Contents lists available atScienceDirect
LWT - Food Science and Technology
journal homepage:www.elsevier.com/locate/lwtMoisture sorption isotherm, isosteric heat and adsorption surface area of
whole chia seeds
Sultan Arslan-Tontul
Selçuk University, Agricultural Faculty, Department of Food Engineering, 42130, Konya, Turkey
A R T I C L E I N F O Keywords: Water activity Moisture content Sorption isotherm Isosteric heat Oilseed A B S T R A C T
This study aimed to evaluate moisture sorption isotherm of whole chia seed. The equilibrium moisture content (EMC) of seeds were detected by saturated salt solutions which have the water activity (aw) range of 0.2–0.9. The isosteric sorption heat was calculated by the Clausius-Clapeyron equation using three different sorption tem-peratures (15 °C, 25 °C and 35 °C). The adsorption surface area of seeds was also calculated by monolayer moisture content obtained from BET and GAB equation. The EMC content of seeds had an increasing trend and determined as 18–20 g H2O/100 g solid at the highest awlevel. The whole chia seeds became less hygroscopic with the rising sorption temperatures at constant aw. The moisture sorption isotherm was determined as Type II. The monolayer moisture content was determined as 2.39–2.91 g H2O/100 g solid. BET and Peleg were the best-fitted models. The isosteric and net isosteric heat were 77.74 and 34.74 kJ/mol at lowest moisture content, respectively. Additionally, the adsorption surface area changed between 95.31 and 102.72 m2/g.
1. Introduction
Chia (Salvia hispanica L.) is an annual summer herbaceous plant that classified in the family Lamiaceae, subfamily Nepetoideae, and genus Salvia. The native land of chia is Guatemala and Mexico, but today, it is cultivated in Australia, Bolivia, Colombia, Guatemala, Peru and Argentina (Grancieri, Martino, & de Mejia, 2019; Moreira, Chenlo, Prieto, & Torres, 2012). Nowadays, the demand for chia seeds is in-creasing in the food industry due to its high nutritional quality. It is reported that whole chia seed contains high amounts of fatty acids, dietaryfibres, proteins, antioxidants, vitamins and minerals (Ayerza & Coates, 2011;Grancieri et al., 2019;Muñoz, Cobos, Diaz, & Aguilera, 2012;Valdivia-López & Tecante, 2015). It is known as one of the most important sources of omega-3, classified as polyunsaturated fatty acids (PUFA) (Ayerza & Coates, 2009,2011;Moreira et al., 2012). It is re-commended for the preventing of various chronic diseases such as obesity, cardiovascular diseases, diabetes, and cancer (Grancieri et al., 2019).
In the last decade, chia seeds have been added in the formulation of various foods such as bakery products (Brites et al., 2019;Zhu & Chan, 2018), yoghurt (Kwon, Bae, Seo, & Han, 2019), cheese (Munoz-Tebar et al., 2019), cereal bar (Iuliano, Gonzalez, Casas, Moncayo, & Cote, 2019), ice-cream (Campos, Ruivo, Scapim, Madrona, & Bergamasco, 2016) and frankfurter (Fernandez-Lopez et al., 2019) to improve nu-tritional quality and gain the functionality to the end product. This
popularity makes important transportation of chia to all over the world since it is harvested mostly in tropical and subtropical regions. When the storage conditions are not optimised, seeds can quickly deteriorate as oxidatively because of high PUFA content (Bodoira, Penci, Ribotta, & Martínez, 2017; Bordón, Meriles, Ribotta, & Martinez, 2019). Ad-ditionally, high relative humidity of environment and water activity of food lead to the microbial spoilage (Abdullah, Nawawi, & Othman, 2000). Sorption isotherms can control unstable storage conditions such as relative humidity, temperature and water activity and moisture content of the product.
A sorption isotherm is a relationship between equilibrium moisture content (EMC) and water activity (aw) at constant temperature and
pressure. Sorption isotherms are crucial to know the water sorption mechanism and interactions between food components and water. Therefore, it gives useful information in modelling of the drying process and equipment, optimisation, predicting the shelf life of product, de-termining critical moisture level, mixing products with various awand
the selection of packaging material (Koua, Koffi, Gbaha, & Toure, 2014; Panjagari, Singh, Ganguly, & Indumati, 2015;Shanker, Kumar, Juvvi, & Debnath, 2019;Soleimanifard & Hamdami, 2018). Besides these prac-tical applications, the isotherm is also important for evaluating the thermodynamic functions of the water, which is adsorbed in foods (Chirife & Iglesias, 1978).
The isosteric heat of sorption is a thermodynamic parameter cal-culated from sorption isotherm, carried at least two temperature. It
https://doi.org/10.1016/j.lwt.2019.108859
Received 2 October 2019; Received in revised form 15 November 2019; Accepted 18 November 2019 E-mail addresses:sultan.arslan@selcuk.edu.tr,sltnarslan07@gmail.com.
Available online 29 November 2019
0023-6438/ © 2019 Elsevier Ltd. All rights reserved.
implies the amount of energy required to change unit mass of a product from liquid to vapour at a certain temperature and aw. From the point of
view, the energy requirement for drying of a material can be evaluated by isosteric heat. The moisture content at which the net isosteric sorption heat is approximately equal to the latent evaporation tem-perature of pure water is indicator of bound water. As food is dried to the lower moisture levels, the heat of adsorbed water increase above the vaporisation of pure water (Khawas & Deka, 2017; Koua et al., 2014).
