Contents lists available at
ScienceDirect
Food Research International
journal homepage:
www.elsevier.com/locate/foodres
New insights into the chemical profiling, cytotoxicity and bioactivity of four
Bunium species
Gokhan Zengin
a,⁎, Mehmet Yavuz Paksoy
b, Muhammad Zakariyyah Aumeeruddy
c,
Jasmina Glamocilja
d, Marina Sokovic
d, Alina Diuzheva
e, József Jekő
f, Zoltán Cziáky
f,
Maria João Rodrigues
g, Luisa Custodio
g, Mohamad Fawzi Mahomoodally
caDepartment of Biology, Science Faculty, Selcuk University Campus, Konya, Turkey
bDepartment of Environmental Engineering, Faculty of Engineering, University of Munzur, Tunceli, Turkey cDepartment of Health Sciences, Faculty of Science, University of Mauritius, 230 Réduit, Mauritius dInstitute for Biological Research “Siniša Stanković” University of Belgrade, Belgrade, Serbia eDepartment of Analytical Chemistry, Pavol Jozef Šafárik University in Košice, Košice, Slovakia
fAgricultural and Molecular Research and Service Institute, University of Nyíregyháza, Nyíregyháza, Hungary
gFaculty of Sciences and Technology, Centre of Marine Sciences, University of Algarve, Ed. 7, Campus of Gambelas, 8005-139 Faro, Portugal
A R T I C L E I N F O Keywords: Bunium phytoconstituents cytotoxicity antioxidant enzyme inhibitors A B S T R A C T
Bunium species have been reported to be used both as food and in traditional medicines. The scientific com-munity has attempted to probe into the pharmacological and chemical profiles of this genus. Nonetheless, many species have not been investigated fully to date. In this study, we determined the phenolic components, anti-microbial, antioxidant, and enzyme inhibitory activities of aerial parts of four Bunium species (B. sayai, B. pin-natifolium, B. brachyactis and B. macrocarpum). Results showed that B. microcarpum and B. pinnatifolium were strong antioxidants as evidenced in the DPPH, ABTS, CUPRAC, and FRAP assays. B. brachyactis was the most effective metal chelator, and displayed high enzyme inhibition against cholinesterase, tyrosinase, amylase, glucosidase, and lipase. The four species showed varied antimicrobial activity against each microorganism. Overall, they showed high activity against P. mirabilis and E. coli (MIC and MBC < 1 mg mL−1). B. brachyactis
was more effective against Aspergillus versicolor compared to the standard drug ketoconazole. B. brachyactis was also more effective than both ketoconazole and bifonazole against Trichoderma viride. B. sayai was more effective than ketoconazole in inhibiting A. fumigatus. B. sayai was most non-toxic to HEK 293 (cellular viability = 117%) and HepG2 (cellular viability = 104%). The highest level of TPC was observed in B. pinnatifolium (35.94 mg GAE g−1) while B. microcarpum possessed the highest TFC (39.21 mg RE g−1). Seventy four compounds were detected
in B. microcarpum, 70 in B. brachyactis, 66 in B. sayai, and 51 in B. pinnatifolium. Quinic acid, chlorogenic acid, pantothenic acid, esculin, isoquercitrin, rutin, apigenin, and scopoletin were present in all the four species. This study showed that the four Bunium species are good sources of biologically active compounds with pharma-ceutical and nutrapharma-ceutical potential.
1. Introduction
Since ancient times, nature has been the prime rescue for diseases.
Although there was not enough information on the etiology of
life-threatening diseases, treatments were based on trials and experience
using natural resources such as herbs and animal products (
Awortwe,
Bruckmueller, & Cascorbi, 2019
). The healing properties of medicinal
plants were identified, noted, and passed on to the successive
genera-tions via oral communication and also written documents and preserved
monuments. This interest in medicinal plants has brought about today's
modern fashion of their processing and usage (
Mollica et al., 2017
;
Petrovska, 2012
). In fact, there has been a tremendous surge in the
https://doi.org/10.1016/j.foodres.2019.05.013
Received 1 January 2019; Received in revised form 5 May 2019; Accepted 8 May 2019
Abbreviations: ABTS, 2,2′-azino-bis(3-ethylbenzothiazoline)-6-sulfonic acid; ACAE, acarbose equivalent; AChE, acetylcholinesterase; BChE, butyrylcholinesterase; CUPRAC, cupric reducing antioxidant capacity; DPPH, 1,1-diphenyl-2-picrylhydrazyl; EDTAE, EDTA equivalent; FRAP, ferric reducing antioxidant power; GAE, gallic acid equivalent; GALAE, galatamine equivalent; HEK, human embryonic kidney; KAE, kojic acid equivalent; MBC, minimum bactericidal concentration; MIC, minimum inhibitory concentration; RE, rutin equivalent; TE, trolox equivalent; TPC, Total phenolic content; TFC, Total flavonoid content
⁎Corresponding author.
E-mail address:gokhanzengin@selcuk.edu.tr(G. Zengin).
Available online 10 May 2019
0963-9969/ © 2019 Elsevier Ltd. All rights reserved.
public’s acceptance and interest in natural therapies in recent years,
especially herbal remedies, which are available not only in drug stores,
but also in food stores and supermarkets (
Ekor, 2014
).
The genus Bunium is close to Carum. The seeds and essential oils of
members of these two genera have been used as food and for
ther-apeutic purposes all over the world since ancient times (
Bousetla,
Zellagui, Derouiche, & Rhouati, 2015
). The genus Bunium (Family:
Apiaceae), consists of 50 accepted species (
http://www.theplantlist.
org
). Plants from this genus are distributed in Asia, Europe, and North
Africa (
Çelik & Bağci, 2017
). Interestingly, Turkey has the highest
number of species density of Apiaceae family (about 486 species), out
of which 8 species from the genus Bunium are endemic to Turkey, with
an endemism ratio of 40% (
Çelik & Bağci, 2017
).
Several Bunium species are consumed as traditional food in
Mediterranean regions including Turkey. For instance, B. persicum
(Boiss.) B. Fedtsch. also known as black cumin, is an important
aro-matic food plant whose seeds are consumed widely as a condiment and
has economic value (
Demirci & Ozkan, 2014
). The seeds are regarded as
stimulants, carminatives and also useful for the treatment of diarrhea
and dyspepsia (
Azizi, Davareenejad, Bos, Woerdenbag, & Kayser,
2009
). Another species, B. paucifolium var. brevipes, is used for urinary
inflammations (
Khatun, Parlak, Polat, & Cakilcioglu, 2012
) and the
tuber and whole plant are eaten after the bark is peeled (
Çakır, 2017
).
Moreover, several parts of Bunium macrocarpum (Boiss.) Freyn Bunium
paucifolium DC. and B. mauritanicum L. are taken raw orally as food and
used to make food products such as bread (
Doğan, Bulut, Tuzlacı, &
Şenkardeş, 2014
). In addition, they also claimed to manage allergy,
bronchitis, and cough (
Benarba et al., 2015
). In Morocco, a herbal
mixture known as Msahan contains 13 medicinal plants including B.
bulbocastanum, and is used for general health improvement, and for
gynecological and musculoskeletal problems (
Teixidor-Toneu, Martin,
Ouhammou, Puri, & Hawkins, 2016
).
Among the Bunium species, most scientific studies have probed into
the biological potential of B. persicum. Pharmacological studies have
proved its antibacterial, antifungal (
Bhat, Gani, & Zargar, 2017
),
anti-oxidant (
Sharafati Chaleshtori, Saholi, & Sharafati Chaleshtori, 2018
),
antinociceptive and anti-inflammatory activities (
Hajhashemi, Sajjadi,
& Zomorodkia, 2011
). As for other species, two coumarins, scopoletin
and scoparone, were identified in the roots of Algerian B. incrassatum
(
Bousetla et al., 2015
). Two prenylated isocoumarins were detected in
the roots and fruits of B. paucifolium var. paucifolium (
Appendino, Ozen,
& Jakupovic, 1994
). Ten main components isolated and identified in
the seeds of B. cylindricurn were dillapiole, myristicin, 1(-)-bornyl
acetate, α-, β-, γ-elemene, β-selinene, 7(11)-selinen-4-ol (juniper
cam-phor), elemol, and 4-methyl-4-hydroxy-penten-2-oic acid (
Agarwal,
Vashist, & Atal, 1974
).
Nonetheless, many species of this genus have not been investigated
to date. Given the pharmacological potential of Bunium species, the
present study aimed to shed light on the biological and chemical profile
of four commonly consumed species in Turkey. To determine the
bio-logical properties, namely antimicrobial, antioxidant, and enzyme
in-hibitory activities of four Bunium species (B. sayai, B. pinnatifolium, B.
brachyactis, and B. microcarpum). The in vitro cytotoxicity of the extracts
were also evaluated against three mammalian cell lines. The
char-acterization of the phenolic composition of the extracts, which are
known bioactive compounds, was undertaken using HPLC-MS/MS, in
an endeavour to support any biological activities observed.
2. Materials and Methods
2.1. Plant material and preparation of extracts
Bunium species (Bunium brachyactis (Post) Wolff; Bunium
pinnatifo-lium Kljuykov; Bunium sayai Yıld and Bunium microcarpum subsp.
mi-crocarpum (Boiss.) Freyn) were collected in different regions of Turkey
in season 2018 (June). The taxonomical classification was performed by
the botanist Dr. Mehmet Yavuz Paksoy (Munzur University, Tunceli).
The location details are given in
Table 1
. The aerial parts (including
flowers) were divided and dried for 10 days at room temperature (RT)
(25 °C, in shade). Then, these samples were powdered with a laboratory
mill.
