ContentslistsavailableatScienceDirect
Colloids
and
Surfaces
B:
Biointerfaces
jou rn a l h om ep ag e :w w w . e l s e v i e r . c o m / l o c a t e / c o l s u r f b
Protocols
Encapsulation
of
living
bacteria
in
electrospun
cyclodextrin
ultrathin
fibers
for
bioremediation
of
heavy
metals
and
reactive
dye
from
wastewater
Nalan
Oya
San
Keskin
a,b,c,∗∗,
Asli
Celebioglu
a,d,
Omer
Faruk
Sarioglu
a,d,
Tamer
Uyar
a,d,∗,
Turgay
Tekinay
c,e,∗∗aUNAM-NationalNanotechnologyResearchCenter,BilkentUniversity,Ankara06800,Turkey bPolatlıScienceandLiteratureFaculty,BiologyDepartment,GaziUniversity,Ankara06900,Turkey cLifeSciencesApplicationandResearchCenter,GaziUniversity,Ankara06830,Turkey
dInstituteofMaterialsScienceandNanotechnology,BilkentUniversity,06800Ankara,Turkey
eFacultyofMedicine,DepartmentofMedicalBiologyandGenetics,GaziUniversity,Ankara06560,Turkey
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:Received23February2017
Receivedinrevisedform11October2017 Accepted17October2017
Availableonline18October2017 Keywords: Electrospinning Cyclodextrin Nanofibers Bacteria Encapsulation Heavymetals Reactivedye
a
b
s
t
r
a
c
t
Cyclodextrins(CD)arecyclicoligosaccharidesproducedfromtheenzymaticdegradationofstarchasa whitepowderform;ontheotherhand,theycanbetransformedintoultrathinelectrospunfiberformby electrospinningtechnique.Theelectrospuncyclodextrinfibers(CD-F)canbequiteattractivematerials toencapsulatebacteriaforbioremediationpurposes.Forinstance,CD-Fnotonlyserveasacarriermatrix butalsoitservesasafeedingsourcefortheencapsulatedbacteria.Inthepresentstudy,wedemonstrate afacileapproachbyencapsulationofbacteriaintoCD-Fmatrixforwastewatertreatmentapplication. Thenaturalandnon-toxicpropertiesofCD-Frenderabetterbacterialviabilityforfibrousbiocomposite. TheencapsulatedbacteriainCD-Fexhibitcellviabilityformorethan7daysat4◦Cstoragecondition. Fur-thermore,wehavetestedthebioremediationcapabilityofbacteria/CD-Fbiocompositeforthetreatment ofheavymetals(Nickel(II)andChromium(VI))andtextiledye(ReactiveBlack5,RB5).Thebacteria/CD-F biocompositehasshownremovalefficiencyofNi(II),Cr(VI)andRB5as70±0.2%,58±1.4%and82±0.8, respectively.Asanticipated,thepollutantsremovalcapabilitiesofthebacteria/CD-Fwashigher com-paretofreebacteriasincebacteriacanuseCDasanextracarbonsourcewhichpromotestheirgrowth rate.ThisstudydemonstratesthatCD-Faresuitableplatformsfortheencapsulationofbacterialcellsto developnovelbiocompositesthathavebioremediationcapabilitiesforwastewatertreatment.
©2017ElsevierB.V.Allrightsreserved.
1. Introduction
The use of biomass such as bacteria, fungi and algae as a biosorbenthavegreat potentials for cleaningup environmental contaminantsduetotheavailabilityofwiderangeofsorptionsites which retainmetals andmetallic ions,aswellasother organic compounds[1,2].Avarietyofapproachesareemployedin bioreme-diation,encapsulationofbacterialcellsisconsideredtobethemost promisingduetotheirpotentialinremovingspecificcontaminants within a carrier matrix [3]. Encapsulationhas many promising
∗ Correspondingauthorat:UNAM-NationalNanotechnologyResearchCenter, BilkentUniversity,Ankara,06800,Turkey.
∗∗ Correspondingauthorsat:PolatlıScienceandLiteratureFaculty,Biology Depart-ment,GaziUniversity,Ankara,06900,Turkey.
E-mailaddresses:oyasan@gazi.edu.tr(N.O.SanKeskin), uyar@unam.bilkent.edu.tr(T.Uyar),ttekinay@gazi.edu.tr(T.Tekinay).
applicationsinabroadrangeoffieldsfrombioreactorstomedicine. Advantagesofencapsulatedcellsinclude,easeofseparation, pro-tectionfromharshexternalconditions,andreducedsusceptibility tocontaminationbyforeignorganisms.Themostcommon encap-sulationtechniquesarespray-drying[4],emulsifying-crosslinking [5]orcoacervation[6].However,thesetechniquesinvolveuseof hightemperatureororganicagentsinatleastoneoftheproduction steps,leadingtopotentialdestructionofsensitiveencapsulated nutrients aswell as toxicity problems associated withresidual organic agents [7]. Hence, to overcome these limitations, new encapsulationtechnologiesthatdonotinvolveharshconditions suchhightemperatureandtoxicsolventsarebeinginvestigated, which can provide effective encapsulation for sensitive guest agents.
