Contents lists available atScienceDirect
Industrial Crops & Products
journal homepage:www.elsevier.com/locate/indcropPhytochemical composition and in vitro pharmacological investigations of
Neurada procumbens L. (Neuradaceae): A multidirectional approach for
industrial products
Umair Khurshid
a, Saeed Ahmad
a,**
, Hammad Saleem
b,c,*
, Hafiz Awais Nawaz
c, Gokhan Zengin
d,
Marcello Locatelli
e, Mohamad Fawzi Mahomoodally
f, Syafiq Asnawi Zainal Abidin
g,
Muhammad Imran Tousif
h, Nafees Ahemad
baDepartment of Pharmacy, The Islamia University of Bahawalpur, Pakistan
bSchool of Pharmacy, Monash University Malaysia, Jalan Lagoon Selatan, 47500 Bandar Sunway, Selangor Darul Ehsan, Malaysia cInstitute of Pharmaceutical Sciences (IPS), University of Veterinary & Animal Sciences (UVAS), Lahore, Pakistan
dDepartment of Biology, Faculty of Science, Selcuk University, Campus/Konya, Turkey eDepartment of Pharmacy, University ‘G. d’Annunzio” of Chieti-Pescara, 66100, Chieti, Italy fDepartment of Health Sciences, Faculty of Science, University of Mauritius, Mauritius
gLiquid Chromatography Mass Spectrometry (LCMS) Platform, Monash University, Jalan Lagoon Selatan, 47500 Bandar Sunway, Selangor Darul Ehsan, Malaysia hDepartment of Chemistry, Dera Ghazi Khan Campus, University of Education Lahore, 32200 Dera Ghazi Khan, Pakistan
A R T I C L E I N F O Keywords: UHPLC-MS Extraction Enzyme inhibition Antioxidant Bioactive compounds A B S T R A C T
The plants of genus Neurada have been utilized for medicinal purposes. This study probed into the biological propensities and endeavored to achieve the detailed phytochemical profile, via ultra-high performance liquid chromatography -UHPLC-MS analysis and high performance liquid chromatography photodiode array -HPLC-PDA of different polarity extracts (methanol, n-hexane, chloroform, and n-butanol) of Neurada procumbens L. The biological features were studied by determining the antioxidant potential via 2,2-Diphenyl-1-picrylhydrazyl -DPPH, 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid -ABTS, ferric reducing antioxidant power -FRAP, cupric reducing antioxidant power -CUPRAC, phoshomolybdenum and metal chelation assays and clinically significant major enzymes (cholinesterases, α-amylase, α-glucosidase and tyrosinase inhibition). Moreover, the correlation among the biological activities and total bioactive contents of the extracts were studied via multi-variate statistical analysis. The methanol and n-butanol extracts revealed the presence of total phenolic and flavonoid contents in highest concentrations which tend to correlate with their maximum anti-oxidant capacities for radical scavenging and reducing power assays. The n-hexane extract was most active in the phosphomo-lybdenum assays whereas the chloroform extracts showed the highest metal chelation potential. The UHPLC-MS phytochemical profiling of the methanolic extract (both positive and negative ionization mode) revealed the existence of eighteen phytochemicals representing four different classes (phenolics, flavonoids, sesquiterpenoids, and alkaloids). The n-butanol extract inhibited acetylcholinesterase (4.10 mg galantamine equivalent -GALAE/g) and tyrosinase (127.65 mg kojic acid equivalent -KAE/g) significantly as compared to the control. The n-hexane extract exhibited highest inhibition against butyrylcholinesterase (1.93 mg GALAE/g) and α-glucosidase (59.0 mmol acarbose equivalent -ACAE/g), while chloroform extract exhibited prominent inhibitory action in-imical to α-amylase (0.65 mmol ACAE/g). Overall, it was observed that FRAP and tyrosinase activities were the most contributive biological activities for the formation of first component with p values of 0.018 and 0.023, respectively, whereas, for the formation of second component, glucosidase activity was the most contributive assay with p value of 0.002. It can be concluded that the manifestation of dynamic phytochemicals with multi-pharmacological potential marks N. procumbens as a prospective origin of health-promoting molecules warrants further exploration as a unique therapeutic medicinal plant.
https://doi.org/10.1016/j.indcrop.2019.111861
Received 6 May 2019; Received in revised form 9 October 2019; Accepted 10 October 2019
⁎Corresponding author at: School of Pharmacy, Monash University Malaysia, Jalan Lagoon Selatan, 47500 Bandar Sunway, Selangor Darul Ehsan, Malaysia. ⁎⁎Corresponding author.
E-mail addresses:rsahmed_iub@yahoo.com(S. Ahmad),hammad.saleem@monash.edu(H. Saleem). Industrial Crops & Products 142 (2019) 111861
Available online 06 November 2019
0926-6690/ © 2019 Elsevier B.V. All rights reserved.