To prepare methanol extracts, dried samples were stirred overnight
(24 h, 250 rpm in a shaker (Lab Companion SI-300 Benchtop Shaker) at
RT (5 g in 100 mL solvent). After filtration, the extracts were
con-centrated using a rotary evaporator under vacuum at 40 °C. The extracts
were completely dried at 40 °C in the oven (Memmert- UNB 400) and
they were stored at 4 °C until further analysis.
2.2. Chemicals and reagents
All reagents and standards were of analytical grade. Most of
che-micals were supplied by Sigma-Aldrich (Germany), including DPPH,
ABTS, cupric chloride, ferric chloride, ferrous sulphate, trolox, EDTA,
neucuproine, 2,4,6-Tri(2-pyridyl)-s-triazine (TPTZ), ammonium
acetate, sulphuric acid (96%), gallic acid, rutin, aluminum chloride,
Folin-Ciocalteau reagent, DTNB (5,5-dithiobis (2-nitrobenzoic) acid),
and AChE (acetylcholinesterase (Electric ell
acetylcholinesterase,Type-VI-S, EC 3.1.1.7)), BChE (butyrylcholinesterase (horse serum
ylcholinesterase, EC 3.1.1.8)), acetylthiocholine iodide (ATCI),
butyr-ylthiocholine chloride (BTCl), α-amylase (ex-porcine pancreas, EC
3.2.1.1), acarbose, α-glucosidase solution (from Saccharomyces
cerevi-siae, EC 3.2.1.20), tyrosinase (from mushroom, EC1.14.18.1),
l-2,3-di-hydroxyphenylalanine (L-DOPA), kojic acid, orlistat, pancreatic lipase
(type I, from porcine pancreas, EC. 3.1.1.3), streptomycin, ampicillin,
ketoconazole and bifonazole, MTT
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium). Methanol (HPLC grade) was purchased from
Merck (Germany).
2.3. Profile of bioactive compounds and HPLC-MS/MS analysis
With reference to our previous studies (
Uysal et al., 2017
), the total
amount of phenolics (TPC) (by standard Folin-Ciocalteu method) and
flavonoids (TFC) (by AlCl
3method) were determined. Standard
com-pounds (gallic acid (mg GAE g
−1) for TPC and rutin (mg RE g
−1) for
TFC, respectively) were used to express the obtained results.
All HPLC-MS/MS experiments were carried out on a Dionex HPLC
Table 1Locations, extraction yields, total phenolic and flavonoids contents of the Bunium species.
Samples Locations Yields (%) Total phenolic content
(mg GAE g−1extract) Total flavonoid content(mg RE g−1extract)
B. sayai Hakkari; Depin, Sümbül River around, 1300 m. 5.79 27.52 ± 0.36c⁎ 13.95 ± 0.15d
B. pinnatifolium İzmir; Kemalpaşa, Nif Mounth, 650 m 6.21 35.94 ± 1.89a 38.33 ± 0.22b
B. brachyactis Kahramanmaraş; Ahir Mounth, Çallıbalma aound, 1800 m 2.31 28.18 ± 0.37c 23.60 ± 0.16c
B. microcarpum Antalya, Elmalı-Avlan, 1000 m 8.62 32.87 ± 0.18b 39.21 ± 0.06a
⁎ Values expressed are means ± S.D. of three parallel measurements. GAE: Gallic acid equivalent; RE: Rutin equivalent. Different letters indicate the differences in
Ultimate 3000RS UHPLC instrument. A chromatographic method was
developed using a Thermo Accucore C18 (100 mm × 2.1, mm i. d., 2.6
μm) column (
Zengin et al., 2018
). All analytical details were given in
supplementary material.
2.4. Assays for enzyme inhibition and antioxidant capacity
Lipase, tyrosinase, α-amylase, α-glucosidase and cholinesterases
were selected as target enzymes and the procedures of these assays are
described in our earlier work (
Grochowski et al., 2017
;
Uysal et al.,
2017
). Standard inhibitors (acarbose (for α-amylase and
α-glucosi-dase), galantamine (for AChE and BChE), kojic acid (for tyrosinase) and
orlistat (for lipase)) were used to express the enzyme inhibitor
prop-erties.
The antioxidant capacity of the extracts was spectrophotometrically
screened by different experiments, namely the ferrozine assay (for
chelating abilities), phosphomolybdenum, reduction potentials (by
FRAP and CUPRAC assays) and radical scavenging (using DPPH and
ABTS radicals). Standard compounds (Trolox equivalent –TE g
−and
ethylenediaminetetraacetic acid equivalent – EDTAE g
−1) were used to
express the antioxidant properties. Trolox was used as a standard in
phosphomolybdenum, radical scavenging and reduction abilities, while
EDTA was a reference in metal chelating activity. The procedures of
assays are reported in our earlier work (
Uysal et al., 2017
).
2.5. Antibacterial and antifungal activities
Antimicrobial and antifungal activities were determined using the
microdilution method as described previously (
Zengin et al., 2017
). The
antibacterial activity of the studied extracts was evaluated using several
bacterial strains. Escherichia coli (ATCC 35210), Pseudomonas aeruginosa
(ATCC 27853) and Salmonella Typhimurium (ATCC 13311), were used
as Gram-negative bacteria. For Gram-positive bacteria, Proteous
mir-abilis (human isolate), Enterobacter cloacae (ATCC 35030), Bacillus
cereus (clinical isolate), Micrococcus flavus (ATCC 10240), and
Staphy-lococcus aureus (ATCC 6538) were used. The results were expressed as
minimum inhibitory (MICs) and minimum bactericidal concentrations
(MBCs). Streptomycin and ampicillin were used as standards in the
antibacterial assay.
The antifungal activity of the extracts was evaluated using different
fungal species, including Aspergillus versicolor (ATCC 11730), A.
fumi-gatus (plant isolate), A. terreus (soil isolate), A. niger (ATCC 6275),
Penicillium ochrochloron (ATCC 9112), P. funiculosum (ATCC 36839), P.
verrucosum (food isolate) and Trichoderma viride (IAM 5061). Antifungal
results were expressed by MICs and minimum fungicidal concentrations
(MFCs). Ketoconazole and bifonazole were used as standards in the
antifungal assay.
2.6. Cell culture
The murine RAW 264.7 macrophages, the human embryonic kidney
(HEK) 293, and human hepatocellular carcinoma (HepG2) cell lines
were respectively provided by the Faculty of Pharmacy and Centre for
Neurosciences and Cell Biology (University of Coimbra, Portugal), the
Functional Biochemistry and Proteomics, and the Marine Molecular
Bioengineering groups (Centre of Marine Sciences, Portugal). RAW cells
were maintained in RPMI 1640 culture media, while HEK and HepG2
cells were cultured in DMEM culture media, both supplemented with
10% heat-inactivated fetal bovine serum (FBS), 1% L-glutamine (2
mM), and 1% penicillin (50 U mL
−1)/streptomycin (50 μg mL
−1), and
were kept at 37°C in moistened atmosphere with 5% CO
2.
2.7. Determination of the cytotoxicity of the extracts
Cells at 80% confluency were seeded in 96-well microplates at a
density of 1 × 10
4cells well
−1(RAW 264.7) and 5 × 10
3cells well
−1(HEK 293 and HepG2) and left to adhere for 24h. Then, the extracts
were applied at the concentration of 100 μg mL
−1for 72h, and control
cells were treated with DMSO at maximum concentration used in the
extracts (0.2%). Cellular viability was determined by the MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide colorimetric
assay, using a microplate reader (Biotek Synergy 4), as described
pre-viously (
Rodrigues et al., 2014
). Results were expressed as cellular
viability (%).
2.8. Data evaluation
The obtained results were expressed as mean ± standard deviation
(SD) and statistically evaluated by one-way ANOVA (by Tukey test,
p < 0.05), to indicate differences among the tested extracts. To further
statistical evaluation, Pearson correlation, heat map and Principal
component (PCA) analysis were carried out to observe variabilities of
the tested extracts. The statistical procedures were performed by R
software v. 3.5.1.
3. Results and discussion
3.1. Chemical composition
The quest for phytochemical plays a major role in the search for
novel chemical entities with potential as leads for drug discovery (
Gu
et al., 2014
). Plants contain several constituents which are generally
believed to act together synergistically (
Ekor, 2014
).
In order to evaluate the total bioactive compounds of the four
Bunium species, TPC and TFC were determined (Table 1
). TPC ranged
between 27.52 and 35.94 mg GAE g
−1. The highest level was observed
in B. pinnatifolium (35.94 mg GAE g
−1) followed by B. microcarpum
(32.87 mg GAE g
−1), while the lowest amount was displayed by B.
sayai (27.52 mg GAE g
−1). The level of TFC was also quite similar to
TPC, ranging from 13.95 to 39.21 mg RE g
−1. The species B.
micro-carpum exhibited the highest TFC (39.21 mg RE g
−1). B. pinnatifolium
also had a quite similar amount (38.33 mg RE g
−1) while the two other
plants revealed lower levels, with the least amount in B. sayai (13.95
mg RE g
−1).
With regards to the individual phytocompounds, 74 compounds
were detected in B. microcarpum, 70 in B. brachyactis, 66 in B. sayai, and
51 in B. pinnatifolium (
Tables 2–5
). The chromatograms for the extracts
are reported in Supplementary materials (Figs. S1–S4). Quinic acid,
chlorogenic acid, pantothenic acid, esculin, isoquercitrin, rutin,
api-genin, and scopoletin were present in all species. Vitexin, cosmosiin,
diosmin, luteolin, angelicin, salcolin B, and vicenin-2 were present in B.
brachyactis, B. microcarpum, and B. sayai. Naringenin was identified in
B. brachyactis, B. microcarpum, and B. pinnatifolium. In addition,
kaempferol was detected in B. microcarpum, B. pinnatifolium, and B.
sayai. Afzelin was detected in B. brachyactis, B. pinnatifolium, and B.
sayai, while orientin was present only in B. brachyactis and B.
micro-carpum. Several studies have been performed on chemical composition
of essential oils from the members of the Bunium genus (
Sharafati
Chaleshtori et al., 2018
;
Talebi, Moghaddam, & Pirbalouti, 2018
), but
there is still insufficient data for phytochemical characterization of their
extracts. At this point the present work could provide valuable
in-formation for the genus.