Recently,electrospinningtechniquehasattractedcertain atten-tionin which activeagents suchaspharmaceuticals [8,9], food additives [10–12] are being simultaneously encapsulated into fibermatrixviaelectrospinningprocess.Electrospunfibershave https://doi.org/10.1016/j.colsurfb.2017.10.047
receiveda great deal of attention owing to their high surface area,verylight-weight,nano-porousnature,andflexibilityin spe-cific physical and chemical functionalization [13–15]. Thereby, electrospunfibersandtheirwebsareverypromisingcandidates for membranes/filters and environmental applications [16–20]. Forexample,electrospunwebswerealsousedasimmobilization matrixforlivingmicroorganismforremediationofwastewater [21–24].Therearealsoveryfewreportsaboutencapsulationof microorganisminelectrospunfibers.For example,Salalhaetal. [25]showedencapsulationofEscherichiacoliandStaphylococcus albusby electrospinningof bacteriasuspensions withpolyvinyl alcohol (PVA).Further, Funget al. [26] has successfully encap-sulatedprobiotics inagrowaste-based nanofibers,and Liuet al. [27]hasfabricatedaninsolublefibrousmaterialvia electrospin-ningwhichencapsulateindustriallyimportantbacteria.Another reportbyVajdaietal.[28]demonstratessuccessfultrackingofcell viabilityofStenotrophomonasmaltophiliabacteriainPVAfibrous webs.Furthermore,Lee andBelcherfabricatedone-dimensional (1D)micro-andnanosizeddiameterfiberswithlongrod-shaped M13viruses[29].Encapsulationtechniquemayrepresent excel-lentalternativetolyophilizationforthepreservationoforganisms. Furthermore,noneedtowaitbacterialattachment(3–21days)and haslowercontaminationrisk.Eventhoughfewstudiesreported successful encapsulation of bacteria in electrospun matrix, the proof-of-concept studiesfor thepotential applicationsof these fibershavenotbeendemonstratedsatisfactorily.Oneofourrecent studybioremediatingbacterial cells weresuccessfully encapsu-latedintopolyvinylalcohol(PVA)andpolyethyleneoxide(PEO) polymericmatriceswhilekeepingthebacteriabioactiveandthe viablecellnumbersindesirableamounts[30].Moreover,thechoice offibermatrixwasoftena water solublepolymer suchasPVA orPEO since aqueoussolution waspreferablyused as an elec-trospinningsolutioninordertoprovidesuitableenvironmentfor thebacteriabeforeelectrospinning.Yet,aftertheelectrospinning, thesesyntheticpolymericmatrixesarenotveryappropriateonce thebacteriaareencapsulatedwithinthematrixsincebacterianeed carbonsourcefortheirsurvival.Hence,carbohydrate-basedfiber wouldbemuchpreferablematrixfortheencapsulationofbacteria. Therefore,inthisstudywechoosecyclodextrin(CD)electrospun fibermatrixtoencapsulatebacteriaforbioremediationpurposes. CDsareclassifiedascyclicoligosaccharides whichareproduced fromstarchasawhitepowderform,andtheycanbetransformed intofiberformbyelectrospinningtechnique[31–34].Ingeneral, polymericmaterialsareneededfortheelectrospinningoffibers sincechainentanglementandoverlappingbetweenthepolymer chainsare therequirementstosustainthecontinuousjet elon-gationduringtheelectrospinning process[13,14].Nevertheless, inourrecent studieswehave shownsuccessfulelectrospinning of fibers from CD without using any polymeric carrier matrix. AlthoughCDarecyclicoligosaccharides,whichareconsideredtobe verysmallmoleculescomparedtopolymers,theself-assemblyand aggregationcharacteristicsofCDmoleculesintheirconcentrated solutionsenablestheelectrospinningoffibersfromCDwithoutthe needofcarrierpolymericmatrix[31–34].
Inourstudy,wesuccessfullydemonstratedtheencapsulation ofLysinibacillussp.NOSKbacteriainwaterbasedand biocompati-blenon-polymericcyclodextrinfibers(CD-F)usingelectrospinning process. The CD-Fprotects bacterial cells to reduce theeffects ofexteriorenvironment;besidetheyservedasanencapsulating matrixandcarbon-sourceforthebacteria.Furthermore,these bac-teriaencapsulatedwater-solublefibersexhibitedaninstantrelease mechanism.The potentialapplicationof thisbacteria/CD-F bio-composite in bioremediationof heavy metals (Ni(II) and Cr(VI) ions)andreactivedyefromaqueoussolutionwassystematically investigated.Themorphologicalcharacteristicsofthepristineand bacteriaencapsulatedfibers wereevaluated.Our resultsclearly
Fig.1.Schematicviewofencapsulationofbacteriainelectrospunfibersby electro-spinningprocess(CD:Cyclodextrin,F:Fiber).
showedthatencapsulatedLysinibacillussp.NOSKwereviable,and theirmetabolicactivitywasnotaffectedbytheencapsulationinto electrospunCD-F.Onthebasisofpresentresults,wecansuggest thatthisnovelbacteria/CD-Fbiocompositecouldbeofinterestfor wastewatertreatment.
2. Materialandmethods
2.1. Materials
The hydroxypropyl--cyclodextrin (HPCD) (degree of sub-stitution:∼0.6,Cavasol®W7HP Pharma)waskindlydonatedby WackerChemieAG(Germany).Thedeionizedwaterwasobtained fromtheMilliporeMilli-QUltrapureWaterSystem.Thestock solu-tionofNi(II)waspreparedbydissolvingnickelsulfateheptahydrate (SigmaAldrich,99.9%)indeionizedwater.Potassiumdichromate (SigmaAldrich,≥99.0%)wasusedtoprepareCr(VI)aqueous solu-tionbydissolvingindeionizedwater.Reactivedye(ReactiveBlack 5(RB5))wasobtainedfromSETAS¸ChemistryFactory(Tekirdag, Turkey).Appropriatevolumeofthestocksolutionswasaddedto thenutrientbroth(NB)medium(Peptonefrommeat5.0g/L;Meat extract3.0g/L)forpreparationoftheexperimentalsamples. 2.2. Cultivationofbacteriuminnutrientbroth(NB)
TheLysinibacillussp.NOSKcultureisolatedfromwastewater treatmentsystem[35]wasroutinelymaintainedinNutrientBroth (NB)medium.Theworkingbacterialculturewaspreparedfrom thestock culture stored at−20◦C in 70%(v/v) sterile glycerol.