In literature, there is only one study investigating the sorption iso-therms of whole chia seeds.Moreira et al. (2012)used the GAB equa-tion (Guggenheim–Andersen–de Boer equation) to determine moisture sorption properties of whole chia seeds. On the other hand, to reveal the moisture sorption characteristics of chia and control storage conditions have great importance due to its quick oxidative deterioration with high oil and PUFA content. For this purpose in the designed study, it is aimed to determine the safe storage conditions (humidity and water activity) by evaluating different sorption models and calculate the isosteric heat and water adsorption surface area of chia seeds.
2. Materials and methods
2.1. Material
Black chia seeds, which were harvested from Argentina, were ob-tained by a commercial importer (Yayla Agro Food, Mersin) in Turkey. The seeds removed from broken parts and foreign materials. The di-mensions of 20 random seeds were determined as follow; 1.97 mm length, 1.13 width, and 0.89 mm thickness. The proximate analysis of seeds was determined by the procedure of AACC (1999). The total protein, fat,fibre, ash and moisture content of seeds were detected as 20.36, 38.12, 18.28, 4.54 and 7.47 g/100 g, respectively. Prior to sorption experiments, seeds were pre-dried in a vacuum oven at 50 °C for five days (Moreira et al., 2012). After the drying, the moisture content and awwere decreased to 1.76 g/100 g and 0.19, respectively.
The seeds were kept in the refrigerator, untill the sorption experiment. Saturated salts solutions of CH3CO2K (CAS No: 127-08-2, Merck,
Darmstadt, Germany) MgCl2(CAS No: 7786-30-3, Sigma, Taufkirchen,
Germany), K2CO3 (CAS No: 584-08-7, Merck, Darmstadt, Germany),
NaBr (CAS No: 7647-15-6, Carlo Erba, Val de Reuil, France), KI (CAS No: 761-11-0, Merck, Darmstadt, Germany), NaCl (CAS No: 7647-14-5, Sigma, Taufkirchen, Germany), BaCl2(CAS No: 1026-27-9, Carlo Erba,
Val de Reuil, France) and K2SO4 (CAS No: 7778-80-5, Sigma,
Taufkirchen, Germany) were used for the obtaining various awrange.
2.2. Sorption procedure
The EMC of chia seeds was determined at 15 °C, 25 °C and 35 °C using eight saturated salt solutions with the aw range of 0.19–0.94
(Table 1). The awvalues of each saturated salt solutions were equal to
the relative humidity divided by 100 (aw = RH/100). The static
gravimetric method was applied for the determination of adsorption isotherms of seeds (Bell & Labuza, 2000). The saturated salt solutions were prepared at boiling water by dissolving the salts until saturation and left them to cool to the room temperature. The saturated salt so-lutions were placed in desiccators and conditioned for 7 days prior to sorption experiment. The awof the saturated salt solutions at different
holding temperatures were measured by using the awmeter (Aqualab,
Washington, USA). Triplicate samples each of 0.45 g ( ± 0.01 g) were weighed in the beaker and placed in desiccators containing saturated salt solutions. At high awabove 0.6, 2 mL toluene was added in a beaker
and it was placed in the desiccators in order to prevent the fungal spoilage of seeds. The sample weighing was performed daily, and EMC was detected when the samples reached constant weight ( ± 0.001) at 15 °C, 25 °C and 35 °C. The moisture content of samples was determined by drying in a drying chamber at 105 °C to a constant weight.
2.3. Analysis of experimental data
The moisture sorption isotherms of whole chia seeds were de-termined by plotting of EMC values obtained from each temperature against the corresponding aw. The description of relationship between
EMC, equilibrium relative humidity and temperature were verified ac-cording to BET (Brunauer–Emmett–Teller) (Aguerre, Suarez, & Viollaz, 1989), GAB (Van den, 1981), Halsey (Halsey, 1948), Henderson (Iglesias & Chirife, 1982), Iglesias & Chirife (Chirife & Iglesias, 1978), Caurie (Chirife & Iglesias, 1978), Oswin (Oswin, 1946), Peleg (Peleg, 1993), Smith (Smith & Smith, 1947) and White & Eiring (Sormoli & Langrish, 2015) (Table 2). The curve fitting and regression analysis were performed using a mathematical software program (Origin Lab Corp, Massachusetts, USA). Thefittest sorption model was selected by the regarding of minimum root of mean square error (RMSE), minimum means absolute percentage error (P %), and the maximum degrees of freedom adjusted R-square (Radj2) of thefit (Sormoli & Langrish, 2015).
RMSE and Radj2were obtained from mathematical software program,
and P was calculated from Equation (1) by experimental (YE) and
predicted data (YP) obtained from thefit.