3.2. Antimicrobial activity
Due to the emergence of infectious diseases and the rise of microbial
resistance, new strategies are being explored to tackle this issue.
Because of the fact that plants have been used for centuries to treat
infections, they are considered as an important source of new
anti-microbial agents (
Bereksi, Hassaïne, Bekhechi, & Abdelouahid, 2018
).
With regards to the antibacterial activity of the four tested Bunium
species (see
Table 6
), the plants showed varied activity depending on
Table 2
Chemical composition of B. brachyactis.
No. Compounds Formula Rt, min [M + H]+ [M–H]− Fragment 1 Fragment 2 Fragment 3 Fragment 4 Fragment 5
1 Quinic acid C7H12O6 1.23 191.0556 173.0448 171.0289 127.0389 111.0438 85.0280
2 Pantothenic acid C9H17NO5 5.42 220.1185 202.1079 184.0974 174.1131 116.0347 90.0556
3 Neochlorogenic acid (5-O-Caffeoylquinic acid) C16H18O9 8.95 355.1029 163.0393 145.0289 135.0445 117.0340 89.0391
4 Esculin (Esculetin-6-O-glucoside) C15H16O9 12.28 341.0873 179.0344 151.0393 133.0289 123.0446
5 Coumaroylquinic acid isomer 1 C16H18O8 12.59 337.0924 191.0553 173.0444 163.0391 119.0489 93.0328
6 Esculetin (6.7-Dihydroxycoumarin) C9H6O4 14.10 179.0344 151.0393 133.0288 123.0445 105.0341 89.0391
7a Chlorogenic acid (3-O-Caffeoylquinic acid) C16H18O9 14.29 355.1029 163.0393 145.0287 135.0445 117.0340 89.0392
8 Chryptochlorogenic acid (4-O-Caffeoylquinic acid) C16H18O9 15.62 355.1029 163.0393 145.0287 135.0445 117.0340 89.0391
9 Syringic acid C9H10O5 15.93 197.0450 182.0215 166.9976 153.0547 138.0311 123.0074
10 Dihydroxy-methoxycoumarin-O-hexoside C16H18O10 15.99 369.0822 207.0294 206.0216 192.0058 190.9980 163.0025
11 Ethyl syringate C11H14O5 16.82 227.0920 181.0500 155.0706 140.0472 123.0443 95.0496
12 Coumaroylquinic acid isomer 2 C16H18O8 16.89 337.0924 191.0555 173.0446 163.0390 119.0489 93.0331
13 Naringenin-6.8-di-C-glucoside C27H32O15 16.99 595.1663 505.1402 475.1247 415.1037 385.0941 355.0830
14 Feruloylhexose C16H20O9 17.04 355.1029 235.0608 193.0501 175.0392 149.0598
15 Coumaroylquinic acid isomer 3 C16H18O8 17.57 337.0924 191.0557 173.0447 163.0391 119.0489 93.0331
16 Sinapoylhexose C17H22O10 17.95 385.1135 267.0725 249.0612 223.0609 208.0374 164.0469
17 Feruloylquinic acid C17H20O9 18.01 367.1029 193.0500 191.0555 173.0447 134.0362 93.0331
18 Scopoletin (7-Hydroxy-6-methoxycoumarin) C10H8O4 18.61 193.0501 178.0264 165.0549 149.0595 137.0602 133.0288
19 Vicenin-2 (Apigenin-6.8-di-C-glucoside) C27H30O15 19.04 593.1507 575.1415 503.1197 473.1094 383.0780 353.0673
20 Indole-4-carbaldehyde C9H7NO 19.08 146.0606 118.0656 117.0580 91.0549
21 Coumaroylquinic acid isomer 4 C16H18O8 19.19 337.0924 191.0556 173.0448 163.0392 119.0492 93.0333
22 Apigenin-C-hexoside-C-pentoside isomer 1 C26H28O14 19.74 563.1401 473.1088 443.1016 413.0864 383.0781 353.0673
23 N-(2-Phenylethyl)acetamide C10H13NO 20.16 164.1075 122.0969 105.0705 103.0549 90.9484 79.0550
24 Trimethoxyphenylacetyl hexose C17H24O10 20.26 387.1291 225.0766 210.0530 195.0294 71.0124 59.0125
25 Apigenin-C-hexoside-C-pentoside isomer 2 C26H28O14 20.38 563.1401 473.1101 443.0979 413.0902 383.0775 353.0674
26 Coumaroylshikimate C16H16O7 20.44 319.0818 163.0391 155.0338 119.0489
27 Orientin (Lutexin. Luteolin-8-C-glucoside) C21H20O11 20.45 449.1084 431.0984 413.0879 353.0662 329.0663 299.0556
28 Tetrahydroxyflavanone-O-(deoxyhexosyl)hexoside C27H32O15 20.77 595.1663 287.0567 175.0024 151.0027 135.0442 107.0126
29 Apigenin-C-hexoside-C-pentoside isomer 3 C26H28O14 20.78 563.1401 473.1086 443.0974 413.0878 383.0781 353.0673
30 Isoorientin (Homoorientin. Luteolin-6-C-glucoside) C21H20O11 20.80 449.1084 431.0983 413.0877 353.0662 329.0662 299.0556
31 Apigenin-O-(acetyl)hexoside C23H22O11 20.81 473.1084 269.0466 268.0380 151.0018
32 4-Acetamidobenzoic acid C9H9NO3 21.05 178.0504 134.0600 92.0491
33a Vitexin (Orientoside. Apigenin-8-C-glucoside) C21H20O10 21.44 433.1135 415.1032 397.0928 337.0710 313.0712 283.0606
34 Luteolin-O-glucuronide C21H18O12 22.31 461.0720 285.0410 217.0506 199.0391 151.0026 133.0280
35 Isovitexin (Avroside. Apigenin-6-C-glucoside) C21H20O10 22.34 433.1135 415.1031 397.0926 337.0712 313.0712 283.0606
36 Di-O-caffeoylquinic acid isomer 1 C25H24O12 22.39 515.1190 353.0883 191.0555 179.0341 173.0447 135.0440
37 Luteolin-7-O-glucoside (Cynaroside) C21H20O11 22.43 447.0927 327.0515 285.0411 284.0332 256.0375 151.0027
38 Tetrahydroxyflavone-O-deoxyhexoside C21H20O10 22.46 431.0978 285.0411 284.0332 255.0301 227.0347
39 Methoxy-trihydroxyflavone-C-hexoside C22H22O11 22.71 463.1240 445.1139 427.1034 343.0819 313.0713 287.0557
40a Isoquercitrin (Hirsutrin. Quercetin-3-O-glucoside) C21H20O12 22.96 463.0877 301.0360 300.0279 271.0252 255.0302 151.0026
41a Rutin (Quercetin-3-O-rutinoside) C27H30O16 23.06 611.1612 465.1011 303.0504 129.0551 85.0291 71.0499
42 Apigenin-O-(deoxyhexosyl)hexoside C27H30O14 23.97 579.1714 433.1138 271.0605
43 Apigenin-O-glucuronide C21H18O11 24.02 445.0771 269.0459 175.0238 113.0231
44a Cosmosiin (Apigetrin. Apigenin-7-O-glucoside) C21H20O10 24.04 433.1135 271.0605 153.0184 145.0280 119.0495
45 Di-O-caffeoylquinic acid isomer 2 C25H24O12 24.19 515.1190 353.0883 191.0555 179.0342 173.0446 135.0441
46 Methoxy-trihydroxyflavone-O-glucuronide C22H20O12 24.29 477.1033 301.0712 286.0479 258.0525
47 Methoxy-trihydroxyflavone-O-hexoside C22H22O11 24.31 461.1084 446.0864 299.0574 298.0487 283.0253 255.0300
48a Diosmin (Diosmetin-7-O-rutinoside) C28H32O15 24.59 609.1820 463.1247 301.0712 286.0477 129.0552 85.0291
49 Psoralen C11H6O3 24.79 187.0395 159.0446 143.0495 131.0496 115.0547
50 Tetrahydroxyflavone-O-(deoxyhexosyl)hexoside C27H30O15 24.90 593.1507 285.0410 284.0331 255.0300 227.0347 151.0023
51 Isorhamnetin-O-hexoside C22H22O12 24.98 477.1033 315.0511 314.0441 299.0195 285.0409 271.0258
52 Isorhamnetin-O-(deoxyhexosyl)hexoside C28H32O16 25.25 623.1612 315.0517 314.0439 300.0281 299.0202 271.0253
53 Bergapten (5-Methoxypsoralen) C12H8O4 25.32 217.0501 202.0266 174.0314 146.0362 131.0496 118.0415
54 Psoralen isomer C11H6O3 25.65 187.0395 159.0445 143.0496 131.0497 115.0547
55 Afzelin (Kaempferol-3-O-rhamnoside) C21H20O10 26.46 431.0978 285.0411 284.0332 255.0301 227.0347
56 Dillapiole C12H14O4 27.05 223.0970 208.0734 195.0657 180.0421 165.0913 133.0652
57a Naringenin C15H12O5 27.25 271.0607 177.0187 165.0188 151.0027 119.0489 107.0125
58a Luteolin (3'.4'.5.7-Tetrahydroxyflavone) C15H10O6 27.89 285.0399 217.0502 199.0395 175.0392 151.0026 133.0283
59a Apigenin (4'.5.7-Trihydroxyflavone) C15H10O5 29.70 269.0450 225.0554 201.0551 151.0025 149.0234 107.0125
60 Dimethoxy-trihydroxy(iso)flavone C17H14O7 29.90 329.0661 314.0438 299.0202 271.0254 61 Salcolin A (Tricin-4'-O-(erythro-β-guaiacylglyceryl) ether) C27H26O11 29.93 525.1397 477.1179 329.0670 314.0442 299.0203 195.0657 62 Methoxy-trihydroxy(iso)flavone C16H12O6 30.01 299.0556 284.0331 256.0379 227.0341 151.0025 107.0124 63 Angelicin C11H6O3 30.51 187.0395 159.0443 143.0496 131.0497 115.0546 64 Salcolin B (Tricin-4'-O-(threo-β-guaiacylglyceryl) ether) C27H26O11 30.73 525.1397 329.0671 314.0439 299.0201 195.0659 165.0547 65 Tetrahydroxyflavone-O-(cinnamoyl)hexoside C30H26O12 32.20 577.1346 285.0410 284.0331 255.0300 227.0346 66 Imperatorin C16H14O4 32.99 271.0970 203.0344 175.0394 147.0444 131.0495 69.0707 67 Dihydroxy-dimethoxy(iso)flavone C17H14O6 34.85 313.0712 298.0488 283.0252 255.0300 68 Dihydroxy-trimethoxy(iso)flavone C18H16O7 34.95 343.0818 328.0592 313.0361 298.0121 69 Isopropyl-methoxy-furocoumarin C15H14O4 34.97 259.0970 244.0736 229.0499 215.0342 185.0613 171.0805 70 Selinidin C19H20O5 35.84 329.1389 229.0864 201.0915 187.0395 175.0394 159.0445 a Confirmed by standard.