ThefrozenstockcultureswererevivedthreetimesinNBmedium priortoexperimentaluse.Thereactivatedculturewasgrownin Erlenmeyer flasks containing NBmedium to getenough active inoculum.Thefreshlyculturedcellswerecollectedby centrifuga-tionat5000rpmfor10minandthendefinedvolumeofbiomass usedforelectrospinningprocess.
2.3. Electrospinningofbacteriaencapsulatedcyclodextrinfibers (bacteria/CD-F)andbacteria-freeCD-F
Theelectrospinningofbacteria encapsulatedCD-Fwere per-formedbyusinghighlyconcentratedaqueousCDsolution(200%, w/v) with 0.5%(w/v) of skim milk media(Fig.1). For bacteria encapsulationprocess,electrospinningwascarriedoutbymaking
asuspensionofbacteriaintheCDaqueoussolution.Theamount ofbacteriaintheCDaqueoussolutionwasvariedas0.25%,0.5%, 1%and 2% (w/w)(respect toCD concentration) inorder toget enoughbacterialcountinCD-F.Theelectrospinningofbacteria-free CD solution and bacteria/CD suspension wereperformed sepa-ratelybyusing1mLsyringewithaneedleofaninnerdiameter of0.4mm.Oneoftheelectrodesofthehighvoltagepowersupply (MatsusadaPrecision,AUSeries)wasclampedtothemetalneedle tip,andtheotherwasclampedtothegroundedcylindrical alu-minumcollector.Theelectrospinningparameterswereoptimized asfollows:appliedvoltage:15kV,tip-to-collectordistance:10cm, andthesolutionflowrate:0.5mL/h.Alltheelectrospinning pro-cesseswereperformedinaUVsterileelectrospinningcabinatroom temperature(∼24◦C)and25%relativehumidityconditions.After
electrospinningprocess,thebacteriaencapsulatedCDfibershave beenstoredat4◦C(inarefrigerator)insmallplasticpetridishes whichwereenclosedbyparaffinbandsforpreventionofairand humiditypassage.Petridishesweresterile(UV-sterilized),dryand havingminimizedheadspacevolume(byoverspreadwithbacteria CD-F)toenhancethestabilityoftheencapsulatedbacteria. 2.4. Viabilitytesting
Inordertocheckbacterialviabilityandtheirsuccessful encap-sulation within the electrospun CD-F, Lysinibacillus sp. NOSK cellswerestainedwithfluorescentstains(LIVE/DEADBacLightTM kit)priortotheelectrospinningprocess.Microscopicassessment of LIVE/DEAD-stained bacterial cells is usually simplified with “green”-labeledcells foralivebacteria. Photographsweretaken byusinganopticalmicroscopewithanattachedfluorescenceunit (Leica,DMI4000B).TheviabilityofLysinibacillussp.NOSKeitherin electrospinningsolutionsorencapsulatedinCD-Fwasalso deter-minedascolony-formingunits(CFU)usingthepour-plateassay. Toevaluatetheencapsulationefficiency,piecesoftheCD-Ffibrous materialwereweighed(10mg)whichcontainthelivingorganisms. Nutrientmedium (1.0mL) wasadded tothesepiecesin micro-centrifugetubes,heldfor60minatroomtemperatureandserial 10-folddilutionsweremadeonthesesamplesandplatedon Nutri-entagar.Afterincubationatoptimalconditions,thenumberofCFU wasdetermined.Alltestswereperformedintriplicate.
2.5. Characterization
Themorphologicalinvestigationofthefiberswascarriedoutby usingscanningelectronmicroscopy(SEM)(200FEG,FEI).The sam-plesweresputtercoated(PECS-682)withagold-palladiumalloy for∼5nmthicknesspriortoSEMimaging.
Raman spectra of the samples were recorded on a WITec alpha300S Confocal Raman spectrometer. The near-infrared 785nmlinewasusedforexcitation.Forallpresentedspectra,the laserpoweronthesample didnot exceed3mW, and 10scans werecollectedin15s.Allspectrawererecordedwithaspectral resolutionof4cm−1.
2.6. Removalanalysis
Duringtheincubationperiod,a3mLsamplewastakendaily fromeachflask.Sampleswerecentrifugedtoprecipitatesuspended biomass at 10.000g for 5min.Cell free nutrient brothmedium wasusedastheblank.TheconcentrationsofNi(II)inthe super-natantwasdeterminedspectrophotometricallyat340nmbyusing sodiumdiethyl dithiocarbamate (from Merck) as a complexing agent. Residual Cr(VI) in the supernatant was measured using the 1,5-diphenylcarbazide (from Alfa Aesar, A Johnson Mathey Company,USA)methodat540nmbyUVspectrophotometer.The concentrationofdye(RB5)ineachaqueoussolutionwasmeasured
onanUV–visspectrophotometer whiletakingtheabsorptionat 597nm.Alltestswereperformedintriplicate.
2.7. Adsorptionisothermsandkineticsstudies
Adsorption coefficients were estimated via seven isotherm models (Langmuir, Langmuir with linear partitioning, Fre-undlich, Freundlich with linear partitioning, Generalized Langmuir–Freundlich, Toth, Linear) using the isotherm param-eter fitting software IsoFit [35]. The mean of three individual determinationswasusedtocalculatecellcounts.