∑
= − = P N Y Y Y 100 i N E P E 1 Eq. 12.4. Determination of isosteric heat of sorption
The net isosteric sorption heat is defined by the difference between total isosteric sorption heat and condensation heat. It was calculated by equations(2) and (3);
Table 1
Water activity (aw) of different saturated solutions of salt at different tem-peratures. Salt aw 15 °C 25 °C 35 °C Potassium acetate 0.22 ± 0.001 0.20 ± 0.001 0.20 ± 0.003 Magnesium chloride 0.34 ± 0.001 0.33 ± 0.001 0.31 ± 0.001 Potassium carbonate 0.44 ± 0.003 0.43 ± 0.001 0.42 ± 0.001 Sodium bromide 0.60 ± 0.003 0.58 ± 0.001 0.58 ± 0.001 Potassium iodide 0.70 ± 0.001 0.69 ± 0.001 0.69 ± 0.001 Sodium chloride 0.76 ± 0.001 0.76 ± 0.001 0.76 ± 0.001 Barium chloride 0.89 ± 0.001 0.89 ± 0.001 0.88 ± 0.006 Potassium sulphate 0.94 ± 0.001 0.94 ± 0.002 0.94 ± 0.001 Table 2
The model equations forfitting the sorption isotherms of chia seed.
Model type Equation Reference
BET = × × − + − − × X Xm C aw aw C aw aw [(1 ) ( 1)((1 ) )] Aguerre et al. (1989) GAB = × × × − × − × + × × X Xm C k aw k aw k aw C k aw [(1 )(1 )] Van den (1981) Caurie X=exp(A+B×aw) Chirife and Iglesias
(1978) Halsey = −
(
)
X A lnaw B1 Halsey (1948)Henderson aw=1−exp( (−B×XA)) Iglesias and Chirife (1982) Iglesias & Chirife = + × − X A B aw aw
1 Chirife and Iglesias
(1978) Oswin = × − X A aw aw 1 Oswin (1946) Peleg X=A×awB+C×awD Peleg (1993)
Smith X=A−B×(ln(1−aw)) Smith and Smith (1947) White & Eiring =
+ × X
A B aw
1 Sormoli and Langrish
= −
( )
d Lnaw d q R ( ) T St 1 Eq. 2 = − qSt QSt ΔHVap Eq. 3 aw= Water activity T = Selected temperature (15 °C, 25 °C or 35 °C) QSt= Isosteric heat of sorptionqSt= Net isosteric heat of sorption
R = 8.314 kJ/molK ΔHVap= 43 kJ/mol
The heat of sorption was determined from the slopes of ln awagainst
1/T plots by linear regression analysis, with the assumption that they are constant over the temperature range studied. QStis a measure of
interaction between water vapour and the adsorbent food material (Ayranci & Duman, 2005).
2.5. Determination adsorption surface area
Adsorption surface area of seeds was calculated from equation(4) using monolayer moisture content obtained from BET and GAB as fol-lows (Koua et al., 2014);
= × × × − × ×
SA X
g mol m molecules mol
1
18 / (1.06 10 ) (6 10 / )
M 19 2 23
Eq. 4
3. Results and discussion
3.1. Adsorption isotherm of whole chia seeds
The moisture sorption isotherm of whole chia seeds is given in Fig. 1. The EMC content of seeds had an increasing trend by the rising of awvalue. It was an expected result caused by increasing of surrounding
vapour pressure of food led increasing of the vapour pressure within. This effect was also reported byMoreira et al. (2012)andShanker et al. (2019). The sorption capacity of material is highly related to chemical composition and structure. Materials with hydrophilic structures such as sugar have more water adsorption ability.Lazouk et al. (2015) no-tified that the composition of oilseed fractions and total moisture con-tent designed the distribution of water in the seed.
At the highest aw, the seeds were adsorbed 18–20 g H2O/100 g
solid. This value was lower than that of reported most of the grains, but it showed similarity with oilseeds. The oil content of chia, nearly 30–38 g/100 g. It might show hygroscopic effect and limit adsorption of water from the surface. Giner and Gely (2005) found that the EMC
content of sunflower was less than wheat, and the researchers explained this result by steric difficulties for water adsorption in the presence of oil.Moreira et al. (2012)determined EMC of chia as 16.6 g H2O/100 g
solid. The EMC of rapeseed was found to be 15 g/100 g solid (Lazouk et al., 2015).
In the study, chia seeds became less hygroscopic with the increasing sorption temperatures at constant aw. It could be a result of that when
temperature increases, the water molecules gain more activity which leads to an increase in the intermolecular distance due to the rise in their energy level. Thus, they become less stable and break away easily from the water binding sites of the food. This phenomenon has been reported from previous studies (Bup et al., 2013; Koua et al., 2014; Mbarga, Nde, Mohagir, Kapseu, & Nkenge, 2017; Singh, Mishra, & Saha, 2011;Soleimanifard & Hamdami, 2018;Taitano, Singh, Lee, & Kong, 2012).
As can be seen inFig. 1, the isotherm has sigmoidal shape due to the two bending zone at 0.2–0.4 and 0.6–0.8 aw. Therefore, the moisture
sorption isotherm of whole chia seeds was determined as Type II ac-cording to Brunauer classification. Additionally, C value is an isotherm constant that is calculated from BET equation. C constant higher than one means that the moisture sorption isotherm must be classified in type II (Sormoli & Langrish, 2015). This type has been reported for the various kind of foods such as juice powder (Sormoli & Langrish, 2015), millet grain (Singh et al., 2011), almond (Taitano et al., 2012), whole wheat and riceflours (Abebe & Ronda, 2015), bananaflour (Khawas & Deka, 2017) and neem kernel (Mbarga et al., 2017) and chia (Moreira et al., 2012). Additionally, type II isotherms are generally described for oilseeds (Al-Muhtaseb, McMinn, & Magee, 2002;Lazouk et al., 2015).