Table 3
Chemical composition of B. microcarpum.
No. Compounds Formula Rt. min [M + H]+ [M–H]− Fragment 1 Fragment 2 Fragment 3 Fragment 4 Fragment 5
1 Quinic acid C7H12O6 1.23 191.0556 173.0447 171.0292 127.0389 111.0437 85.0280
2 Salvianic acid A (Danshensu. 3-(3.4-Dihydroxy)
phenyllactic acid) C9H10O5 4.55 197.0450 179.0343 135.0440 123.0439 72.9916
3 Pantothenic acid C9H17NO5 5.40 220.1185 202.1079 184.0974 174.1125 116.0348 90.0556
4 Esculin (Esculetin-6-O-glucoside) C15H16O9 12.23 341.0873 179.0343 151.0395 133.0288 123.0445
5 Kynurenic acid C10H7NO3 12.89 190.0504 162.0553 144.0441 116.0498 89.0394
6a Chlorogenic acid (3-O-Caffeoylquinic acid) C16H18O9 14.25 355.1029 163.0393 145.0288 135.0445 117.0340 89.0390
7 Chryptochlorogenic acid (4-O-Caffeoylquinic acid) C16H18O9 15.59 355.1029 163.0393 145.0288 135.0445 117.0340 89.0391
8 Dihydroxy-methoxycoumarin-O-hexoside C16H18O10 15.99 369.0822 207.0294 206.0216 192.0058 190.9980 163.0024
9 Ethyl syringate C11H14O5 16.82 227.0920 181.0500 155.0706 140.0472 123.0446 95.0496
10 Coumaroylquinic acid isomer 1 C16H18O8 16.87 337.0924 191.0555 173.0446 163.0391 119.0489 93.0331
11 Naringenin-6.8-di-C-glucoside C27H32O15 16.99 595.1663 505.1400 475.1262 415.1034 385.0934 355.0824
12 Feruloylhexose C16H20O9 17.02 355.1029 235.0609 193.0500 175.0389 149.0597
13 4-Hydroxymellein C10H10O4 17.04 195.0657 177.0550 167.0706 149.0600 103.0552
14 Quercetin-di-O-hexoside C27H30O17 17.23 625.1405 463.0894 462.0822 301.0358 299.0203 271.0247
15 Coumaroylquinic acid isomer 2 C16H18O8 17.53 337.0924 191.0556 173.0446 163.0392 119.0489 93.0331
16 Sinapoylhexose C17H22O10 17.93 385.1135 267.0728 249.0610 223.0608 208.0373 164.0469
17 Feruloylquinic acid C17H20O9 18.00 367.1029 193.0501 191.0555 173.0447 134.0363 93.0331
18 Caffeoylshikimic acid C16H16O8 18.04 335.0767 179.0342 161.0234 135.0440
19 Scopoletin (7-Hydroxy-6-methoxycoumarin) C10H8O4 18.59 193.0501 178.0265 165.0547 149.0600 137.0601 133.0288
20 Vicenin-2 (Apigenin-6.8-di-C-glucoside) C27H30O15 19.02 593.1507 575.1456 503.1200 473.1096 383.0778 353.0672
21 Indole-4-carbaldehyde C9H7NO 19.06 146.0606 118.0656 117.0578 91.0549
22 Coumaroylquinic acid isomer 3 C16H18O8 19.17 337.0924 191.0554 173.0448 163.0392 119.0489 93.0331
23 Methyl O-caffeoylquinate C17H20O9 19.62 367.1029 191.0557 179.0342 161.0234 135.0440 85.0280
24 Luteolin-C-hexoside-C-pentoside isomer 1 C26H28O15 19.67 579.1350 489.1043 459.0943 429.0846 399.0727 369.0620
25 Trimethoxyphenylacetyl hexose C17H24O10 20.24 387.1291 225.0765 210.0529 195.0293 71.0117 59.0125
26 Apigenin-C-hexoside-C-pentoside isomer 1 C26H28O14 20.37 563.1401 473.1091 443.0993 413.0861 383.0781 353.0674
27 Coumaroylshikimate C16H16O7 20.44 319.0818 163.0391 155.0338 119.0489
28 Orientin (Lutexin. Luteolin-8-C-glucoside) C21H20O11 20.46 449.1084 431.0983 413.0875 353.0661 329.0661 299.0555
29 Tetrahydroxyflavanone-O-(deoxyhexosyl)hexoside C27H32O15 20.75 595.1663 287.0567 175.0025 151.0026 135.0440 107.0126
30 Apigenin-C-hexoside-C-pentoside isomer 2 C26H28O14 20.77 563.1401 473.1096 443.0987 413.0885 383.0779 353.0672
31 Isoorientin (Homoorientin. Luteolin-6-C-glucoside) C21H20O11 20.80 449.1084 431.0982 413.0873 353.0662 329.0662 299.0555
32 Apigenin-O-(acetyl)hexoside C23H22O11 20.82 473.1084 269.0466 268.0458 151.0026
33 Luteolin-C-hexoside-C-pentoside isomer 2 C26H28O15 20.99 579.1350 489.1072 459.0942 429.0837 399.0739 369.0613
34a Vitexin (Orientoside. Apigenin-8-C-glucoside) C21H20O10 21.44 433.1135 415.1032 397.0927 337.0712 313.0713 283.0606
35 Quercetin-O-(pentosyl-deoxyhexosyl)hexoside C32H38O20 21.63 741.1878 301.0359 300.0281 271.0263 255.0295
36 Methyl O-(p-coumaroyl)quinate C17H20O8 21.69 351.1080 163.0389 145.0284 119.0489
37 Quercetin-O-(hexosyl)hexoside C27H30O17 21.87 625.1405 301.0356 300.0281 271.0256 255.0302
38 Methyl O-feruloylquinate C18H22O9 22.15 381.1186 193.0501 175.0390 160.0155 149.0596 134.0362
39 Luteolin-O-glucuronide C21H18O12 22.30 461.0720 285.0411 217.0506 199.0391 151.0026 133.0280
40 Isovitexin (Avroside. Apigenin-6-C-glucoside) C21H20O10 22.34 433.1135 415.1032 397.0923 337.0711 313.0711 283.0605
41 Di-O-caffeoylquinic acid isomer 1 C25H24O12 22.37 515.1190 353.0881 191.0554 179.0341 173.0446 135.0440
42 Luteolin-7-O-glucoside (Cynaroside) C21H20O11 22.43 447.0927 327.0527 285.0409 284.0330 256.0379 151.0026
43a Narirutin (Naringenin-7-O-rutinoside) C27H32O14 22.49 581.1870 435.1290 315.0866 273.0762 153.0185 85.0291
44 Methoxy-trihydroxyflavone-C-hexoside C22H22O11 22.75 463.1240 445.1143 427.1040 343.0817 313.0710 287.0559
45a Isoquercitrin (Hirsutrin. Quercetin-3-O-glucoside) C21H20O12 22.94 463.0877 301.0357 300.0279 271.0252 255.0299 151.0025
46a Rutin (Quercetin-3-O-rutinoside) C27H30O16 23.06 611.1612 465.1024 303.0504 129.0551 85.0291 71.0499
47 Quercetin-O-(malonyl)hexoside C24H22O15 23.71 549.0881 505.0996 463.0859 301.0359 300.0279 271.0252
48 Apigenin-O-(deoxyhexosyl)hexoside C27H30O14 23.97 579.1714 433.1137 271.0604
49 Apigenin-O-(crotonoyl)hexoside C25H24O11 23.99 499.1240 431.0988 269.0459 268.0380 93.0332
50a Cosmosiin (Apigetrin. Apigenin-7-O-glucoside) C21H20O10 24.04 433.1135 271.0605 153.0182 145.0293 119.0496
51 Apigenin-O-glucuronide C21H18O11 24.08 445.0771 269.0478 175.0238 113.0229
52 Di-O-caffeoylquinic acid isomer 2 C25H24O12 24.19 515.1190 353.0882 191.0555 179.0341 173.0446 135.0440
53 Methoxy-trihydroxyflavone-O-hexoside C22H22O11 24.32 461.1084 446.0850 299.0565 298.0489 283.0255 255.0298
54 Quercetin-O-(acetyl)hexoside C23H22O13 24.51 505.0982 463.0869 301.0356 300.0279 271.0251 255.0301
55 Hydroxy-methoxy(iso)flavone-O-hexoside C22H22O9 24.55 431.1342 269.0814 254.0580 237.0550 213.0914
56a Diosmin (Diosmetin-7-O-rutinoside) C28H32O15 24.59 609.1820 463.1243 301.0710 286.0476 129.0552 85.0291
57 Tetrahydroxyflavone-O-(deoxyhexosyl)hexoside C27H30O15 24.90 593.1507 285.0409 284.0331 255.0299 227.0346 151.0028
58 Isorhamnetin-O-hexoside C22H22O12 24.98 477.1033 315.0520 314.0438 299.0201 285.0409 271.0252
59 Isorhamnetin-O-(deoxyhexosyl)hexoside C28H32O16 25.25 623.1612 315.0516 314.0438 300.0279 299.0202 271.0252
60 Bergapten (5-Methoxypsoralen) C12H8O4 25.32 217.0501 202.0265 174.0314 146.0363 131.0494 118.0418
61 Psoralen isomer C11H6O3 25.65 187.0395 159.0444 143.0493 131.0496 115.0547
62a Naringenin C15H12O5 27.25 271.0607 177.0188 165.0188 151.0026 119.0489 107.0125
63a Luteolin (3'.4'.5.7-Tetrahydroxyflavone) C15H10O6 27.89 285.0399 217.0503 199.0394 175.0390 151.0026 133.0283
64a Kaempferol (3.4'.5.7-Tetrahydroxyflavone) C15H10O6 29.35 287.0556 258.0527 213.0550 165.0184 153.0183 121.0286
65a Apigenin (4'.5.7-Trihydroxyflavone) C15H10O5 29.71 269.0450 225.0553 201.0552 151.0026 149.0233 107.0126
