea (Cicer arietinum L.). Ind J Gen 62: 319–321.
Chauhan R, Tiwari A, Singh NP (2003) Differential requirement of mature and immature embryo of chickpea (Cicer arietinum L.) for in vitro regeneration. Ind J Plant Physiol 8: 28–33. Chen J, Witham FH, Heuser CW (1995) Inhibition of NAA-induced
adventitious roots in mung bean cuttings by kinetin, zeatin, ethidium bromide and other DNA intercalators. World Wide Web J Biol 1: 1–8.
Fratini R, Ruiz ML (2003) A rooting procedure for lentil (Lens culinaris Medik.) and other hypogeous legumes (pea, chickpea and Lathyrus) based on explant polarity. Plant Cell Rep 21: 726–732.
Hita C, Lafarga C, Gaerra H (1997). Somatic embryogenesis from chickpea (Cicer arietinum L.) immature cotyledons. The effect of zeatin, gibberellic acid and indole-3-butyric acid. Acta Physiol Plant 19: 333–338.
Islam R, Riazuddin S (1994) Somatic embryogenesis from cotyledons of chickpea. Pak J Bot 26: 197–199.
Jayanand B, Sudarsanam G, Sharma KK (2003) An efficient protocol for the regeneration of whole plants of chickpea (Cicer arietinum L.) by using axillary meristem explants derived from in vitro-germinated seedlings. In Vitro Cell Dev Biol Plant 39: 171–179.
Kar S, Basu D, Das S, Ramakrishnan NA, Mukherjee P, Nayak P, Sen SK (1997) Expression of CryIA(C) gene of Bacillus thuringiensis in transgenic chickpea plants inhibits development of pod borer (Heliothis armigera) larvae. Transgenic Res 6: 177–185. Kar S, Johnson TM, Nayak P, Sen SK (1996) Efficient transgenic plant
regeneration through agrobacterium-mediated transformation of chickpea (Cicer arietinum L.). Plant Cell Rep 16: 32–37. Khawar KM, Ozcan S (2004) Hairy root transformation in Turkish
chickpea (Cicer arietinum L) cultivars. Biotechnol Biotechnol Equip 18: 51–54.
Kiran G, Kaviraj CP, Jogeswar G, Kishor PBK, Rao S (2005) Direct and high-frequency somatic embryogenesis and plant regeneration from hypocotyls of chickpea (Cicer arietinum L.), a grain legume. Curr Sci 89: 1012–1018.
Kumar VD, Kirti PB, Sachan JKS, Chopra VL (1994) Plant regeneration via somatic embryogenesis in chickpea (Cicer arietinum L.). Plant Cell Rep 13: 468–472.
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497.
Paul V, Chandra R, Khetarpal S, Polisetty R (2000) Effect of BA induction period on shoot differentiation from seedling explants of chickpea (Cicer arietinum L.). J Plant Biol 27: 235– 239.
Polisetty R, Patil P, Deveshwar JJ, Khetarpal S, Chandra R (1996) Rooting and establishment of in vitro grown shoot tip explants of chickpea (Cicer arietinum L.). Ind J Exp Biol 34: 806–809. Polisetty R, Paul V, Deveshwar JJ, Khetarpal S, Suresh K, Chandra
R (1997) Multiple shoot induction by benzyladenine and complete plant regeneration from seed explants of chickpea (Cicer arietinum L.). Plant Cell Rep 16: 565–571.
Rao BG, Chopra VL (1987). The influence of media and organogenesis in chickpea. Int Chickpea Newsl 17: 7–10.
Rizvi SMH, Singh RP (2000) In vitro plant regeneration from immature leaflet-derived callus cultures of Cicer arietinum L. via organogenesis. Plant Cell Biotechnol Mol Biol 1: 109–114. Sagare AP, Suhasini K, Krishnamurthy KV (1993) Plant regeneration
via somatic embryogenesis in chickpea (Cicer arietinum L.). Plant Cell Rep 12: 652–655.
Sanyal I, Singh AK, Kaushik M, Amla DV (2005) Agrobacterium mediated transformation of chickpea (Cicer arietinum L.) with Bacillus thuringiensis cry1Ac gene for resistance against pod borer insect Helicoverpa armigera. Plant Sci 168: 1135–1146. Sharma KK, Ortiz R (2000). Program for the application of genetic
transformation for crop improvement in the semi-arid tropics. In Vitro Cell Dev Biol Plant 36: 83–92.
Singh S, Hazra S (2009) Somatic embryogenesis from the axillary meristems of peanut (Arachis hypogaea L.). Plant Biotech Rep 3: 333–340.
Snedecor GW, Cochran WG (1967). Statistical Methods. Iowa State University Press, Ames, Iowa, USA, pp. 327–329.
Staikidou I, Selby C, Hanks G (2006) Stimulation of in vitro bulblet growth in Galanthus species with sucrose and activated charcoal. Acta Hortic 725: 421–426.
Suhasini K, Sagare AP, Krishnamurthy KV (1994) Direct somatic embryogenesis from mature embryo axis in chickpea (Cicer arietinum L.). Plant Sci 102: 189–194.
Surekha C, Arundhati A, Seshagiri Rao G (2007) Differential response of Cajanus cajan varieties to transformation with different strains of Agrobacterium. J Biol Sci 7: 176–181.