3. Resultsanddiscussion
3.1. Electrospinningofbacteriaencapsulatedcyclodextrinfibers (bacteria/CD-F)andbacteria-freeCD-F
TheelectrospinningofpureCD-Fwithoutcontainingbacteria wasperformedtoobtaincontrolsample.TherepresentativeSEM imagesofelectrospunbacteria-freeCD-FareshowninFig.S1a–b. TheaveragefiberdiameteroftheelectrospunCD-Fwascalculated as605±205nmbymeasuringabout100fibersfromdifferentSEM images.Inordertoachievecontinuousfiberproductionby electro-spinning,oneoftheimportantoptimizationparameterisfinding theoptimalbacteriaamountforencapsulationprocess.Regardto this,theoptimizationofbacteriaamountforencapsulationinCD fiberswasinvestigatedbyusing0.25%,0.5%,1%and2%(w/w)of bac-teriaandthemorphologiesofthefibers’structureswereanalyzed bySEM.
SEMmicrographofthebacteriumisshowninFig.S2.InFig.2, SEMimagingconfirmedthesuccessfulelectrospinningofCD aque-ous solution into bead-free and uniform fibers having smooth morphology.TheincorporationofLysinibacillussp.NOSKintoCD solutiondid not significantlyaffect theelectrospinning process and in all bacteria concentrationused, fiberswere successfully produced(Fig.2a–d).However,wehadsomedifficultiesforthe electrospinningofCDsolutioncontaining2%(w/w)ofbacteria,the electrospinningjetwasdisturbedfrequently(Fig.2d).Therefore, wedecidedthat1%(w/w)ofbacteriawasanoptimal concentra-tionforthecontinuouselectrospinningofbacteria/CD-Ftoobtain homogenouslydistributedfibrouswebs(Fig.2c).Inacloserlook, thebacterialcells werecompletelyencapsulatedwithintheCD fibermatrix,forminglocalwideningofthefiber.Enlargementand wideningofsomeregionsinthefibersforthebacteria/CD-F sam-pleswaspreliminaryevidenceforbacterialincorporationinside theCD-FasdepictedinFig.2a–d.FromFig.2athruFig.2d,itwas alsoevidentthatwhenbacterialnumberwasincreasedinthe elec-trospinningsolution,theencapsulatedcellnumberwasincreased aswellintheelectrospunCD-Fmatrix.
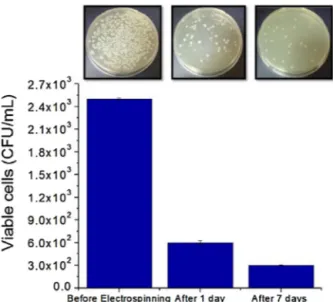
3.2. Viability
ViabilityofLysinibacillussp.NOSKcellswasdeterminedas num-berofcolony-formingunits(CFU)inCDsolutionandinCD-Fweb atrefrigerationtemperature(4◦C)atdifferenttimeintervals(24h to7days)(Fig.3).Over a periodof time,bacterialcell viability wasdecreased,onlyasignificantpercentageofbacteriaretained theirviabilityattheendof7days.Thecellviabilityof Lysinibacil-lussp. NOSKwasfoundto be2.5×103 CFU/mLin CD solution
priortoencapsulation,whichwasdecreasedto6.0×102CFU/mLin
bacteria/CD-Fstoredat4◦Conday1.Thebacterialcellwasfurther decreasedto3.0×102CFU/mLuponthebacteria/CD-Fsstorageat
4◦Cfor7days.Further,fluorescencestainingtechniquewas per-formedinordertogetanaccuratecellviabilityassessmentand cellcount.Thelossincellviabilitycouldbeattributedtotheeffect ofelectrostaticfieldgeneratedduringtheelectrospinningprocess
Fig.2.RepresentativeSEMimagesofcyclodextrin-fiber(CD-F)encapsulating(a)0.25%(w/w)(b)0.5%(w/w)(c)1%(w/w)and(d)2%(w/w)ofbacteria.Thecirclesshow someoftheencapsulatedbacteriumintotheelectrospunfibermatrix.
Fig.3.ViabilityofLysinibacillussp.NOSKasnumberofcolony-formingunits(CFU) inCDsolutionandbacteria/CD-Fat4◦Canddifferentstoragetimes(1dayto7days).
Insetphotographsshowsbacteriacountontheagarsurface.
duetotheapplicationofhighvoltagebetweenthemetalcapillary andthecollector[8,9].Furthermore,rapidevaporationof water duringelectrospinningprocessisalsoanticipatedtocausedrastic changesintheosmoticenvironmentofthebacteria.Theeffectof storageontheviabilityofencapsulatedbacteriaisofgreatconcern fortheirpotentialapplicationinindustrial-scaleprocesses,since applicationofthesenovelbioactivebiocompositesrequirestobe intactandfunctionalatthetimeofuseindesiredsite.