66 Dimethoxy-trihydroxy(iso)flavone C17H14O7 29.89 329.0661 314.0437 299.0201 271.0252 67 Salcolin A (Tricin-4'-O-(erythro-β-guaiacylglyceryl) ether) C27H26O11 29.94 525.1397 477.1179 329.0678 314.0433 299.0209 195.0649 68 Methoxy-trihydroxy(iso)flavone C16H12O6 30.02 299.0556 284.0331 256.0373 227.0340 151.0023 107.0125 69 Angelicin C11H6O3 30.51 187.0395 159.0442 143.0494 131.0497 115.0548 70 Salcolin B (Tricin-4'-O-(threo-β-guaiacylglyceryl) ether) C27H26O11 30.73 525.1397 329.0674 314.0446 299.0209 195.0651 165.0547 71 Dihydroxy-trimethoxy(iso)flavone C18H16O7 31.29 343.0818 328.0594 313.0361 298.0127 285.0417 270.0170
each tested bacteria. Overall, the four species showed high activity
against P. myrabilis and E. coli with MIC and MBC values of less than 1
mg mL
−1. The four species were equally effective against S. aureus
(MIC = 1.13 mg mL
−1; MBC = 1.50 mg mL
−1) and E. coli (MIC = 0.56
mg mL
−1; MBC = 0.75 mg mL
−1). Besides, B. cereus (MIC = 0.28 mg
mL
−1; MBC = 0.37 mg mL
−1), P. aeruginosa (MIC = 0.18 mg mL
−1;
MBC = 0.37 mg mL
−1), and S. Typhimurium (MIC = 1.50 mg mL
−1;
MBC = 3.00 mg mL
−1) were most susceptible to B. brachyactis. In fact,
B. brachyactis was more effective than the standard, ampicillin
(MIC = 0.30 mg mL
−1; MBC = 0.50 mg mL
−1) against P. aeruginosa
and was slightly less effective compared to streptomycin (MIC = 0.10
mg/ml; MBC = 0.20 mg mL
−1). On the other hand, P. myrabilis was
most susceptible to B. microcarpum with a MIC value of 0.14 mg mL
−1and MBC value of 0.18 mg mL
−1, which was comparable to
strepto-mycin (MIC = 0.10 mg mL
−1; MBC = 0.20 mg mL
−1). In addition,
among the four species, B. pinnatifolium exhibited the highest
Table 3 (continued)No. Compounds Formula Rt. min [M + H]+ [M–H]− Fragment 1 Fragment 2 Fragment 3 Fragment 4 Fragment 5
72 Tetrahydroxyflavone-O-(cinnamoyl)hexoside C30H26O12 32.13 577.1346 285.0409 284.0331 255.0300 227.0347
73 Imperatorin C16H14O4 32.98 271.0970 203.0344 175.0394 147.0442 131.0497 69.0707
74 Dihydroxy-dimethoxy(iso)flavone C17H14O6 34.85 313.0712 298.0488 283.0253 255.0300
a Confirmed by standard.
Table 4
Chemical composition of B. pinnatifolium.
No. Compound Formula Rt. min [M + H]+ [M–H]− Fragment 1 Fragment 2 Fragment 3 Fragment 4 Fragment 5
1 Quinic acid C7H12O6 1.20 191.0556 173.0445 171.0288 127.0388 111.0439 85.0280
2 Pantothenic acid C9H17NO5 5.39 220.1185 202.1082 184.0976 174.1130 116.0348 90.0557
3 Esculin (Esculetin-6-O-glucoside) C15H16O9 12.28 341.0873 179.0344 151.0389 133.0291 123.0446
4 Kynurenic acid C10H7NO3 12.89 190.0504 162.0553 144.0447 116.0499 89.0393
5 Esculin isomer C15H16O9 13.67 341.0873 179.0344 151.0393 133.0287 123.0447
6a Chlorogenic acid (3-O-Caffeoylquinic acid) C16H18O9 14.25 355.1029 163.0394 145.0288 135.0445 117.0340 89.0392
7 Chryptochlorogenic acid (4-O-Caffeoylquinic acid) C16H18O9 15.60 355.1029 163.0393 145.0288 135.0445 117.0340 89.0392
8 Syringic acid C9H10O5 15.92 197.0450 182.0214 166.9976 153.0550 138.0313 123.0075
9 Dihydroxy-methoxycoumarin-O-hexoside C16H18O10 15.99 369.0822 207.0295 206.0217 192.0060 190.9981 163.0030
10 Feruloylhexose C16H20O9 16.73 355.1029 235.0613 193.0502 175.0392 149.0598
11 Ethyl syringate C11H14O5 16.80 227.0920 181.0501 155.0706 140.0472 123.0446 95.0498
12 Coumaroylquinic acid isomer 1 C16H18O8 16.88 337.0924 191.0556 173.0447 163.0392 119.0490 93.0331
13 Naringenin-6.8-di-C-glucoside C27H32O15 16.98 595.1663 505.1361 475.1265 415.1043 385.0934 355.0829
14 Caffeoylshikimic acid isomer 1 C16H16O8 17.21 335.0767 179.0343 161.0233 135.0441
15 Coumaroylquinic acid isomer 2 C16H18O8 17.56 337.0924 191.0556 173.0447 163.0393 119.0490 93.0332
16 Sinapoylhexose C17H22O10 17.93 385.1135 267.0725 249.0619 223.0609 208.0374 164.0469
17 Feruloylquinic acid C17H20O9 18.00 367.1029 193.0501 191.0556 173.0447 134.0362 93.0331
18 Caffeoylshikimic acid isomer 2 C16H16O8 18.05 335.0767 179.0342 161.0234 135.0441
19 Scopoletin (7-Hydroxy-6-methoxycoumarin) C10H8O4 18.58 193.0501 178.0266 165.0551 149.0602 137.0603 133.0289
20 Indole-4-carbaldehyde C9H7NO 19.06 146.0606 118.0656 117.0580 91.0547
21 Coumaroylquinic acid isomer 3 C16H18O8 19.20 337.0924 191.0556 173.0447 163.0391 119.0488 93.0331
22 Ferulic acid C10H10O4 19.38 193.0501 178.0264 149.0597 137.0233 134.0362 121.0281
23 Coumaroylshikimate C16H16O7 20.44 319.0818 163.0390 155.0341 119.0490
24 Quercetin-O-(pentosyl-deoxyhexosyl)hexoside C32H38O20 21.62 741.1878 301.0362 300.0281 271.0253 255.0300
25 Methyl O-(p-coumaroyl)quinate C17H20O8 21.70 351.1080 163.0391 145.0287 119.0489
26 Quercetin-O-(hexosyl)hexoside C27H30O17 21.71 625.1405 301.0358 300.0280 271.0255 255.0294
27 Dihydrokaempferol (Aromadendrin. Katuranin) C15H12O6 21.97 287.0556 259.0617 243.0650 201.0554 177.0549 125.0231
28 Methyl O-feruloylquinate C18H22O9 22.14 381.1186 193.0503 175.0390 160.0154 149.0598 134.0362
29 Di-O-caffeoylquinic acid isomer 1 C25H24O12 22.37 515.1190 353.0883 191.0555 179.0342 173.0446 135.0441
30 Hyperoside (Hyperin. Quercetin-3-O-galactoside) C21H20O12 22.73 463.0877 301.0361 300.0280 271.0253 255.0300 151.0026
31a Isoquercitrin (Hirsutrin. Quercetin-3-O-glucoside) C21H20O12 22.92 463.0877 301.0359 300.0281 271.0253 255.0301 151.0026
32a Rutin (Quercetin-3-O-rutinoside) C27H30O16 23.05 611.1612 465.1037 303.0505 129.0551 85.0291 71.0499
33 Quercetin-O-pentoside isomer 1 C20H18O11 23.30 433.0771 301.0370 300.0279 271.0252 255.0291
34 Quercetin-O-pentoside isomer 2 C20H18O11 23.54 433.0771 301.0359 300.0282 271.0254 255.0302
35 Di-O-caffeoylquinic acid isomer 2 C25H24O12 24.19 515.1190 353.0884 191.0557 179.0343 173.0447 135.0441
36 Astragalin (Kaempferol-3-O-glucoside) C21H20O11 24.72 447.0927 327.0523 285.0410 284.0332 255.0300 227.0347
37 Tetrahydroxyflavone-O-(deoxyhexosyl)hexoside C27H30O15 24.87 593.1507 285.0410 284.0332 255.0301 227.0347 151.0024
38 Isorhamnetin-O-glucuronide C22H20O13 25.19 491.0826 315.0519 300.0281 271.0257 255.0302
39 Isorhamnetin-O-(deoxyhexosyl)hexoside C28H32O16 25.25 623.1612 315.0518 314.0439 300.0281 299.0202 271.0254
40 Kaempferol-O-(malonyl)glucoside C24H22O14 25.64 533.0931 489.1053 285.0411 284.0332 255.0299 227.0355
41 Afzelin (Kaempferol-3-O-rhamnoside) C21H20O10 26.45 431.0978 285.0410 284.0331 255.0300 227.0347
42a Quercetin C15H10O7 27.01 303.0505 285.0395 257.0447 229.0500 153.0184 137.0237
43a Naringenin C15H12O5 27.23 271.0607 177.0181 165.0188 151.0028 119.0490 107.0125
44a Kaempferol (3.4'.5.7-Tetrahydroxyflavone) C15H10O6 29.34 287.0556 258.0520 213.0554 165.0186 153.0185 121.0288
45a Apigenin (4'.5.7-Trihydroxyflavone) C15H10O5 29.69 269.0450 225.0551 201.0547 151.0026 149.0234 107.0129
46a Isorhamnetin (3'-Methoxy-3.4'.5.7-tetrahydroxyflavone) C16H12O7 29.84 315.0505 300.0280 283.0250 271.0250 164.0104 151.0027 47 Dimethoxy-trihydroxy(iso)flavone C17H14O7 29.88 329.0661 314.0442 299.0205 271.0252 48 Methoxy-trihydroxy(iso)flavone C16H12O6 29.92 299.0556 284.0331 256.0374 227.0340 151.0023 49 Tetrahydroxyflavone-O-(cinnamoyl)hexoside C30H26O12 32.12 577.1346 285.0410 284.0332 255.0300 227.0347 50 Dimethylallyl-methoxycoumarin C15H16O3 34.96 245.1178 189.0551 161.0600 159.0444 131.0496 103.0550 51 Isoimperatorin C16H14O4 35.72 271.0970 203.0343 175.0395 147.0445 131.0495 69.0707 a Confirmed by standard.