3.2. Monolayer moisture content
The monolayer moisture content is critical moisture content to control and extend quality shelf life of products. At this moisture level, most of the degradation and food spoilage reactions such as en-zymatical browning and oxidation, physical changes in food products such as loss of crispiness, caking and stickiness are slow down. Additionally, it helps determination of the surface potential of moisture sorbed in food (Singh et al., 2011;Sormoli & Langrish, 2015).
The monolayer moisture content (XM) of whole chia seeds were
determined to be 2.39–2.91 g H2O/100 g solid according to BET and
GAB models. The results were in agreement withMoreira et al. (2012) who calculated the XMof chia seed by the GAB model as 1.5–2.2 g H2O/
100 g at tested temperatures. Similar results were also reported by containing high oil seeds and nuts. XMcontent of various nuts (almond,
Brazilian nut, cashew, hazelnut, macadamia nut, pecan, pine nut, pis-tachio, walnut) was determined as 1.1–2.9 H2O/100 g for BET and
1.5–3.3 H2O/100 g for GAB (Venkatachalam & Sathe, 2006). The
reported XMfor hazelnut kernel was 2.17–2.52 (Jung, Wang, McGorrin & Zhao, 2018).Taitano et al. (2012)determined XMbetween 2.38 and
2.48 g H2O/100 g in glanced almonds.Lazouk et al. (2015)calculated
the XMvalue of whole rapeseed, sunflower and linseed were 3, 4.9 and
6 g/100 g respectively.
The general opinion is that the XMvalue decreases with increasing
sorption temperatures due to breaking away of water molecules from their sorption sites easily at high energy levels (Samapundo et al., 2007). However, in this study, XMcalculated by GAB decreased with
increasing sorption temperatures, whereas XMobtained BET was not
affected by temperature. This result might be due to the fact that the BET model can only be applied in the awrange of 0.1–0.5; therefore, did
not represent all experimental data points. Similar results were also reported by Sormoli and Langrish (2015) andMbarga et al. (2017). Additionally, XMobtained by BET was lower than GAB parameters.
According toTaitano et al. (2012), XMis critical data for designing
storage conditions with minimum changes in the food. Consequently, the obtained data from this study imply that the awand relative
hu-midity should be lower 0.25 and 25 g/100 g, respectively, for a long and quality storage of whole chia seeds.
3.3. Model equations
The results obtained from the regression analysis are presented in Table 3. Some statistical parameters are considered for interpreting the fittest equations. Radj2 is one of these statistical parameters and
generally the values are higher than≥0.98 is acceptable. The studies model except GAB andIglesias and Chirife (1982)ensured goodfitness in expressing sorption isotherm of chia seeds. Interestingly, GAB model has been used for explanation sorption properties most of foods. For example,Moreira et al. (2012) recommended the GAB equation for fitting the experimental data of chia seed.Koua et al., 2014announced the GAB model as adequately predicted EMC of cassava for the range of temperatures and aw. However, in this study, thefitness of GAB model
was low.
The mean absolute percentage of error (P%) is another statistical fitness parameter. The limit level of P% is controversial. According to Lomauro, Bakshi, and Labuza (1985), it should be lower than 5% for goodfitness, but in the most of the previous studies it has been reported as 10% (Kaymak-Ertekin & Sultanoglu, 2001;Koua et al., 2014;Sormoli & Langrish, 2015). FromTable 3, when all working temperatures were considered, thefitness of model according to P% can be ordered as BET > GAB > Peleg > Oswin > Halsey > Henderson. The goodness to thefit of some models such as Smith and White & Eiring get worse with the increasing sorption temperatures. It can be concluded from the results that these equations can be used only lower sorption temperatures to state moisture properties of chia seeds.
In most of the literature, the RMSE value of thefittest model was lower than 1. Therefore the nearest RMSE value below 0 to 1 is ac-ceptable for a good modelfit. From the results, it can be said that the RMSE value of models was in acceptable range except Iglesias and Chirife (1982). In addition, it can be noted that the lowest values were
Table 3
Predicted parameters of thefitted models to the experimental data for moisture sorption isotherm of chia seed.
Model type Temp. (°C) Modelfit parameters Model coefficients
RAdj2 P % RSME A B C D BET 15 0.990 0.179 0.001 – 2.41; XM 8.03 – 25 0.998 0.559 0.001 – 2.39; XM 8.47 – 35 0.996 1.113 0.003 – 2.57; XM 8.80 – GAB 15 0.975 2.413 0.004 0.90; k 2.91; XM 5.13 – 25 0.977 2.351 0.004 0.92; k 2.76; XM 6.42 – 35 0.978 2.669 0.004 0.91; k 2.70; XM 4.25 – Caurie 15 0.985 11.789 0.603 −0.425 3.493 – – 25 0.985 14.160 0.730 −0.751 3.864 – – 35 0.978 17.672 1.024 −1.410 4.152 – – Halsey 15 0.995 1.666 0.068 20.78 1.878 – – 25 0.985 4.875 0.095 5.224 1.419 – – 35 0.986 8.288 0.108 3.094 1.230 – – Henderson 15 0.990 3.226 0.044 0.269 2.228 – – 25 0.995 2.947 0.056 0.347 1.876 – – 35 0.984 8.682 0.109 0.474 1.604 – –
Iglesias & Chirife 15 0.952 13.798 1.227 5.283 1.088 – –
25 0.944 26.332 1.450 3.089 1.178 – – 35 0.981 21.039 1.060 2.378 0.468 – – Oswin 15 0.993 6.156 0.339 5.313 0.487 – – 25 0.994 6.183 0.415 4.319 0.551 – – 35 0.991 4.543 0.551 3.539 0.584 – – Peleg 15 0.997 3.857 0.284 9.146 1.254 15.990 8.172 25 0.998 3.523 0.233 8.434 0.939 19.489 8.248 35 0.995 5.321 0.392 18.090 11.077 9.459 1.409 Smith 15 0.993 7.430 0.466 −0.295 6.468 – – 25 0.989 10.227 0.621 −0.252 6.528 – – 35 0.986 14.948 0.724 −0.768 6.358 – –
White & Eiring 15 0.999 2.321 0.232 0.308 −0.277 – –
25 0.991 11.203 0.562 0.420 −0.394 – –
determined in BET and GAB equations.