1. Introduction
Ethnobotanics and ethno-pharmacological considers are equally supported by the World Health Organization (WHO) as a profitable intend to valorize therapeutic plants as origin of products/molecules for both customary as well as present day medication (Baessa et al., 2019). Throughout the journey of man since old time as far back as Mesopo-tamia till now, reliance on plant to relieve sufferings was still con-sidered with much awareness. Tenants for the advocacy of traditional and/or indigenous medicinal system was based mainly on the as-sumption that these are safer but as the same time affordable and ac-cessible (Abioye et al., 2019). Therefore in present era, interest in the elucidation of biological potential and chemical constituent of plant is ever budding among the naturopathies (Mamadalieva et al., 2019).
Although growth in pharmaceutical field and dramatic expansion in the knowledge of organic chemistry deep embedded the preference for synthetic medications, yet, its negative side picture is more insecure in term of its related side effects and high-cost, which renewed the at-traction of investigators towards natural products from various sources for drug. WHO, declared that about 11% of the 252 drugs, are entirely from plant source while variety of synthetic drugs natural precursors (Cvetanović et al., 2018; Saleem et al., 2019a). Especially, with the world-wide spread of diseases such as diabetes mellitus (DM), cancer and Alzheimer’s disease (AD) are being focused for safe and consistent natural drugs over the synthetic and side effects (Mocan et al., 2016). The family Neuradaceae comprises of ten species with three genera and spreading from North Africa, the East Mediterranean, Sinai, Sudan, Ethiopia, and Saudi Arabia to the Indian desert (Boulos, 1999). The genus Neurada belongs to family Neuradaceae, is an monotypic genus and N. procumbens, a desert plant commonly known as ”Als'dan”, thought about edible by Bedouin and has been utilized customarily as a therapeutic herbal plant for treating diarrhea and dysentery and also utilized as a stimulant to boost heart and respiration (James, 2016; Marzouk et al., 2014). Previously, this plant has been put into in-vestigations for its anti-viral (Shahzad et al., 2019) and anticancer (Ali et al., 2014) activities. After oral administration of aqueous extract of this plant, the blood pressure of anaesthetized normotensive rats has been reported to be elevated (Chen et al., 2004). Furthermore, isolation of some flavonoids named taxifolin, taxifolin 3-O-a-rhamnopyranoside, vitexin 2’’-O-a-rhamnopyranoside,vitexin, 2’’-O-a-rhamnopyranoside, orientin, and isoorientin 2’’-O-a-rhamnopyranoside were also reported from aerial parts of N. procumbens (Marzouk et al., 2014). In spite of the wealth of research concerning the therapeutic properties of this genus, data regarding N. procumbens antioxidant potential and their capability to inhibit key enzymes of clinical relevance is restricted. Regarding background information, this research explored the multidirectional biological and chemical aspects of this plant species including, in vitro enzyme inhibition capabilities of N. procumbens methanol, chloroform,
n-hexane and n-butanol extracts on compounds related with
neurode-generative infirmities (AChE and BChE), diabetes (glucosidase and α-amylase) and skin hyperpigmentation (tyrosinase). Similarly, samples were evaluated for radical scavenging activity (RSA) on 2, 2-diphenyl-1-picrylhydrazyl (DPPH) and 2, 2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic corrosive) (ABTS) radicals and for metal chelating action on copper and iron particles. Moreover, samples were explored for the assurance of their all-out substance in various phenolic and flavonoid gatherings, by spectrophotometric technique. Likewise, the methanol extract was also evaluated for secondary metabolites composition via UHPLC-MS analysis. Moreover, the correlation among the total bioac-tive contents and the biological assays of the extracts were also studied
via multivariate statistical analysis and the determination of the
prin-cipal component analysis (PCA).
2. Materials and methods
2.1. Plant material and extraction
The collection of fully matured plant was done in May, 2016 from Cholistan desert, district Bahawalpur, Pakistan. The identified plant was authenticated and given voucher number “452/LS” by taxonomist from life science department of The Islamia University of Bahawalpur, Pakistan. The air dried plant was coarsely powdered and extracted with 80% methanol. The extract was filtered and concentrated using pres-sure reduced rotary evaporator at 40 °C to obtain a viscous semi-solid material. Methanolic extract was further fractioned (liquid-liquid) with different solvents in ascending order of polarity i.e., n-hexane, chloro-form and n-butanol using separating funnel. All the extracts were dried using Roravapor.
2.2. Total phenolic and flavonoid content
Total phenolic and flavonoid contents were evaluated in crude ex-tract and its fractions as previously elaborated (Grochowski et al., 2017) by means of a well-known procedure as follow. 1 mL of diluted (1:9, v/v) Folin–Ciocalteu reagent (1 mL) was added to 0.25 mL of the sample solution (1 mg/mL). After 3 min, 0.75 mL of a Na2CO3solution
(1%) was added and the mixture was left at room temperature for 2 h in an incubator. Absorbance was measured at 760 nm. Total flavonoid contents were estimated by adding 1 mL of aluminium chloride (2%) in methanol to 1 mL of sample solution (1 mg/mL). The mixture was in-cubated for 10 min at room temperature and the absorbance reading was taken at 415 nm. The outcomes of total phenolic constituents were reported as equivalents of gallic acid (mg GAE/g extract) while the results of total flavonoid constituents were recorded as equivalents of quercetin (mg QE/g extract).