3.3. Characterization
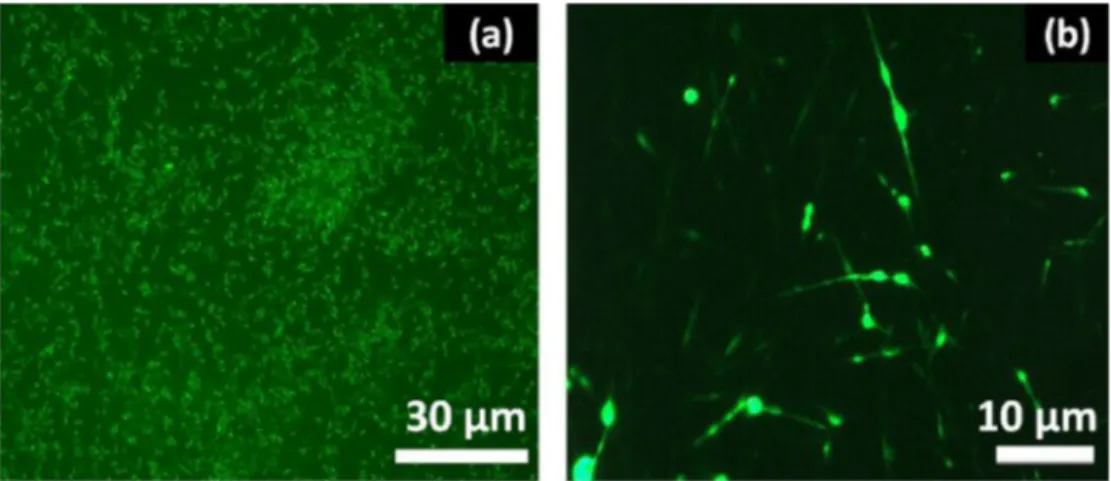
InadditiontotheSEMimage,presenceofLysinibacillussp.NOSK wasfurthersupportedbyfluorescencemicroscopyimages.Fig.4a clearlyindicatesthatthestainedLysinibacillussp.NOSKbacterial cellsemitgreenfluorescenceowingtotheirviabilitynaturewhich confirmstheirsuccessfulencapsulationinCD-Fmatrix(Fig.4b). Althoughbacterialcellsweregenerallylocalizedindividuallyinside thefibermatrix,somemoreagglomeratedcellswerealsoobserved incertainareasofthefibrousweb.
Raman spectroscopy is considered to be an molecular fin-gerprinting technique for studying structure of polymers and microorganisms[36].RamanspectraofpristineCD-F,pristine bac-teriaandbacteria/CD-FwithmainRamanshiftsandassignments oftheRamanbandsaregiveninFig.5.Theobservedpeaksaround 851cm−1intheRamanspectrumofpristineCD-Fisduetoskeletal CCH.PristinebacteriaRamanspectrashowstheamideIvibrationat 1650cm−1,andtheCH2deformationvibrationat1420cm−1.After
encapsulationprocess,presenceofproteinsinthebacteria/CD-F wasconfirmedfromthepeakaround1005cm−1andaswelltwo prominentpeaksi.e.,∼1048and∼995cm−1observedat polysac-charideregion(1200–900cm−1)couldbearisingfromvibrationsin thepeptidoglycanofbacterialcellwall26whichfurtherconfirmed
thepresenceofthebacterialcellsinthefibrousmatrix. 3.4. Removalanalysis
Theheavymetalremovalcapabilityofthebacteria/CD-Fwas investigatedinabatchsystemwithregardtotheinitialpHand Ni(II)concentrations.Uninoculatederlenmeyerflaskscontaining heavymetals,reactivedyeandCD-Fwereusedascontrol
sam-Fig.4. Fluorescencemicroscopyimagesofbacteria(a)beforeencapsulationprocess(b)afterencapsulationinCD-Fmatrix.
Fig.5. Ramanspectraof(a)pristineCD-F(b)pristinebacteriaand(b)bacteria/CD-F.InsetsshowthemicrographofpristineCD-F,pristinebacteriaandbacteria/CD-F.
plestoobserveanyreactionsofthemediawithheavymetaland dye(Fig.S3).Inthesesamples,removalyieldwas0.8%forNi(II), 0.3%forCr(VI)and%0.5forRB5.Therewasnosignificantdifference inthebioremovalyieldofNi(II),Cr(VI)andRB5bypristineCD-F, respectively.
Thepercentageuptakeofheavymetalsandreactivedyewere calculatedfromthedifferencebetweentheinitialandfinalvalues usingEq.(A.1)
Removal(%)=(C0−Ceq)/C0×100 (A.1)
HereC0andCeqaretheinitialandequilibriumconcentrationsof
heavymetalsandreactivedye(mgL−1),respectively.Theremoval capacity canbe determinedby themass balance principle(Eq. (A.2)).
qm=(C0−Cf)/Xm×100 (A.2)
Inthesetwoequations,qm(themaximumheavymetalanddye
uptake)representsthemaximumamountofheavymetal/dyeper unitofdryweightofmicrobialcells(mgL−1),Xmrepresentsthe
maximumdriedcellmass(gL−1),andC0andCfrepresenttheinitial
andfinalconcentrations(mgL−1),respectively.
TheinitialpHvalueofthesolutionisanimportantparameterto investigatehigherremovalcapacities.TheRB5andCr(VI)removal yieldsofthebacterialcellsweresimilarlyhighatpH8.0asfound inourpreviousstudy[35].Furthermore,inthisstudy,theeffect ofpHwasexaminedafter24hofbacterialincubationforthe sam-plesataninitialNi(II)concentrationof30mgL−1,butatdifferent initialpHvalueswithintherangeof4–9.AsshowninFig.6,the removalefficienciesafter24hwerefoundas%77.0,%77.5,%78.0, %76.1,%72.1and%71.7atpHvaluesof4.0,5.0,6.0,7.0,8.0and9.0, respectively.TheoptimalpHrangeforNi(II)removalwasfoundto be4.0–6.0.Anear-neutralpHconditionispreferablesince
opera-Fig.6. TheeffectofpHontheNi(II)removalyieldofthefreebacteriaat10mgL−1
ofinitialNi(II)concentration(T:30◦C;stirringrate:100rpm).Errorbarsrepresent
standarddeviationofthemeanvaluesforindependentreplicates(n=3).