As a conclusion when all statistical parameters were considered, the BET equation gave the bestfit to sorption data with the minimum P% and RMSE and maximum Radj2at 15 °C, 25 °C and 35 °C in awrange
between 0.2 and 0.5. On the other hand, the experimental data were satisfactorilyfitted using the Peleg model in the whole studied a range of awand temperatures with the values of P < 5.32, RMSE < 0.392
and Radj2> 0.99. Therefore, BET and Peleg model can be applied for
adequately predicted EMC of whole chia seeds for the range of tem-peratures and awstudied. There have been previous studies found the
Peleg model as suitable to explain the sorption activities of foods (Khawas & Deka, 2017;Shanker et al., 2019).
3.4. Net isosteric heat of sorption
The isosteric heat of sorption is a useful method for determining the effect of temperature to the foods. It defines as the amount of energy required to change unit mass of a product from liquid to vapour at a particular temperature and aw. The isosteric heat is generally modelled
by Clasius-Clapeyron equation. The application of this method requires data at least at two or more experimental temperatures. The net isos-teric heat of sorption can be used to estimate the energy requirements of drying and provides important information on the state of water in foodstuffs (Koua et al., 2014).
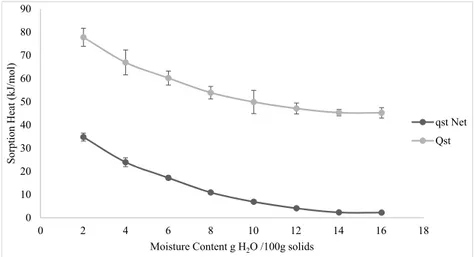
Fig. 2shows the isosteric heat and net isosteric heat of sorption. At the lowest moisture content, QStand qStwere calculated to be 77.74
and 34.74 kJ/mol and tended to decrease with increasing the moisture content. Moreira et al. (2012) found that a 10 times increase of moisture content caused to 7.2 times reduces in isosteric heat.Singh et al. (2011)reported that the isosteric heat, calculated using Clausiu-s–Clapeyron equation, varied between 46.76 and 61.71 kJ/mol at moisture levels 7–21 g/100 g for barnyard millet grain. The net isosteric heat of sorption decreased from 28 to 5 kJ/kg by the increase of moisture content 2–7 g/100 g in hazelnut kernels (Jung et al., 2018). Tarigan, Prateepchaikul, Yamsaengsung, Sirichote, and Tekasakul (2006) noted that net isosteric heat decreased until 0 kJ/mol with raising of moisture content.
Fig. 2clearly illustrated that the decrease of isosteric heat occurred more sharply in the moisture content range of 2–10 g/100 g and after that no more change was observed. The similar result was also obtained byPanjagari et al. (2015)who found that the maximum heat of ad-sorption (93.79 kJ/mol) was obtained between the moisture content of 1–2 g/100 g on dry basis. However, between 2 and 5 g/100 g moisture, the isosteric heat of sorption decreased sharply, and after that, it was in line. At the high level of moisture content of food, the energy necessary for vaporisation is low due to weak hydrophilic bounds of
macromolecules and free water. On the contrary, during drying, moisture content decreases continuously since only the monolayer moisture is left. As a result of this process, the water molecules become tightly bound to the surface of food and the sorption sites. At the same time, the heat of sorption increases above the heat of vaporisation of pure water, making it difficult to remove water from the surface (Iglesias & Chirife, 1982; Kaya & Kahyaoglu, 2006; Panjagari et al., 2015;Sormoli & Langrish, 2015). Moreover,Khawas and Deka (2017) attributed the decrease of qStwith the increase in EMC values to strong
water-solid interaction and sorption occurred on the less active sites giving lower qSt.
3.5. Adsorption surface area
The specific surface area plays an essential role in determining the water-binding capacity of a material (Hidar et al., 2018). Adsorption surface area of chia seeds was calculated by monolayer moisture con-tent obtained from GAB. SA was determined to be 102.72, 97.43 and 95.31 m2/g at 15 °C, 25 °C and 35 °C, respectively. From the results, it can be concluded that making hydrogen bound capacity of seeds creased with increasing temperature. This behaviour has been de-scribed as a reduction in the number of active sites because of physical and chemical changes induced by temperature (Hidar et al., 2018). The surface interaction, structure and chemical composition affected the water sorption capacity of seeds.Koua et al. (2014)indicated micro-porous structure of food lead to increase adsorption surface area.Bup et al. (2013)determined for shea nut as 72.32–175.65 m2/g and
re-ported a significant reduction of the surface area of both raw and cooked kernels with increasing temperature.