2.3. Antioxidant activities
The estimation of free radical scavenging activity by two methods (DPPH, ABTS), reducing power assay (FRAP, CUPRAC) followed by phosphomolybdenum (total antioxidant capacity) and metal chelating activity (ferrous ion chelation) was done by utilizing techniques nar-rated by (Grochowski et al., 2017).
2.3.1. DPPH free radical scavenging activity
For this antioxidant activity, the DPPH solution (0.267 mM 4 mL, 0.004% methanol solution) was mixed with 1 mL sample solution fol-lowed by 30 min of incubation and ultimately absorbance was mea-sured at 517 nm. Unit of measurement was in mg of trolox equivalents/ g of dry extract (TEs/g extract).
2.3.2. ABTS radical cation scavenging activity
The reaction of 7 mM ABTS solution with 2.45 mM potassium per-sulfate led to the formation of ABTS+radical cation. 1 mL of test
so-lution was mixed with 2 mL of ABTS soso-lution followed by measurement of absorbance at 734 nm after 30 min. The articulation of results was done in mg of trolox equivalents/g of dry extract (TEs/g extract).
2.3.3. Phosphomolybdenum method
In this assay, 3 mL of reagent solution which comprised of (0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium mo-lybdate) was mixed in 0.3 mL of sample solution and the resultant ab-sorbance was measured at 695 nm after 90 min of incubation. Measuring units were in mmol of trolox equivalents/g of dry extract (TEs/g extract).
2.3.4. Cupric ion reducing (CUPRAC) method
This method was executed by taking 0.5 mL of the sample solution and mixing it with [CuCl2 (1 mL, 10 mM), neocuproine (1 mL, 7.5 mM),
NH4Ac buffer (1 mL, 1 M, pH 7.0)] and after 30 min of incubation, the
absorbance was estimated at 450 nm. The measurement of results was done in mg of trolox equivalents/g of dry extract (TEs/g extract).
2.3.5. Ferric reducing antioxidant power (FRAP) method
This method involved the addition of 0.1 mL of sample solution to 2 mL reagent in acetate buffer (0.3 M, pH 3.6), 2, 4, 6-tris (2-pyridyl)-s-triazine (TPTZ) (10 mM) in 40 mM HCl and ferric chloride (20 mM) in (v/v/v) ratio of 10:1:1 leading to the measurement of absorbance at 593 nm after an incubation period of 30 min. The articulation of results was done in mg of trolox equivalents/g of dry extract (TEs/g extract).
2.3.6. Metal chelating activity on ferrous ions
This method was effectuated by adding 0.05 mL of a solution of FeCl2(2 mM) to 2.0 mL of sample solution. Later 0.2 mL of ferrozine
(5 mM) was utilized in order to instigate the reaction followed by measurement of absorbance at 562 nm after 10 min of incubation and the expression of results was done in milligrams of ethylenediamine-tetraacetic acid equivalents per gram of dry extract (EDTAEs/g extract).
2.4. Enzyme inhibition assays
The potential inhibitory capacity of all the concentrates against the enzymes including acetylcholinesterase (AChE), butyrylcholinesterase (BChE), tyrosinase, α-amylase and α-glucosidase were explored making use of standard bio-assays (Grochowski et al., 2017).
2.4.1. Cholinesterase
In either of the enzyme substrates i.e. [acetylthiocholine iodide (15 mM ATCI) or butyrylthiocholine chloride (1.5 mM BTCl, 25 μL)], 50 μL sample solution was added along with DTNB (3 mM 125 μL) and 25 μL enzyme solution (0.265 u/mL AChE or 0.026 u/mL BChE) in Tris-HCl buffer (pH 8.0). Reaction was allowed to be completed in 15 min of incubation and absorbance was measured at 405 nm. The results were articulated as mg of galantamine equivalent/g of dry extract (GALAEs/g extract).
2.4.2. α-Amylase
25 μL of sample solution was mixed with 50 μL of the α-amylase solution (10 u/mL) already dissolved in phosphate buffer (pH 6.9 with 6 mM sodium chloride). The above reaction mixture was added to 50 μL of the starch solution (0.05%) and the reaction was made to stop by adding 25 μL of HCl (1 M). Finally, 100 μL of the iodine-potassium io-dide solution was incorporated into the above mixture. The absorbance reading was logged after an incubation period of 10 min. Millimoles of acarbose equivalents per gram of dry extract (ACAEs/g extract) were the units in which measurements were made.
2.4.3. α-Glucosidase
This assay comprehended with the addition of 50 μL of sample so-lution in equal concentrations 50 μL of glutathione (0.5 mg/mL) and α-glucosidase solution (0.2 u/mL) in phosphate buffer (pH 6.8) and PNPG (10 mM). The reaction was stopped after 15 min with the addition of 50 μL of sodium carbonate solution (0.2 M) and ultimately absorbance was measured at 400 nm. The expression of results was done in milli-moles of acarbose equivalents per gram of dry extract (ACAEs/g ex-tract).