Table 5
Chemical composition of B. sayaii.
No. Compound Formula Rt. min [M + H]+ [M–H]− Fragment 1 Fragment 2 Fragment 3 Fragment 4 Fragment 5
1 Quinic acid C7H12O6 1.20 191.0556 173.0446 171.0290 127.0390 111.0439 85.0281
2 Pantothenic acid C9H17NO5 5.51 220.1185 202.1078 184.0974 174.1129 116.0347 90.0556
3 Esculin (Esculetin-6-O-glucoside) C15H16O9 12.32 341.0873 179.0343 151.0391 133.0288 123.0446
4 Kynurenic acid C10H7NO3 12.98 190.0504 162.0552 144.0441 116.0497 89.0392
5a Chlorogenic acid (3-O-Caffeoylquinic acid) C16H18O9 14.31 355.1029 163.0393 145.0287 135.0445 117.0340 89.0391
6 Chryptochlorogenic acid (4-O-Caffeoylquinic acid) C16H18O9 15.66 355.1029 163.0392 145.0287 135.0445 117.0339 89.0392
7 Dihydroxy-methoxycoumarin-O-hexoside C16H18O10 15.97 369.0822 207.0296 206.0219 192.0060 190.9982 163.0030
8 Feruloylhexose isomer 1 C16H20O9 16.70 355.1029 235.0609 193.0501 175.0396 149.0598
9 Ethyl syringate C11H14O5 16.86 227.0920 181.0500 155.0705 140.0471 123.0445 95.0497
10 Coumaroylquinic acid isomer 1 C16H18O8 16.87 337.0924 191.0557 173.0448 163.0392 119.0491 93.0332
11 Naringenin-6.8-di-C-glucoside C27H32O15 16.97 595.1663 505.1399 475.1243 415.1036 385.0934 355.0832
12 Feruloylhexose isomer 2 C16H20O9 17.02 355.1029 235.0611 193.0500 175.0396 149.0598
13 Coumaroylquinic acid isomer 2 C16H18O8 17.52 337.0924 191.0558 173.0448 163.0392 119.0489 93.0332
14 Sinapoylhexose C17H22O10 17.92 385.1135 267.0726 249.0619 223.0611 208.0376 164.0471
15 Feruloylquinic acid C17H20O9 17.98 367.1029 193.0504 191.0557 173.0448 134.0364 93.0332
16 Caffeoylshikimic acid C16H16O8 18.04 335.0767 179.0344 161.0235 135.0442
17 Scopoletin (7-Hydroxy-6-methoxycoumarin) C10H8O4 18.64 193.0501 178.0265 165.0550 149.0600 137.0601 133.0288
18 Vicenin-2 (Apigenin-6.8-di-C-glucoside) C27H30O15 19.01 593.1507 575.1433 503.1203 473.1097 383.0780 353.0675
19 Indole-4-carbaldehyde C9H7NO 19.13 146.0606 118.0656 117.0578 91.0549
20 Coumaroylquinic acid isomer 3 C16H18O8 19.18 337.0924 191.0557 173.0445 163.0391 119.0490 93.0332
21 Ferulic acid C10H10O4 19.36 193.0501 178.0264 149.0598 137.0234 134.0363 121.0283
22 Quercetin-O-(hexosyl)hexoside isomer 1 C27H30O17 20.02 625.1405 301.0360 300.0285 271.0252 255.0306
23 Apigenin-C-hexoside-C-pentoside isomer 1 C26H28O14 20.09 563.1401 473.1096 443.0971 413.0907 383.0784 353.0669
24 Apigenin-C-hexoside-C-pentoside isomer 2 C26H28O14 20.36 563.1401 473.1103 443.0992 413.0885 383.0781 353.0676
25 Coumaroylshikimate C16H16O7 20.43 319.0818 163.0393 155.0341 119.0490
26 Apigenin-C-hexoside-C-pentoside isomer 3 C26H28O14 20.74 563.1401 473.1101 443.0992 413.0887 383.0782 353.0675
27a Vitexin (Orientoside. Apigenin-8-C-glucoside) C21H20O10 21.47 433.1135 415.1033 397.0923 337.0708 313.0714 283.0606
28 Quercetin-O-(pentosyl-deoxyhexosyl)hexoside C32H38O20 21.60 741.1878 301.0359 300.0284 271.0258 255.0303
29 Quercetin-O-(hexosyl)hexoside isomer 2 C27H30O17 21.87 625.1405 301.0360 300.0283 271.0250 255.0298
30 Methyl O-feruloylquinate C18H22O9 22.12 381.1186 193.0504 175.0389 160.0153 149.0600 134.0363
31 Luteolin-O-glucuronide C21H18O12 22.27 461.0720 285.0412 217.0506 199.0361 151.0026 133.0283
32 Di-O-caffeoylquinic acid isomer 1 C25H24O12 22.35 515.1190 353.0886 191.0557 179.0343 173.0448 135.0442
33 Isovitexin (Avroside. Apigenin-6-C-glucoside) C21H20O10 22.36 433.1135 415.1036 397.0927 337.0712 313.0712 283.0606
34 Tetrahydroxyflavone-O-(deoxyhexosyl)hexoside
isomer 1 C27H30O15 22.48 593.1507 285.0412 284.0334 199.0396 151.0028
35 Hyperoside (Hyperin. Quercetin-3-O-galactoside) C21H20O12 22.71 463.0877 301.0361 300.0282 271.0254 255.0302 151.0027
36a Isoquercitrin (Hirsutrin. Quercetin-3-O-glucoside) C21H20O12 22.93 463.0877 301.0360 300.0282 271.0255 255.0303 151.0028
37a Rutin (Quercetin-3-O-rutinoside) C27H30O16 23.10 611.1612 465.1038 303.0504 129.0551 85.0291 71.0499
38 Quercetin-O-pentoside isomer 1 C20H18O11 23.27 433.0771 301.0362 300.0281 271.0255 255.0300
39 Quercetin-O-pentoside isomer 2 C20H18O11 23.53 433.0771 301.0362 300.0282 271.0255 255.0302
40 Quercetin-O-(malonyl)hexoside C24H22O15 23.70 549.0881 505.0999 463.0884 301.0360 300.0281 271.0254
41 Apigenin-O-(deoxyhexosyl)hexoside C27H30O14 24.00 579.1714 433.1137 271.0604
42 Apigenin-O-glucuronide C21H18O11 24.02 445.0771 269.0461 175.0238 113.0232
43a Cosmosiin (Apigetrin. Apigenin-7-O-glucoside) C21H20O10 24.07 433.1135 271.0605 153.0186 145.0293 119.0494
44 Di-O-caffeoylquinic acid isomer 2 C25H24O12 24.17 515.1190 353.0885 191.0557 179.0343 173.0448 135.0441
45 Tetrahydroxyflavone-O-(deoxyhexosyl)hexoside
isomer 2 C27H30O15 24.23 593.1507 285.0413 284.0333 255.0303 227.0349 151.0025
46 Methoxy-trihydroxyflavone-O-hexoside C22H22O11 24.28 461.1084 446.0865 299.0568 298.0489 283.0256 255.0301
47a Quercitrin (Quercetin-3-O-rhamnoside) C21H20O11 24.55 449.1084 303.0505 229.0501 129.0552 85.0291 71.0499
48a Diosmin (Diosmetin-7-O-rutinoside) C28H32O15 24.62 609.1820 463.1245 301.0711 286.0477 129.0553 85.0291
49 N-trans-Feruloyltyramine C18H19NO4 24.73 314.1392 177.0550 145.0287 121.0653
50 Isorhamnetin-O-hexoside isomer 1 C22H22O12 24.76 477.1033 315.0519 314.0440 299.0206 285.0414 271.0255
51 Tetrahydroxyflavone-O-(deoxyhexosyl)hexoside
isomer 3 C27H30O15 24.86 593.1507 285.0412 284.0333 255.0302 227.0349 151.0028
52 Isorhamnetin-O-hexoside isomer 2 C22H22O12 24.96 477.1033 315.0520 314.0440 299.0204 285.0412 271.0255
53 Isorhamnetin-O-hexoside isomer 3 C22H22O12 25.22 477.1033 315.0522 314.0440 299.0204 285.0412 271.0255
54 Psoralen isomer C11H6O3 25.66 187.0395 159.0444 143.0496 131.0496 115.0547
55 Afzelin (Kaempferol-3-O-rhamnoside) C21H20O10 26.42 431.0978 285.0412 284.0334 255.0303 227.0349
56 Methoxy-tetrahydroxy(iso)flavone-O-deoxyhexoside C22H22O11 26.60 461.1084 315.0518 314.0440 300.0284 299.0205 271.0255
57a Quercetin C15H10O7 27.07 303.0505 285.0404 257.0449 229.0494 153.0190 137.0235
58a Luteolin (3'.4'.5.7-Tetrahydroxyflavone) C15H10O6 27.85 285.0399 217.0499 199.0396 175.0393 151.0029 133.0284
59a Kaempferol (3.4'.5.7-Tetrahydroxyflavone) C15H10O6 29.38 287.0556 258.0527 213.0550 165.0184 153.0183 121.0286
60a Apigenin (4'.5.7-Trihydroxyflavone) C15H10O5 29.68 269.0450 225.0553 201.0553 151.0028 149.0234 107.0125