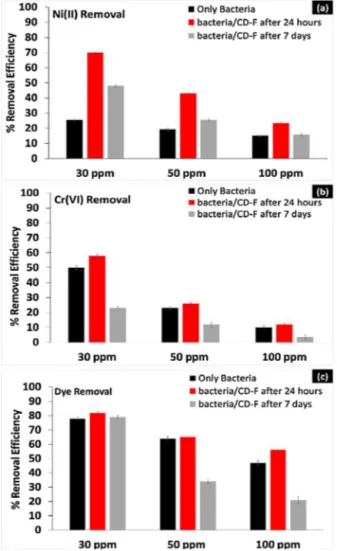
Fig.7.Theeffectofinitial(a)Ni(II)(b)Cr(VI)and(c)ReactiveBlack5concentration ontheremovalyieldofthefreebacteriaandbacteria/CD-Fafter1dayand7days storageat4◦Cduringthe24hincubationperiod(T:30◦C;stirringrate:100rpm).
Errorbarsrepresentstandarddeviationofthemeanvaluesforindependentreplicates (n=3).
tionundernear-neutralconditionscaneasilybeappliedtomost wastewatersystems[37].
TheeffectofinitialNi(II),Cr(VI)andRB5concentrationsonthe bioremovalperformance wasevaluated usingfree-bacteria and bacteria/CD-Fafter24hstorageat4◦C,andafter7daysstorageat 4◦Catdifferentinitialheavymetalsanddyeconcentrations(30mg L−1thru100mgL−1).AsseeninFig.7a,theaverageNi(II)removal yield was calculated as 25.5±0.3% for the free-bacteria. After encapsulationprocess,theNi(II)removalyieldofbacteria/CD-Fwas
around70±0.2%.After7daysstorageat4◦C,Ni(II)removalyieldof bacteria/CD-Freducedto48.07±0.4%.TheNi(II)removal capabili-tiesofthebacteria/CD-Fwerehigherthanthatofthefree-bacteria forallthesampleshavingdifferentinitialNi(II)concentrations. WhenCD-Fmatrixwasdissolvedinthemedium,bacteriausedCD asanextracarbonsourceandachievedanenhancedgrowthrate (OD600nm:2.09).However,sincethefreebacteriahadnoextra
car-bonsource,theirgrowthratewasnotenhanced(OD600nm:1.03)
(Fig.S4).
TheeffectoftheinitialconcentrationofCr(VI)ontheremovalof Cr(VI)isshowninFig.7b.Lysinibacillussp.NOSKcouldreduceCr(VI) 50±1.2%,23±1.3%and9.8±1.3%fromthemediumcontains30,50 and100mgL−1,respectively.Afterencapsulationprocess,the max-imumremovalyieldsofbacteria/CD-F were58±1.4%,26±0.9% and12±0.7%for30,50and100mgL−1Cr(VI)concentrations.After 7days storage at4◦C, Cr(VI) removalyields weredecreased to 23±1.2%,12±1.6%and3.5±1.2%for30,50and100mgL−1Cr(VI). Itisclearthat,after7daysstoragewithdecreaseinbacterialcount, theremovalperformancesofbacteria/CD-Fdecrease.
Fig.7cillustrates the evolution of the dyeremoval yield as a function of the initial dye concentration. At the end of the incubationperiod,themaximumremoval yieldswere78±1.1% at 30mgL−1. The removal capacities of Lysinibacillus sp. NOSK werefoundtoberangingbetween64% and47%in mediawith increasing RB5 concentrations. The pattern of the changes in removalefficiencyofbacteria/CD-Fwasfoundtobequite differ-ent.Bacteria/CD-Fremovedthedyeefficiently,inspiteofincreasing dyeconcentrationsupto300mgL−1level,withthehighestyieldof 82±0.8%.Removalefficiencydecreased,whenthedye concentra-tionincreasedinalloftheothertrialsperformedwithbacteria. After 7days storage, the decolorization of RB5 was 79±1.2%, 34±1.5%and21±2.3%forbacteria/CD-F.
3.5. Adsorptionisothermsandorderofreactions
Inordertoinvestigatetheadsorptionprocessesofheavymetals anddyeonbacteriaandbacteria/CD-F,fivekineticmodelswere used.Adsorptionkineticsparameters andstatistical parameters ofall isothermstested forNi(II)removal arelistedinTable S1. Whilebacteria/CD-F fitswellforeach ofthetestedmodel,only bacteriadoesnotfitanyofthetestedmodels,hencetheestimated adsorptionisothermcoefficientsofthissamplewasnotdiscussed. NolinearcomponentwasfoundinNi(II)removalbybacteria/CD-F afterstorageat4◦Cfor1dayand7days.Linearityassessmentsfor Ni(II)werefoundasnonlinearinLangmuirandFreundlichmodels. Inaddition,Ry2valuesoflinearisothermsarelowerthantheRy2
valuesofotherisotherms.ThebestfittingmodelforNi(II)removal wasLangmuirmodelforbacteria/CD-Fafterboth1daystorageat 4◦C(Ry20.993)and7dstorageat4◦C(Ry20.924).Sincethe
high-estcorrelationsobservedinLangmuirisotherms,Ni(II)removalis likelytobemonolayericbybacteria/CD-F.Themaximumremoval capacities(Qmax)ofbacteria/CD-Fafterstoragefor1dayand7days
undertheLangmuirmodelwereestimatedtobe45.95mgg−1and 30.95mgg−1,respectively.