2.4.4. Tyrosinase
40 μL of tyrosinase solution (200 u/mL) was mixed with 25 μL of the sample solution. To the above mixture, phosphate buffer (40 mM, 100 μL, pH 6.8) was added in a 96-well microplate. Temperature was retained at 25 °C during the incubation period of 15 min. Addition of l-DOPA (10 mM, 40 μL) led to the actuation of reaction. The incubation period was set for 10 min at room temperature and the absorbance was estimated at 492 nm. Milligrams of kojic acid equivalents per gram of
dry extract (KAE/g extract) were the units in which results were ex-pressed.
2.5. Phytochemical composition
The profiling of secondary metabolites in the methanolic extract was estimated by employing the standard RP-UHPLC-MS analysis method as described previously (Saleem et al., 2019b). Agilent 1290 Infinity UHPLC system paired to Agilent 6520 Accurate-Mass Q-TOF mass spectrometer interfaced with a dual ESI source was incorporated with the column Agilent Zorbax Eclipse XDB-C18 (2.1 x 150 mm, 3.5 μm). 4 °C temperature was set and maintained for auto-sampler and 25 °C for column respectively. 0.1% formic acid solution in water was selected as mobile phase (A), while acetonitrile and 0.1% formic acid solution as mobile phase (B) with a flow rate of 0.5 mL/min. 1.0 μL of plant extract dissolved in methanol (HPLC grade) was inserted for 25 min followed by 5 min of post-run time. For nebulization, nitrogen gas was availed with a flow rate of 25 L/hour and for drying purpose its flow rate was adjusted to 600 L/hour with temperature being set at 350 °C. The capillary voltage and fragmentation voltage were main-tained at 3500 V and 125 V respectively (Saleem et al., 2019b). Simi-larly, polyphenolic composition in all the extracts were also determined using HPLC analyses as previously reported (Locatelli et al., 2017).
2.6. Statistical analysis
Final outcomes were taken as a result of mean of three comparable trials and represented as average ± SD of values. One-way ANOVA and SPSS v. 17.0 programs were utilized for result investigation. The p-value of p < 0.05 was assessed to be statistically considerable 3. Result and discussion
3.1. Phytochemical composition
Phenolics and flavonoids are considered as active metabolites, which are produced as a response of environmental stress, and have acquired much recognition among the circle of researchers for possible pharmacological applications (Rahman et al., 2018). In this current research work, various extracts of N. procumbens were assessed for the presence of phenolic and flavonoid contents and the results are pre-sented inTable 1. The n-butanol extract of N. procumbens attributed higher value amount of total phenolic i.e.47.29 ± 0.82 mg GAE/g extract which is comparable with that of NP-M which showed 43.47 ± 1.55 mg GAE/g extract for phenolic content, followed by NP-H (36.57 ± 0.82 mg GAE/g extract) NP-C (17.71 ± 0.10 mg GAE/g extract). Likewise, for the total flavonoid content of the almost same sequence was observed with NP-B with high content 35.16 ± 0.97 mg QE/g extract while NP-C recorded for least amount of flavonoid con-stituent (12.93 ± 0.18 mg QE/g extract) (Table 1). Our results are Table 1
Total phenolic and flavonoid contents of N. procumbens extracts*. Plant
extract Total phenolic content (mgGAE/g extract) Total flavonoid content (mg RE/g extract)
NP-M 43.47 ± 1.55b 18.88 ± 0.10b
NP-C 17.71 ± 0.10d 12.93 ± 0.18c
NP-H 36.57 ± 0.82c 18.76 ± 0.25b
NP-B 47.29 ± 0.82a 35.16 ± 0.97a
Data from three repetitions with mean ± standard deviation. GAE: gallic acid equivalent; RE: rutin equivalent. Different letters indicate significant differ-ences in the tested extracts (p < 0.05).
* NP-M: N. procumbens methanol extract; NP-C: N. procumbens chloroform extract; NP-H: N. procumbens n-hexane extract; NP-B: N. procumbens n-butanol extract.