61 Dimethoxy-trihydroxy(iso)flavone C17H14O7 29.86 329.0661 314.0442 299.0203 271.0255 62 Salcolin A (Tricin-4'-O-(erythro-β-guaiacylglyceryl) ether) C27H26O11 29.90 525.1397 477.1179 329.0678 314.0433 299.0209 195.0649 63 Methoxy-trihydroxy(iso)flavones C16H12O6 29.98 299.0556 284.0332 256.0378 227.0348 151.0017 107.0116 64 Angelicin C11H6O3 30.54 187.0395 159.0445 143.0496 131.0497 115.0545 65 Salcolin B (Tricin-4'-O-(threo-β-guaiacylglyceryl) ether) C27H26O11 30.70 525.1397 329.0674 314.0443 299.0208 195.0656 165.0550 66 Tetrahydroxyflavone-O-(cinnamoyl)hexoside C30H26O12 32.13 577.1346 285.0412 284.0335 255.0303 227.0349 a Confirmed by standard.
antibacterial effect against M. flavus and E. cloacae (MIC = 0.56 mg
mL
−1; MBC = 0.75 mg mL
−1).
As for the antifungal activity of the extracts, B. brachyactis showed
the highest effect against A. versicolor (MIC = 0.18 mg mL
−1;
MFC = 0.37 mg mL
−1), T. viride (MIC = 0.02 mg mL
−1; MFC = 0.03
mg mL
−1), and P. funiculosum (MIC = 0.18 mg mL
−1; MFC = 0.37 mg
mL
−1). B. brachyactis was more effective against A. versicolor compared
to the standard drug ketoconazole (MIC = 0.20 mg mL
−1; MFC = 0.50
mg mL
−1), and was also more effective than both ketoconazole and
bifonazole against T. viride. On the contrary, A. fumigatus was most
susceptible to B. sayai (MIC = 0.14 mg mL
−1; MFC = 0.28 mg mL
−1)
while P. ochrochloron was most susceptible to B. pinnatifolium
(MIC = 0.275 mg mL
−1; MFC = 0.37 mg mL
−1). Moreover, B. sayai
was more effective than ketoconazole (MIC = 0.20 mg mL
−1;
MFC = 0.50 mg mL
−1) in inhibiting A. fumigatus and was slightly less
effective but comparable to bifonazole (MIC = 0.15 mg mL
−1;
MFC = 0.20 mg mL
−1). On the other hand, B. sayai and B. pinnatifolium
were equally effective against A. terreus (MIC = 0.28 mg mL
−1;
MFC = 0.56 mg mL
−1) and P. verrucosum (MIC = 0.28 mg mL
−1;
MFC = 0.37 mg mL
−1) while B. pinnatifolium and B. brachyactis showed
similar activity against A. niger (MIC = 0.56 mg mL
−1; MFC = 0.75 mg
mL
−1).
A number of compounds identified in the Bunium species were
previously found to possess antimicrobial properties. For instance,
apigenin was found to inhibit several bacteria tested in our study,
in-cluding P. aeruginosa, S. Typhimurium, and P. mirabilis (
Nayaka,
Londonkar, Umesh, & Tukappa, 2014
). Chlorogenic acid showed
anti-bacterial effects by significantly increasing the outer and plasma
membrane permeability, resulting in the loss of the barrier function,
and induced slight leakage of nucleotide, which led to cell death (
Lou,
Wang, Zhu, Ma, & Wang, 2011
). Esculin and rutin were also found to
inhibit S. aureus (
Amin, Khurram, Khattak, & Khan, 2015
;
Kostova,
Nikolov, & Chipilska, 1993
). Vitexin also modulated S. aureus cell
surface hydrophobicity and reduced the intracellular adhesion of
planktonic cells to form biofilm (
Das et al., 2018
). Also, luteolin
in-hibited the activity of DNA topoisomerase I and II in S. aureus, which
resulted in some decrease in the nucleic acid and protein synthesis
(
Wang & Xie, 2010
).
Table 6
Antibacterial activity (mg mL−1) of the tested extracts⁎. Antibacterial properties
Bunium species S. aureus B. cereus P. mirabilis M. flavus P. aeruginosa E. coli S. Typhimurium E. cloacae B. sayai MIC 1.13 ± 0.26b 0.56 ± 0.10c 0.56 ± 0.10d 1.13 ± 0.22d 0.28 ± 0.02b 0.56 ± 0.10b 2.25 ± 0.20bc 1.13 ± 0.10c MBC 1.50 ± 0.20b 0.75 ± 0.03d 0.75 ± 0.20d 1.50 ± 0.20c 0.37 ± 0.03b 0.75 ± 0.01b 3.00 ± 0.30b 1.50 ± 0.90c B. pinnatifolium MIC 1.13 ± .0.10b 0.37 ± 0.02b 0.28 ± 0.10c 0.56 ± 0.10c 0.28 ± 0.03b 0.56 ± 0.03b 2.15 ± 0.40bc 0.56 ± 0.20b MBC 1.50 ± 0.25b 0.75 ± 0.04d 0.37 ± 0.03c 0.75 ± 0.10b 0.37 ± 0.04b 0.75 ± 0.04b 3.00 ± 0.50b 0.75 ± 0.03b B. brachyactis MIC 1.13 ± 0.10b 0.28 ± 0.03b 0.56 ± 0.040d 0.75 ± 0.10c 0.18 ± 0.01ab 0.56 ± 0.05b 1.50 ± 0.60b 1.13 ± 0.20c MBC 1.50 ± 0.30b 0.37 ± 0.02c 0.75 ± 0.050d 1.50 ± 0.20c 0.37 ± 0.02b 0.75 ± 0.06b 3.00 ± 0.80b 1.50 ± 0.10c B. microcarpum MIC 1.13 ± 0.10b 1.13 ± 0.10d 0.14 ± 0.003b 1.13 ± 0.25d 0.28 ± 0.03b 0.56 ± 0.02b 3.00 ± 0.90c 1.13 ± 0.20c MBC 1.50 ± 0.28b 1.50 ± 0.20e 0.18 ± 0.02b 1.50 ± 0.26c 0.37 ± 0.04b 0.75 ± 0.03b 4.50 ± 1.20c 1.50 ± 0.10c Streptomycin MIC 0.10 ± 0.10a 0.03 ± 0.002a 0.10 ± 0.03b 0.05 ± 0.003a 0.10 ± 0.02a 0.10 ± 0.02a 0.10 ± 0.03a 0.10 ± 0.03a MBC 0.20 ± 0.18a 0.05 ± 0.003a 0.20 ± 0.02b 0.10 ± 0.02a 0.20 ± 0.02a 0.20 ± 0.03a 0.20 ± 0.03a 0.20 ± 0.03a Ampicillin MIC 0.10 ± 0.012a 0.10 ± 0.020d 0.01 ± 0.002a 0.10 ± 0.03b 0.30 ± 0.03b 0.15 ± 0.01a 0.10 ± 0.02a 0.15 ± 0.002a MBC 0.15 ± 0.10a 0.15 ± 0.020b 0.01 ± 0.001a 0.15 ± 0.02a 0.50 ± 0.04c 0.20 ± 0.03a 0.20 ± 0.02a 0.20 ± 0.03a Antifungal abilities
Bunium species A. fumigatus A. versicolor A. terreus A. niger T. viride P. ochrochloron P. funiculosum P. veruccosum B. sayai MIC 0.14 ± 0.02a 0.75 ± 0.03d 0.28 ± 0.02b 1.13 ± 0.25d 0.09 ± 0.002b 0.56 ± 0.03b 0.28 ± 0.02b 0.28 ± 0.02ab MFC 0.28 ± 0.01a 1.50 ± 0.20e 0.56 ± 0.03b 1.50 ± 0.28d 0.18 ± 0.02b 0.75 ± 0.03b 0.37 ± 0.01ab 0.37 ± 0.02b B. pinnatifolium MIC 0.50 ± 0.03c 0.56 ± 0.02c 0.28 ± 0.01b 0.56 ± 0.02b 0.28 ± 0.03d 0.28 ± 0.01a 0.28 ± 0.02b 0.28 ± 0.03ab MFC 1.13 ± 0.10c 0.75 ± 0.03d 0.56 ± 0.02b 0.75 ± 0.03c 0.37 ± 0.04c 0.37 ± 0.02ab 0.37 ± 0.03ab 0.37 ± 0.03b B. brachyactis MIC 0.42 ± 0.02c 0.18 ± 0.02b 0.56 ± 0.03c 0.56 ± 0.02b 0.02 ± 0.002a 0.37 ± 0.010ab 0.18 ± 0.01a 0.37 ± 0.03b MFC 0.56 ± 0.03b 0.380.03b 1.25 ± 0.20c 0.75 ± 0.03c 0.03 ± 0.003a 0.75 ± 0.02b 0.37 ± 0.02ab 0.75 ± 0.04c B. microcarpum MIC 0.56 ± 0.02c 1.50 ± 0.20e 0.56 ± 0.03c 0.75 ± 0.02c 0.28 ± 0.02d 0.56 ± 0.03b 0.56 ± 0.03c 0.56 ± 0.02c MFC 1.13 ± 0.06c 3.00 ± 0.10f 1.13 ± 0.30c 1.50 ± 0.20d 0.37 ± 0.03c 0.75 ± 0.04b 0.75 ± 0.04c 0.75 ± 0.04c Ketoconazole MIC 0.20 ± 0.02b 0.20 ± 0.02b 0.15 ± 0.02ab 0.20 ± 0.02a 1.00 ± 0.10e 1.00 ± 0.30c 0.20 ± 0.01ab 0.20 ± 0.02a MFC 0.50 ± 0.05b 0.50 ± 0.03c 0.20 ± 0.01a 0.50 ± 0.03b 1.50 ± 0.20d 1.50 ± 0.20c 0.50 ± 0.02b 0.30 ± 0.03b Bifonazole MIC 0.15 ± 0.02a 0.10 ± 0.01a 0.10 ± 0.01a 0.15 ± 0.02a 0.15 ± 0.02c 0.20 ± 0.03a 0.20 ± 0.01ab 0.10 ± 0.01a MFC 0.20 ± 0.03a 0.20 ± 0.03a 0.15 ± 0.01a 0.20 ± 0.02a 0.20 ± 0.01b 0.25 ± 0.02a 0.25 ± 0.02a 0.20 ± 0.02a
⁎ Different letters indicate the differences in the Bunium species for each bacterial and fungal strains (p < 0.05).