TableS2showstheadsorptionkineticsparametersand statis-tical parametersof allisotherms tested forCr(VI) removal.The Langmuirequationcorrelates theCr(VI)adsorption equilibrium datausingbacteria/CD-Fboth1daystorageat4◦C(Ry20.993)and
7daystorageat4◦C(Ry20.944)verywellandsuggestsitsremoval
is likely tobe monolayeric. However, theLangmuir–Freundlich adsorptionmodelsdonotfitthedataaswellastheLangmuir equa-tiondoes.
Adsorptionkineticsparameters and statisticalparameters of allisothermstestedforRB5removalarelistedinTableS3.Toth generalizedmodelswerefoundtobethebestfittingmodelsfor RB5removalusingbothbacteria/CD-F1daystorageat4◦C(Ry2
0.977)and 7daystorageat 4◦C (Ry2 0.999). Thehighest
corre-lationsobservedinTothisothermsuggestsitsremovalisrather heterogenousandmultilayericbybacteria/CD-F.
3.6. Discussion
Electrospun nanofibers have high surface area and porous structurecomparetofilmbasedmaterials,so,suchnanofibrous materialsprovidesbetterperformanceandhigherefficiency. More-over, during the electrospinning process, the additives suchas drugs,foodadditivesandmicroorganismscanbemore homoge-nouslydistributedthrough thefibermatrix.On theotherhand, theelectricalpowerappliedduringtheelectrospinningcanhave somesideeffectsespeciallyonlivingmicroorganisms.This prob-lemmightbetolerated byincreasingthenumberof livingcells intheelectrospinningsolutionpriortoelectrospinning,suchthat, sufficientamountofviablecellscouldbeachievedevenafterthe electrospinningprocess.
In this study,CD-F serves as an extra carbon source which promotesthegrowthrateofbacteria,sotheutilizationof free-standingCD-Fbasedfibrousmaterialsasencapsulationmatrixis anattractiveapproachforthelateronuseofthese microorgan-isms.However,CDcannotformafilmstructureandresultedina powdermaterialafterdryingofitssolutionwithoutany encapsu-lationofbacteriawhichcancreatechallengesduringthepractical applications.Onthecontrary,CD-Fnanofibrousmatcanbe eas-ilyhandledandfoldedasfree-standingmaterialwhichprovides certainadvantagesduringitspractices.
AsobservedinthefluorescencemicroscopyandSEMimages, eventhesizeofbacteriaisbiggerthanthenanofiberdiameter, bac-teriaweredispersedprettyhomogenousthroughthefibermatrix. Here,microorganismswereencapsulatedinCD-Fhavingdifferent concentrations(0.25,0.5,1and2%(w/w))andtheremediationtests wereperformedbyusing1%(w/w)concentratedCD-Fsincethere weresomedifficultiesfortheelectrospinningofCDsolution con-taining2%(w/w)ofbacteria.Nevertheless,asitcanbeobserved fromtheresultsofremovaltests,contaminantswereremediatedby highefficiencyfromtheirliquidenvironment(70±0.2%,58±1.4% and82±0.8%for 30mgL−1 concentratedsolutionsof theNi(II), Cr(VI)andReactiveBlack5,respectively).30mgL−1concentration isalreadyabovetheacceptablelimitsofenvironmentalconcerns. Therefore,itcanbeconcludedthattheencapsulationefficiencyof CD-Fisquitewellenoughfortheintendedapplications.
4. Conclusions
Inthisstudy,wehaveshownsuccessfulencapsulationofstrain Lysinibacillussp.NOSKinCD-Fbyusingelectrospinningprocess. TheCD, which isa cyclic oligosaccharideobtainedfromstarch, wasusedasanencapsulation matrixanda feedingsourcethus facilitateaneasyrevivalofbacteria.Thesuitableconditionsofthe electrospinningprocessforencapsulationofbacteriaand maintain-ingtheirviabilitywereoptimized.Althoughthe%loadingofthe bacteriawaslimited becauseofthedifficultyofelectrospinning offibers,theobservedresultsconfirmedthatsignificantamount ofcellssurvivedandretainedbiologicalfunctionalityduringthe processeveninthedrasticosmoticchangeandelectrostaticfield generatedduringelectrospinningprocess.Moreover,Lysinibacillus sp.NOSKremainedviablewithintheCD-Fsfor7daysat refriger-atorconditions(4◦C).Electrospunfiberencapsulationtechnology alsoofferssignificantadvantagesforstudyingthebehaviorofcells under confinement.Cells, either bacterial or mammalian,were foundtoaltertheirbehaviorinaconfinedenvironment.Webelieve thatthebioactivityofencapsulatedLysinibacillussp.NOSKcells remainssameintheCD-Fthatcanallowaneasyrecoveryof
bac-terialcells duringapplication.Theobtainedresultssuggestthat theCD-Fbiocompositesystemwellsuitableforpotential applica-tioninNi(II),Cr(VI)andReactivedyebioremovalfromwastewater systems.Therefore,electrospinning techniquemayrepresentan excellentalternativetolyophilizationforthepreservationof organ-ismsforstraincollections,andforapplicationssuchaswastewater treatment.
Acknowledgements
The Scientific and Technological Research Council of Turkey (TUBITAK, project #114Y264) is acknowledged for funding the research.Dr.UyaracknowledgesTheTurkishAcademyofSciences - Outstanding Young Scientists Award Program (TUBA-GEBIP) for partialfundingof theresearch. A. Celebiogluacknowledges TUBITAKproject#113Y348forapostdoctoralfellowship.O.F. Sar-iogluacknowledges TUBITAKBIDEB(2211-C)for NationalPh.D. Scholarship.