supported by the previous literature reporting the presence of flavonoid constituent in N. procumbens (Marzouk et al., 2014). Generally, the methanol extract is able to recovery the highest amount of phenolic compounds related to its polarity (equal to 33), while the other solvents herein tested show different values (n-hexane = 2.0; chloroform = 4.8;
n-butanol = 18). Follow this polarity order, butanol and methanol
ex-tracts show similar (and higher) contents, while n-hexane and chloro-form the lower amount. In the same way, also the choice of the solvent polarity could influence the selective extraction of a specific compound. Liquid chromatography mass spectrometry investigation of the methanolic extract of N. procumbens was executed for knowledge in-sight about the probable phytochemicals it possess. The standard chromatogram with mass spectrometric location both in negative and positive particle modes displayed very unpredictable peaks as appeared inFig. 1. The UHPLC-MS profiling of N. procumbens methanol extract has exposed the occurrence of a total of 18 different secondary meta-bolites; 14 in negative ionization mode (Table 2) and 4 in positive io-nization mode (Table 3). Compound identification was made possible through a database i.e., Metlin. Majority of the compounds belongs to usual flavonoids including ferulic acid 4-sulfate, ferulic acid, (-)-epi-gallocatechin 3-gallate 7-glucoside 4"-glucuronide, tricin 7-[feruloyl-(- > 2)-glucuronyl-(1- > 2)-glucuronide], 6-hydroxyluteolin 3'-methyl ether 7-[6”-(3-hydroxy-3methylglutaryl)glucoside], kaempferol 3-(6”-acetylglucoside)-7-glucoside, isoscutellarein 4'-methyl ether 8-(2”-sul-fatoglucuronide), Phenol (10-Acetoxyligustroside, p-Salicylic acid),
alkaloids (3-O-acetylhamayne, angustine) and terpenoid (veranisatin C, ganoderic acid H, bryononic acid, emmotin A) derivatives. Moreover, one lignin amide (grossamide), carbohydrate (glucoheptonic acid) and chalcone (anthyllisone) were also identified. Marzouk et al, 2013, has reported the isolation of flavonoids including taxifolin, taxifolin 3-O-β-rhamnopyranoside, vitexin, vitexin 2'-O-α-3-O-β-rhamnopyranoside, orientin 2'-O-α- rhamnopyranoside and isoorientin 2'-O-α-rhamnopyranoside, p-hydroxybenzoic acid from N. procumbens (Marzouk et al., 2014). This can be considered as the first detailed study on the UHPLC-MS phyto-chemical investigation of N. procumbens methanol extract.
Further analysis of the plant fractions was accomplished to re-cognize the polyphenolic composition utilizing the Reversed-Phase High-Performance Liquid Chromatography analytical and quantifica-tion techniques. Under analytical condiquantifica-tions used, the chloroform ex-tract of N. procumbens revealed the presence of 3-OH benzoic acid (1.96 ± 0.24 μg/g) and t-ferullic acid (0.28 ± 0.0 μg/g), followed by the n-butanol extract which contains the syringic acid (3.63 ± 0.44 μg/g) and naringin (0.47 ± 0.05 μg/g). However, all the 22 tested phenolic compounds were not detected in methanol and n-hexane extracts.
3.2. Antioxidant assays
Free radicals are responsible for gene mutation and molecular transformation controlled by natural anti-oxidant defense system in Fig. 1. Total ion chromatogram of methanol extract of N.
procumbens.
A (-ve ionization mode), 1: Glucoheptonic acid; 2: 10-Acetoxyligustroside; 3: p-Salicylic acid; 4: Ferulic acid 4-sul-fate; 5: Veranisatin C; 6: Ferulic acid; 7: 3-O-Acetylhamayne; 8: (-)-Epigallocatechin 3-gallate 7-glucoside 4"-glucuronide; 9: Tricin 7-[feruloyl-(- > 2)-glucuronyl-(1- > 2)-glucuronide]; 10: 6-Hydroxyluteolin 3'-methyl ether 7-[6”-(3-hydroxy-3me-thylglutaryl)glucoside]; 11: Kaempferol 3-(6”-acetylgluco-side)-7-glucoside; 12: Isoscutellarein 4'-methyl ether 8-(2”-sulfatoglucuronide); 13: Grossamide; 14: Ganoderic acid H. B (+ve ionization mode) 1: Angustine; 2: Anthyllisone; 3: Emmotin A; 4: Bryononic acid.
Table 2
UHPLC-MS analysis of methanol extract of N. procumbens (negative ionization mode).
S.no RT (min) B. peak(m/z) Comp. identified Mol. formula Compound class Mol. mass
1 0.61 225.0614 Glucoheptonic acid C7H14O8 Carbohydrate 226.0689
2 0.91 581.1859 10-Acetoxyligustroside C27H34O14 Phenol 582.1949
3 6.80 137.0246 p-Salicylic acid C7H6O3 Phenol 138.0317
4 7.88 273.0087 Ferulic acid 4-sulfate C10H10O7S Flavonoid 274.0147
5 8.43 371.0987 Veranisatin C C16H20O10 Sesquiterpenoid 372.1056
6 9.11 193.0511 Ferulic acid C10H10O4 Flavonoid 194.0579
7 9.24 328.1207 3-O-Acetylhamayne C18H19N O5 Alkaloid 329.1263
8 9.69 795.1629 (-)-Epigallocatechin 3-gallate 7-glucoside 4"-glucuronide C34H36O22 Flavonoid 796.1704 9 9.98 857.1792 Tricin 7-[feruloyl-(- > 2)-glucuronyl-(1- > 2)-glucuronide] C39H38O22 Flavonoid 858.1863 10 9.98 621.1475 6-Hydroxyluteolin 3'-methyl ether 7-[6”-(3-hydroxy-3methylglutaryl)glucoside] C28H30O16 Flavonoid 622.1549
11 10.0 651.1572 Kaempferol 3-(6”-acetylglucoside)-7-glucoside C29H32O17 Flavonoid 652.1649
12 10.6 555.0462 Isoscutellarein 4'-methyl ether 8-(2”-sulfatoglucuronide) C22H20O15S Flavonoid 556.0533
13 12.0 623.2385 Grossamide C36H36N2O8 Lignan amide 624.2463
14 16.6 571.2905 Ganoderic acid H C32H44O9 Triterpenoid 572.2978
living organisms. Several herbs, spices and plant extracts and products are used as anti-oxidant in food. So there was need to investigate fur-ther natural sources for it (Dai and Mumper, 2010). The DPPH is an organic compound used to measure antioxidant capacity of plant ex-tract. It is reduced by antioxidant compounds in plant extracts from purple radical to picrylhydrazine which is a pale yellow radical (Aktumsek et al., 2013). In present investigations, a variety of anti-oxidant assays (DPPH, ABTS, FRAP, CUPRAC, phosphomolybdenum and metal chelating assays) were conducted with the aim to get the antioxidant potential of N. procumbens, and the results are presented in Table 4.