4. Conclusion
According to the results, the moisture content of seeds had an in-creasing trend by the rising of awvalue, and the seeds adsorbed 18–20 g
H2O/100 g solid. Chia seeds became less hygroscopic with the
in-creasing sorption temperatures at constant aw. The adsorption isotherm
of seeds detected as Type II according to Branuer classification. The XM
of whole chia seeds were determined as 2.39–2.91 g H2O/100 g solid
according to BET and GAB equations. Additionally, the experimental data were satisfactorilyfitted using the Peleg model in the whole stu-died range of water activities. The isosteric heat decreased more sharply at lower moisture contents, and after that no more change was ob-served. According to SA calculations, it can be concluded that making hydrogen bound capacity of chia seed surface decreased with increasing temperature. Consequently, the obtained data from this study imply that awand relative humidity should be lower 0.25 and 25 g/100 g,
0 10 20 30 40 50 60 70 80 90 0 2 4 6 8 10 12 14 16 18 Sorption Heat (kJ/m ol)
Moisture Content g H2O /100g solids
qst Net Qst
respectively for a long and quality storage of whole chia seeds.
CRediT author statement
Sultan ARSLAN-TONTUL: Investigation, Methodology, Data Curation, Writing- Reviewing and Editing.
Declaration of competing interest
None.
Nomenclature
A, B, C, D, k Model coefficients
GAB Guggenheim, Anderson, de Boer equation BET Brunauer, Emmett, Teller equation EMC Equilibrum moisture content aw Water activity
RH Relative humidity
RMSE Minimum root of mean square error Radj2 Degrees of freedom adjusted R-square
P % Mean absolute percentage error
YE Experimental equilibrium moisture content
YP Predicted equilibrium moisture content form thefit
N Number of data point
T Temperature
QSt Isosteric heat of sorption
qSt Net isosteric heat of sorption
R Universal gas constant
ΔHVap heat of vaporisation of pure water (kJ/mol water)
SA Adsorption surface area XM Monolayer moisture content
Appendix A. Supplementary data
Supplementary data to this article can be found online athttps:// doi.org/10.1016/j.lwt.2019.108859.
References
AACC (1999). Official methods of analysis of aoac international. Minnesota, USA: American Association of Cereal Chemists, Inc (St).
Abdullah, N., Nawawi, A., & Othman, I. (2000). Fungal spoilage of starch-based foods in relation to its water activity (aw). Journal of Stored Products Research, 36(1), 47–54.
https://doi.org/10.1016/S0022-474X(99)00026-0.
Abebe, W., & Ronda, F. (2015). Flowability, moisture sorption and thermal properties of tef Eragrostis tef (Zucc.) Trotter grainflours. Journal of Cereal Science, 63, 14–20. https://doi:10.1016/j.jcs.2015.02.003.
Aguerre, R. J., Suarez, C., & Viollaz, P. E. (1989). New BET type multilayer sorption isotherms. Part II: Modelling water sorption in foods. Lebensmittel-Wissenschaft und -Technologie- Food Science and Technology, 22(4), 192–195.
Al-Muhtaseb, A. H., McMinn, W. A. M., & Magee, T. R. A. (2002). Moisture sorption isotherm characteristics of food products: A review. Food and Bioproducts Processing, 80(2), 118–128.https://doi.org/10.1205/09603080252938753.
Ayerza, R., & Coates, W. (2009). Influence of environment on growing period and yield, protein, oil andα-linolenic content of three chia (Salvia hispanica L.) selections. Industrial Crops and Products, 30(2), 321–324.https://doi.org/10.1016/j.indcrop. 2009.03.009.
Ayerza, R., & Coates, W. (2011). Protein content, oil content and fatty acid profiles as potential criteria to determine the origin of commercially grown chia (Salvia hispa-nica L.). Industrial Crops and Products, 34(2), 1366–1371.https://doi.org/10.1016/j. indcrop.2010.12.007.
Ayranci, E., & Duman, O. (2005). Moisture sorption isotherms of cowpea (Vigna un-guiculata L. Walp) and its protein isolate at 10, 20 and 30 degrees C. Journal of Food Engineering, 70(1), 83–91.https://doi.org/10.1016/j.jfoodeng.2004.08.044. Bell, L. N., & Labuza, T. P. (2000). Moisture sortption:Practical aspects of isotherm
mea-surement and use (2nd ed.). MN, USA: American Association of Cereal Chemists, Inc. Bodoira, R. M., Penci, M. C., Ribotta, P. D., & Martínez, M. L. (2017). Chia (Salvia
his-panica L.) oil stability: Study of the effect of natural antioxidants. Lebensmittel-Wissenschaft und -Technologie- Food Science and Technology, 75, 107–113.https://doi. org/10.1016/j.lwt.2016.08.031.
Bordón, M. G., Meriles, S. P., Ribotta, P. D., & Martinez, M. L. (2019). Enhancement of composition and oxidative stability of chia (Salvia hispanica L.) seed oil by blending
with specialty oils. Journal of Food Science, 84(5), 1035–1044.https://doi.org/10. 1111/1750-3841.14580.