AppendixA. Supplementarydata
Supplementarydataassociatedwiththisarticlecanbefound, intheonlineversion,athttps://doi.org/10.1016/j.colsurfb.2017.10. 047.
References
[1]A.Kumar,B.S.Bisht,V.D.Joshi,T.Dhewa,Int.J.Environ.Stud.Sci.1(6)(2011) 1079.
[2]N.Ali,A.Hameed,S.Ahmed,J.Hazard.Mater.164(1)(2009)322.
[3]S.Klein,J.Kuhn,R.Avrahami,S.Tarre,M.Beliavski,Biomacromolecules10(7) (2009)1751.
[4]M.L.Bruschi,M.L.C.Cardoso,M.B.Lucchesi,M.P.D.Gremião,Int.J.Pharm.264 (1)(2003)45.
[5]T.Ishizaka,K.Endo,M.J.Koishi,Pharm.Sci.70(4)(1981)358. [6]M.C.Mauguet,J.Legrand,L.Brujes,G.Carnelle,C.Larre,Y.Popineau,J.
Microencapsulation19(3)(2002)377.
[7]D.T.Birnbaum,J.D.Kosmala,D.B.Henthorn,L.Brannon-Peppas,Controlled Release65(3)(2000)375.
[8]T.Uyar,E.Kny,ElectrospunMaterialsforTissueEngineeringandBiomedical Applications:Research,DesignandCommercialization,Elsevier,Woodhead PublishingSeriesinBiomaterials,2017(ISBN:9780081010228).
[9]M.F.Canbolat,A.Celebioglu,T.Uyar,ColloidsSurf.B:Biointerfaces115(2014) 15.
[10]A.Fernandez,S.Torres-Giner,J.M.Lagaron,FoodHydrocolloids23(5)(2009) 1427.
[11]F.Kayaci,Y.Ertas,T.Uyar,J.Agric.Food.Chem.61(34)(2013)8156. [12]Z.Aytac,S.I.Kusku,E.Durgun,T.Uyar,Mater.Sci.Eng.C63(2016)231. [13]J.H.Wendorff,S.Agarwal,A.Greiner,Electrospinning:Materials,Processing,
andApplications,JohnWiley&Sons,2012.
[14]S.Ramakrishna,K.Fujihara,W.E.Teo,T.C.Lim,Z.Ma,AnIntroductionto ElectrospinningandNanofibers,WorldScientificPublishingCompany,2005. [15]S.Agarwal,J.H.Wendorff,A.Greiner,Macromol.RapidCommun.31(15)
(2010)1317.
[16]T.Uyar,R.Havelund,J.Hacaloglu,F.Besenbacher,P.Kingshott,ACSNano4(9) (2010)5121.
[17]T.Uyar,R.Havelund,Y.Nur,J.Hacaloglu,F.Besembacher,P.Kingshott,J. Membr.Sci.332(2004)129.
[18]M.Botes,T.EugeneCloete,Crit.Rev.Microbiol.36(1)(2010)68.
[19]S.Ramakrishna,R.Jose,P.S.Archana,A.S.Nair,R.Balamurugan,J.Venugopal, W.E.S.J.Teo,Mater.Sci.45(23)(2010)6283.
[20]A.Senthamizhan,B.Balusamy,A.Celebioglu,T.Uyar,J.Mater.Chem.A4 (2016)2484.
[21]O.F.Sarioglu,A.Celebioglu,T.Tekinay,T.Uyar,J.Environ.Sci.Technol.1(8) (2016)2057.
[22]O.F.Sarioglu,A.Celebioglu,T.Tekinay,T.Uyar,RSCAdv.5(124)(2015) 102750.
[23]M.Gensheimer,M.Becker,A.Brandis-Heep,J.Wendorff,R.K.Thauer,Adv. Mater19(18)(2007)2480.
[24]N.O.San,A.Celebioglu,Y.Tümtas¸,T.Uyar,T.Tekinay,RSCAdv.4(61)(2014) 32249.
[25]W.Salalha,J.Kuhn,Y.Dror,E.Zussman,Nanotechnology17(18)(2006)4675. [26]W.Y.Fung,K.H.Yuen,M.T.J.Liong,J.Agric.Food.Chem.59(15)(2011)8140. [27]Y.Liu,M.H.Rafailovich,R.Malal,D.Cohn,Proc.Natl.Acad.Sci.106(34)(2009)
14201.
[28]A.Vajdai,B.Szabó,K.Süvegh,R.Zelkó,G.Újhelyi,Int.J.Pharm.429(1)(2012) 135.
[29]S.W.Lee,A.M.Belcher,NanoLett.4(3)(2004)387.
[30]O.F.Sarioglu,N.O.S.Keskin,A.Celebioglu,T.Tekinay,T.Uyar,ColloidsSurf.B. Biointerfaces157(2017)245.
[31]A.Celebioglu,T.Uyar,Chem.Commun.46(37)(2010)6903. [32]A.Celebioglu,T.Uyar,Nanoscale4(2012)621.
[33]A.Celebioglu,T.Uyar,J.ColloidInterfaceSci.404(2013)1.
[34]A.Celebioglu,T.Uyar,RSCAdv.3(2013)22891.
[35]N.O.SanKeskin,A.Celebioglu,O.F.Sarioglu,A.D.Ozkan,T.Uyar,T.Tekinay, RSCAdv.5(106)(2015)86867.
[36]E.B.Hanlon,R.Manoharan,T.Koo,K.E.Shafer,J.T.Motz,M.Fitzmaurice,Phys. Med.Biol.45(2)(2000)1.
[37]T.Granato,A.Katovic,K.M.Valkaj,A.Tagarelli,G.J.Giordano,PorousMater.16 (2)(2009)227.