The NP-M of N. procumbens showed highest activity in the DPPH and ABTS assays (DPPH: 83.93 ± 0.57 and ABTS: 170.53 ± 0.68 mg TE/g extract, respectively), NP-H and NP-B shared almost same contributions towards both assays. While least activity was studied for NP-C (DPPH: 6.50 ± 0.78 and ABTS: 13.85 ± 0.70 mg TE/g extract, respectively). CUPRAC and FRAP activities applied for estimation of reducing potential of plant extract by conversion of Cu+2and Fe+3ion into Cu+1
and Fe+2ions by respectively (Saleem et al., 2019c). The FRAP essay
NP-B exhibited highest reducing potential i.e.107.63 ± 0.93 mg TE/g extract, while NP-C (40.00 ± 0.66 mg TE/g extract) was considered as least eloquent in this assay. In CUPRAC assays, NP-B and NP-M have showed almost same reducing potential 183.70 ± 3.08 and 182.10 ± 2.48 mg TE/g extract respectively. Similarly, the NP-M and NP-B shared more persuasive results in free radical scavenging (DPPH and ABTS) and reducing power (FRAP and CUPRAC) assay. In addition, results of the bioactive content also accredited the increased amount of phenolic and flavonoid contents in above two extracts, which tend to corroborate with the reported data, and revealed concurrence between high amount of bioactive content with free radical scavenging and re-ducing power assays (Khan et al., 2019).
In general, the highest radical scavenging antioxidant potential of the methanol extract of N. procumbens is also supported by previous studies by Marzouk et al, 2013, reporing the antioxidant properties and isolation of flavonoids including taxifolin, taxifolin 3-O-β-rhamnopyr-anoside, vitexin, vitexin rhamnopyr3-O-β-rhamnopyr-anoside, orientin 2'-O-α-rhamnopyranoside and isoorientin 2'-O-α-2'-O-α-rhamnopyranoside, p-hydro-xybenzoic acid from N. procumbens (Marzouk et al., 2014). Similarly, the methanolic extracts of N. procumbens has been also reported for DPPH inhibition activity(% inhibition at 50 PPM: 93.5%; EC50:15.9 ± 1.2) (Moustafa et al., 2014).
Total antioxidant capacity measured through phosphomolybdenum
exhibited the following sequence NP-H > NP-M > NP-C > NP-B (2.42 ± 0.14 > 2.37 ± 0.22 > 2.13 ± 0.07 > 1.19 ± 0.02 mg TE/g extract).The metal chelating activity of N. procumbens as is pre-sented inTable 4, where NP-C with least phenolic and flavonoid con-tent declared as strong activity (0.65 ± 0.01 mg EDTAE/g extract) while for other extracts activity was ranging from 16.13 ± 1.20 to 21.63 ± 2.86 (mg EDTAE/g extract). Valuable phosphomolybdenum and the metal chelating power were measured for NP-H and NP-C re-spectively, revealed no linear correlation with bioactive constituents, as literature supported this assumption that it might be possible that some phenolic compounds along with non-phenolic constituent responsible for effective potential for these less polar extract with less bioactive content (Saleem et al., 2018;Zengin et al., 2019).