Brites, L., Ortolan, F., da Silva, D. W., Bueno, F. R., Rocha, T. D., Chang, Y. K., et al. (2019). Gluten-free cookies elaborated with buckwheatflour, millet flour and chia seeds. Food Science and Technology, 39(2), 458–466.https://doi.org/10.1590/fst. 30416.
Bup, D. N., Abi, C. F., Tenin, D., Kapseu, C., Tchiegang, C., & Mouloungui, Z. (2013). Effect of cooking on moisture sorption isotherms of shea nut (Vitellaria paradoxa Gaertn.) kernels part II: Modelling and properties of sorbed water. Food and Bioprocess Technology, 6(11), 3273–3283. https://doi.org/10.1007/s11947-012-0949-8.
Campos, B. E., Ruivo, T. D., Scapim, M. R. D., Madrona, G. S., & Bergamasco, R. D. (2016). Optimization of the mucilage extraction process from chia seeds and application in ice cream as a stabilizer and emulsifier. LWT-Food Science and Technology, 65, 874–883.https://doi.org/10.1016/j.lwt.2015.09.021.
Chirife, J., & Iglesias, H. A. (1978). Equations forfitting water sorption isotherms of foods: Part 1— a review. International Journal of Food Science and Technology, 13(3), 159–174.https://doi.org/10.1111/j.1365-2621.1978.tb00792.x.
Fernandez-Lopez, J., Lucas-Gonzalez, R., Viuda-Martos, M., Sayas-Barbera, E., Navarro, C., Haros, C. M., et al. (2019). Chia (Salvia hispanica L.) products as ingredients for reformulating frankfurters: Effects on quality properties and shelf-life. Meat Science, 156, 139–145.https://doi.org/10.1016/j.meatsci.2019.05.028.
Giner, S. A., & Gely, M. C. (2005). Sorptional parameters of sunflower seeds of use in drying and storage stability studies. Biosystems Engineering, 92(2), 217–227.https:// doi.org/10.1016/j.biosystemsorg.2005.06.002.
Grancieri, M., Martino, H. S. D., & de Mejia, E. G. (2019). Chia seed (Salvia hispanica L.) as a source of proteins and bioactive peptides with health benefits: A review. Comprehensive Reviews in Food Science and Food Safety, 18(2), 480–499.https://doi. org/10.1111/1541-4337.12423.
Halsey, G. (1948). Physical adsorption on non-uniform surfaces. The Journal of Chemical Physics, 16(10), 931–937.https://doi.org/10.1063/1.1746689.
Hidar, N., Ouhammou, M., Idlimam, A., Jaouad, A., Bouchdoug, M., Lamharrar, A., et al. (2018). Investigation of water adsorption and thermodynamic properties of stevia powder. Journal of Food Measurement and Characterization, 12(4), 2615–2625. https://doi.org/10.1007/s11694-018-9879-0.
Iglesias, H., & Chirife, J. (1982). Handbook of food Isotherms: Water sorption parameters for food and food components. New York: Academic Press Inc.
Iuliano, L., Gonzalez, G., Casas, N., Moncayo, D., & Cote, S. (2019). Development of an organic quinoa bar with amaranth and chia. Food Science and Technology, 39, 218–224.https://doi.org/10.1590/fst.25517.
Jung, J., Wang, W. J., McGorrin, R. J., & Zhao, Y. Y. (2018). Moisture adsorption isotherm and storability of hazelnut inshells and kernels produced in Oregon, USA. Journal of Food Science, 83(2), 340–348.https://doi.org/10.1111/1750-3841.14025. Kaya, S., & Kahyaoglu, T. (2006). Influence of dehulling and roasting process on the
thermodynamics of moisture adsorption in sesame seed. Journal of Food Engineering, 76(2), 139–147.https://doi.org/10.1016/j.jfoodeng.2005.04.042.
Kaymak-Ertekin, F., & Sultanoglu, M. (2001). Moisture sorption isotherm characteristics of peppers. Journal of Food Engineering, 47(3), 225–231.https://doi.org/10.1016/ s0260-8774(00)00120-5.
Khawas, P., & Deka, S. C. (2017). Moisture sorption isotherm of underutilized culinary bananaflour and its antioxidant stability during storage. Journal of Food Processing and Preservation, 41(4), 1–10.https://doi.org/10.1111/jfpp.13087.
Koua, B. K., Koffi, P. M. E., Gbaha, P., & Toure, S. (2014). Thermodynamic analysis of sorption isotherms of cassava (Manihot esculenta). Journal of Food Science & Technology, 51(9), 1711–1723.https://doi.org/10.1007/s13197-012-0687-y. Kwon, H. C., Bae, H., Seo, H. G., & Han, S. G. (2019). Short communication: Chia seed
extract enhances physiochemical and antioxidant properties of yogurt. Journal of Dairy Science, 102(6), 4870–4876.https://doi.org/10.3168/jds.2018-16129. Lazouk, M. A., Savoire, R., Kaddour, A., Castello, J., Lanoiselle, J. L., Van Hecke, E., et al.
(2015). Oilseeds sorption isoterms, mechanical properties and pressing: Global view of water impact. Journal of Food Engineering, 153, 73–80.https://doi.org/10.1016/j. jfoodeng.2014.12.008.