Table 7
Antioxidant properties of the Bunium species⁎.
Samples Phosphomolybdenum (mmol TE g−1extract) DPPH (mg TE g −1 extract) ABTS (mg TE g −1 extract) CUPRAC (mg TE g −1 extract) FRAP (mg TE g −1
extract) Metal chelating activity(mg EDTAE g−1extract) B. sayai 1.24 ± 0.16b 41.15 ± 1.27c 68.66 ± 1.09c 118.53 ± 2.39c 89.05 ± 3.10c 40.70 ± 0.60b B. pinnatifolium 1.53 ± 0.08a 51.89 ± 2.75b 96.66 ± 8.99a 155.47 ± 7.60a 128.23 ± 5.40a 18.29 ± 0.63c B. brachyactis 1.51 ± 0.17a 49.79 ± 1.11b 84.87 ± 1.64b 135.28 ± 1.17b 108.64 ± 2.88b 52.61 ± 0.49a B. microcarpum 1.13 ± 0.05b 69.66 ± 1.52a 100.33 ± 3.18a 160.64 ± 4.37a 121.86 ± 8.34a 15.66 ± 0.28d
⁎ Values expressed are means ± S.D. of three parallel measurements. TE: Trolox equivalent; EDTAE: EDTA equivalent. Different letters indicate the differences in
3.3. Antioxidant activity
Six assays were used to assess the antioxidant capacity of the tested
Bunium species (Table 7
), namely DPPH and ABTS scavenging, cupric
and ferric reducing, phosphomolybdenum (all expressed in mg TE
g
−1extract), and metal chelating assay (expressed in mg EDTAE/g
ex-tract). This approach is useful given the variations among the wide
range of antioxidant assays currently used, which do not provide
pre-cise and accurate information on the complete antioxidant power of a
plant sample (
Mahomoodally et al., 2018
).
The most effective DPPH and ABTS scavenger was B. microcarpum
(69.66 and 100.33 mg TE g
−1), respectively) followed by B.
pinnatifo-lium (51.89 and 96.66 mg TE g
−1, respectively), while the least
effec-tive scavenger was B. sayai. Similarly, B. microcarpum showed the
strongest cupric reducing activity (160.64 mg TE g
−1) while B.
pinna-tifolium exhibited the highest ferric reducing effect (128.23 mg TE g
−1).
Again, the weakest cupric and ferric reducer was displayed by B. sayai.
On the other hand, the least effective antioxidant in the
phosphomo-lybdenum and metal chelating assays was B. microcarpum while the
strongest antioxidant among the tested Bunium species in the respective
assays were B. pinnatifolium (1.53 mmol TE g
−1) and B. brachyactis
(52.61 mg EDTAE g
−1), respectively.
The high antioxidant activity of B. pinnatifolium and B. microcarpum
in most assays (with the exception of metal chelating assay) could be
explained by three reasons; first by the high phenolic and flavonoids
content since a number of previous studies have observed that herbal
extracts possessing high TPC and TFC showed high antioxidant effects
(
Sut et al., 2019
;
Zengin et al., 2019
;
Zengin et al., 2019
). This fact was
also confirmed correlation analysis and the results are given in
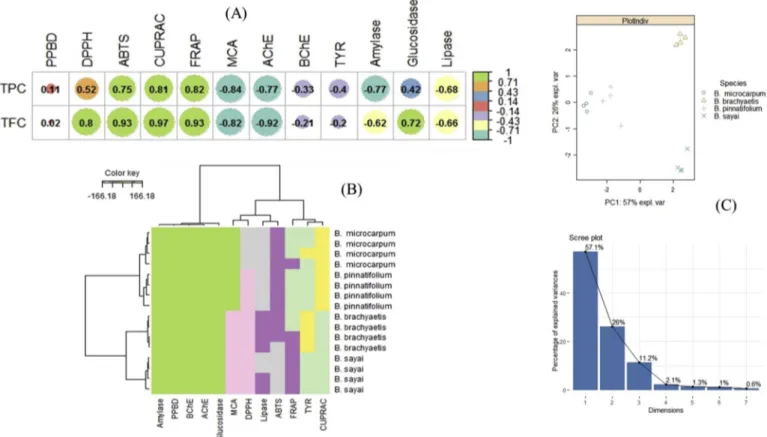
Fig. 1
(A). Strong correlation was observed between total bioactive
compounds and antioxidant properties, especially reducing power
as-says. Secondly, it could be that the compounds present in all the four
species are present in higher amount in these two species, but this
as-sumption need to be further investigated using other analytical
tech-niques to measure the amount of these phenolic compounds in the
extracts. Several compounds present in both species, such as
chloro-genic acid, pantothenic acid, esculin, isoquercitrin, rutin, apigenin,
scopoletin, naringenin, and kaempferol, were previously found to
pos-sess antioxidant properties (
Biljali et al., 2012
;
Cavia-Saiz et al., 2010
;
Li, Jiang, Wang, Liu, & Chen, 2016
;
Malik et al., 2011
;
Romanova,
Vachalkova, Cipak, Ovesna, & Rauko, 2001
;
Vellosa et al., 2011
;
Xu,
Hu, & Liu, 2012
;
Yang, Guo, & Yuan, 2008
).
3.4. Enzyme inhibitory activity
The inhibitory properties of the four Bunium species were studied
against six enzymes, namely AChE, BChE, tyrosinase, amylase,
gluco-sidase, and lipase (
Table 8
). The development of innovative natural
cholinesterase inhibitors are presently gaining much interest for the
symptomatic treatment of Alzheimer’s disease. On the other hand, the
inhibition of α-glucosidase and α-amylase (two main enzymes involved
in starch hydrolysis to glucose) has also become an important strategy
to tackle diabetes mellitus (type 2), while tyrosinase inhibition is
ne-cessary to prevent the misbalance in melanin formation, associated
with a variety of skin diseases such as melasma and hyperpigmentation
(
Mahomoodally et al., 2018
). In addition, inhibition of pancreatic lipase
and the associated reduction of lipid absorption is an attractive
ap-proach to manage obesity and its associated diseases such as diabetes
mellitus and coronary heart diseases (
Buchholz & Melzig, 2015
).
It was observed that B. sayai and B. brachyactis were almost equally
effective in inhibiting AChE (3.53 and 3.42 mg GALAE g
−1,
respec-tively). The most effective BChE inhibitor was B. brachyactis (3.68 mg
GALAE g
−1). In contrast, B. microcarpum displayed the lowest
in-hibitory effect against cholinesterases. In addition, the four species
Fig. 1. Statistical evaluations (A: Correlation coefficients between total bioactive compounds and biological activities (Pearson Correlation Coefficient (R). p < 0.05); B: Heatmap of extracts in according to biological activities (n = 4); C: Distribution of the tested Bunium species on the correlation circle based on PCA; PPBD: Phosphomolybdenum; MCA: Metal chelating assay; TYR: Tyrosinase inhibition assay; TPC: Total phenolics content; TFC: Total flavonoids content).