3.3. Enzyme inhibition potential
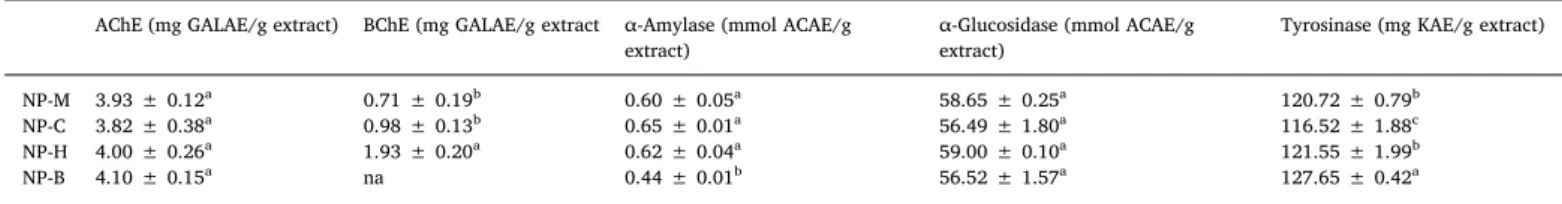
The applications of natural enzyme inhibitions ensuring more pro-mising results as a significant therapeutic approach to get rid of Alzheimer’s disease (AD), diabetes mellitus, and hyperpigmentation amongst others. Therefore, the search for natural key inhibitors for cholinesterase, amylase, glucosidase, and tyrosinase are currently under scrutiny (Zengin et al., 2018). In recent work, the enzyme inhibition study of N. procumbens extracts were evaluated against cholinesterases (AChE and BChE), α-amylase, α-glucosidase and tyrosinase leading to the presentation of results inTable 5.
Neurodegenerative disorders as a major health alarm is of particular concern in many countries where elder population faced neuronal loss and abnormal emotional changes. For treatment of such neurodegen-erative disorders hampering the hydrolysis of AChE and BChE is con-sidered as helping tool (Abirami et al., 2014;Rajakumar et al., 2017). All the extracts of N. procumbens exhibited excellent inhibition potential in acetyl cholinesterases enzymes inhibition assays with the following order (NP-B > NP- > NP-M > NP-C) (4.10 ± 0.15 > 4.10 ± 0.15 > 3.93 ± 0.12 > 3.82 ± 0.38 mg GALAE/g extract).While in BChE assay, NP-H was found more active (1.93 ± 0.20 mg GALAE/g extract) and NP-B was found inactive. Present investigation revealed that NP-B contributed successfully maximum potential, indicating the positive relationship of phenolic content and acetyl cholinesterases enzymes inhibition assays (Ajiboye et al., 2018;Aktumsek et al., 2013). Diabetes mellitus (DM), presently considered as a global epidemic, stand at the eight position in terms of global mortality. Estimated survey in 2011 revealed that about 1.4 million people’s life become Table 3
UHPLC-MS analysis of methanol extract of N. procumbens (positive ionization mode).
S.no RT (min) B. peak(m/z) Comp. identified Mol. formula Compound class Mol. mass
1 10.37 314.1289 Angustine C20H15N3O Alkaloid 313.1216
2 12.78 391.1827 Anthyllisone C25H26O4 Chalcone 390.1816
3 16.93 279.1586 Emmotin A C16H22O4 Sesquiterpenoid 278.1513
4 18.45 455.3497 Bryononic acid C30H46O3 Triterpenoid 454.3420
RT: retention time; B. peak: base peak. Table 4
Antioxidant properties of N. procumbens extracts*. Plant
extracts DPPH (mg TE/gextract) ABTS (mg TE/gextract) FRAP (mg TE/gextract) CUPRAC (mg TE/gextract) Phosphomolybdenum (mmol TE/g extract) Metal chelating (mgEDTAE/g extract) NP-M 83.93 ± 0.57a 170.53 ± 0.68a 107.63 ± 0.93b 182.10 ± 2.48a 2.37 ± 0.22a 16.13 ± 1.20b
NP-C 18.09 ± 1.53d 26.71 ± 2.04c 40.00 ± 0.66d 79.58 ± 4.42c 2.13 ± 0.07a 21.63 ± 2.86a
NP-H 64.68 ± 1.63c 146.64 ± 4.19b 96.36 ± 1.64c 154.73 ± 2.12b 2.42 ± 0.14a 19.79 ± 1.54c
NP-B 68.88 ± 0.84b 146.84 ± 1.87b 142.75 ± 1.81a 183.70 ± 3.08a 1.19 ± 0.02b 0.65 ± 0.01d
* NP-M: N. procumbens methanol extract; NP-C: N. procumbens chloroform extract; NP-H: N. procumbens n-hexane extract; NP-B: N. procumbens n-butanol extract. DPPH: 2-diphenyl-1-picrylhydrazyl; ABTS: 2, 2’-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid; FRAP: ferric reducing antioxidant power; CUPRAC: cupric re-ducing antioxidant power; TE: trolox equivalent; EDTAE: EDTA equivalent. All values expressed are means ± S.D. of three parallel measurements. Different letters indicate significant differences in the tested extracts (p < 0.05).
dark because of this viral disease. Inhibitors of α-amylase and α-glu-cosidase are becoming the target enzymes for possible drug develop-ment. These enzymes play key role in hydrolyzing process of long chain carbohydrates and disaccharides into monosaccharides, respectively (Jhong et al., 2015). The anti-α-amylase assay showed that NP-C, NP-H, and NP-M have exhibited medium potential (0.65 ± 0.01, 0.62 ± 0.04, 0.60 ± 0.05 mmol ACAE/g), while least potential re-corded for NP-B (0.44 ± 0.01 mmol ACAE/g). In α-glucosidase assay similar action was studied for all extracts towards α-glucosidase en-zyme (Table 5).