Lomauro, C. J., Bakshi, A. S., & Labuza, T. P. (1985). Evaluation of food moisture sorption isotherm equations .1. Fruit, vegetable and meat-products. Lebensmittel-Wissenschaft & Technologie, 18(2), 111–117.
Mbarga, M. C. N., Nde, D. B., Mohagir, A., Kapseu, C., & Nkenge, G. E. (2017). Moisture sorption isotherms and properties of sorbed water of neem (Azadirichta indica A. Juss) kernels. Journal of Engineering Physics and Thermophysics, 90(1), 35–42.https://doi. org/10.1007/s10891-017-1536-7.
Moreira, R., Chenlo, F., Prieto, D. M., & Torres, M. D. (2012). Water adsorption isotherms of chia (salvia hispanica L.) seeds. Food and Bioprocess Technology, 5(3), 1077–1082. https://doi.org/10.1007/s11947-010-0400-y.
Munoz-Tebar, N., De la Vara, J. A., de Elguea-Culebras, G. O., Cano, E. L., Molina, A., Carmona, M., et al. (2019). Enrichment of sheep cheese with chia (Salvia hispanica L.) oil as a source of omega-3. LWT-Food Science and Technology, 108, 407–415.https:// doi.org/10.1016/j.lwt.2019.03.092.
Muñoz, L. A., Cobos, A., Diaz, O., & Aguilera, J. M. (2012). Chia seeds: Microstructure, mucilage extraction and hydration. Journal of Food Engineering, 108(1), 216–224. https://doi.org/10.1016/j.jfoodeng.2011.06.037.
Oswin, C. R. (1946). The kinetics of package life III. The isotherm Journal of Chemical Industry, 65, 419–421.
Panjagari, N. R., Singh, A. K., Ganguly, S., & Indumati, K. P. (2015). Beta-glucan rich compositeflour biscuits: Modelling of moisture sorption isotherms and determination of sorption heat. Journal of Food Science and Technology-Mysore, 52(9), 5497–5509. https://doi.org/10.1007/s13197-014-1658-2.
sigmoid moisture sorption isotherms. Journal of Food Process Engineering, 16(1), 21–37.https://doi.org/10.1111/j.1745-4530.1993.tb00160.x.
Samapundo, S., Devlieghere, F., De Meulenaer, B., Atukwase, A., Lamboni, Y., & Debevere, J. M. (2007). Sorption isotherms and isosteric heats of sorption of whole yellow dent corn. Journal of Food Engineering, 79(1), 168–175.https://doi.org/10. 1016/j.jfoodeng.2006.01.040.
Shanker, N., Kumar, M. M., Juvvi, P., & Debnath, S. (2019). Moisture sorption char-acteristics of ready-to-eat snack food enriched with purslane leaves. Journal of Food Science and Technology-Mysore, 56(4), 1918–1926. https://doi.org/10.1007/s13197-019-03657-1.
Singh, K. P., Mishra, H. N., & Saha, S. (2011). Sorption isotherms of barnyard millet grain and kernel. Food and Bioprocess Technology, 4(5), 788–796.https://doi.org/10.1007/ s11947-009-0195-x.
Smith, S. E., & Smith, S. E. (1947). The sorption of water vapor by high polymers. Journal of the American Chemical Society, 69(3), 646–651.https://doi.org/10.1021/ ja01195a053.
Soleimanifard, S., & Hamdami, N. (2018). Modelling of the sorption isotherms and de-termination of the isosteric heat of split pistachios, pistachio kernels and shells. Czech Journal of Food Sciences, 36(3), 268–275.https://doi.org/10.17221/460/2016-cjfs. Sormoli, M. E., & Langrish, T. A. G. (2015). Moisture sorption isotherms and net isosteric
heat of sorption for spray-dried pure orange juice powder. LWT-Food Science and Technology, 62(1), 875–882.https://doi.org/10.1016/j.lwt.2014.09.064. Taitano, L. Z., Singh, R. P., Lee, J. H., & Kong, F. (2012). Thermodynamic analysis of
moisture adsorption isotherms of raw and blanched almonds. Journal of Food Process Engineering, 35(6), 840–850.https://doi.org/10.1111/j.1745-4530.2010.00632.x. Tarigan, E., Prateepchaikul, G., Yamsaengsung, R., Sirichote, A., & Tekasakul, P. (2006).
Sorption isotherms of shelled and unshelled kernels of candle nuts. Journal of Food Engineering, 75(4), 447–452.https://doi.org/10.1016/j.jfoodeng.2005.04.030. Valdivia-López, M.Á., & Tecante, A. (2015). Chapter two - chia (Salvia hispanica): A
re-view of native mexican seed and its nutritional and functional properties. In J. Henry (Vol. Ed.), Advances in food and nutrition research: Vol. 75, (pp. 53–75). Academic Press.
Van den, B. (1981). Pre-eminent audiority on the Convention, in his text. 147.
Venkatachalam, M., & Sathe, S. K. (2006). Chemical composition of selected edible nut seeds. Journal of Agricultural and Food Chemistry, 54(13), 4705–4714.https://doi.org/ 10.1021/jf0606959.
Zhu, F., & Chan, C. (2018). Effect of chia seed on glycemic response, texture, and sensory properties of Chinese steamed bread. LWT-Food Science and Technology, 98, 77–84. https://doi.org/10.1016/j.lwt.2018.08.016.