Tyrosinase is a polyphenolic oxidase that leads to the conversion of
L-tyrosine to dopachrome, which is associated with the production of
melanin which help in protection from ultraviolet rays. However, its over production culminate to skin problems and neurodegenerative disorders (Zengin et al., 2016,2015). Tyrosinase inhibition activities depicted by N. procumbens extract are represented inTable 4and the NP-B extract showed the maximum tyrosinase inhibition (127.65 ± 0.42 mg KAE/g), while tyrosinase inhibitions for remaining extracts were in following decreasing order NP-H > NP-M > NP-C. In
the present study, NP-B which has higher phenolic content and also exhibited good tyrosinase inhibition, which correlates with previous results (Zengin et al., 2015).
3.4. Statistical evaluation
As presented inFig. 2, we determined two primary constituents as 95.2% of total variance in PCA. The concerning variables related to antioxidant specifications (except metal chelation and phosphomo-lybdenum) and enzymes (including AChE and tyrosinase) firmly furn-ished the generation of axis 1 (65.6%), while the variables for BChE, amylase and glucosidase enzyme inhibition as well as metal chelation and phosphomolybdenum activities stoutly conform to the axis 2 (29.6%). Pearson correlation suggests a firm positive correlation be-tween total bioactive contents (r = 0.99-0.5) and DPPH, ABTS, FRAP, and CUPRAC, which are in accordance to previous studies also showing strong positive correlation between phenolics, radical scavenging and reducing potential assays (Llorent-Martínez et al., 2017). A negative relationship was seen between total bioactive contents (r = -0.38-0.98), Table 5
Enzyme inhibition activities of N. procumbens extracts*.
AChE (mg GALAE/g extract) BChE (mg GALAE/g extract α-Amylase (mmol ACAE/g
extract) α-Glucosidase (mmol ACAE/gextract) Tyrosinase (mg KAE/g extract)
NP-M 3.93 ± 0.12a 0.71 ± 0.19b 0.60 ± 0.05a 58.65 ± 0.25a 120.72 ± 0.79b
NP-C 3.82 ± 0.38a 0.98 ± 0.13b 0.65 ± 0.01a 56.49 ± 1.80a 116.52 ± 1.88c
NP-H 4.00 ± 0.26a 1.93 ± 0.20a 0.62 ± 0.04a 59.00 ± 0.10a 121.55 ± 1.99b
NP-B 4.10 ± 0.15a na 0.44 ± 0.01b 56.52 ± 1.57a 127.65 ± 0.42a
* Values expressed are means ± S.D. of three parallel measurements. NP-M: N. procumbens methanol extract; NP-C: N. procumbens chloroform extract; NP-H: N.
procumbens n-hexane extract; NP-B: N. procumbens n-butanol extract. AChE: acetylcholinesterase; BChE: butyrylcholinesterase; GALAE: galantamine equivalent; KAE:
kojic acid equivalent; ACAE: acarbose equivalent; na: not active. Different letters indicate significant differences in the tested extracts (p < 0.05).
Fig. 2. Statistical evaluations, A: Correlation coefficients between total bioactive compounds and biological activities ((r), p < 0.05); B: Eigen values and percentage of variability expressed by the factors; C: Distribution of the tested extracts on the factorial plan and representation of biological activities on the correlation circle based on principal component analysis -PCA; D: Cluster dendrogram.
*TPC: total phenolic content; TFC: total flavonoid content; DPPH: 2-diphenyl-1-picrylhydrazyl; ABTS: 2, 2’-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid; FRAP: ferric reducing antioxidant power; CUPRAC: cupric reducing antioxidant power; AChE: acetylcholinesterase; BChE: butyrylcholinesterase.
metal chelating and phosphomolybdenum activities which may be justified with the antagonistic or synergetic effect of phytochemicals or presence of some non-phenolic chelators (Marini et al., 2018). Simi-larly, a strong positive relationship was recorded among bioactive contents and AChE and tyrosinase enzymes, whereas a negative asso-ciation was present amongst BChE and amylase enzymes with bioactive contents. For glucosidase, a positive relationship was seen with phe-nolic contents (r = 0.31), while a moderated negative relationship was observed for flavonoid contents (r = -0.31). Overall, it was observed that FRAP and tyrosinase activities were the most contributive biolo-gical activities for the formation of first component with p values of 0.018 and 0.023, respectively, whereas, for the formation of second component, glucosidase activity was the most contributive assay with p value of 0,002.
4. Conclusion
The present study presented have comparison of different solvent extracts from N. procumbens in terms of their biological properties and chemical characterization. The methanol and n-butanol extracts re-vealed strong antioxidant propensity with high level of phenolic and flavonoid concentrations. To provide more information regarding che-mical profile, UHPLC-MS analysis of methanol extract was performed and phenolic, flavonoids, sesquiterpenoids and alkaloids were noticed as main groups. Regarding inhibitory effects against key enzymes, all tested extracts exhibited different ability on these enzymes. Based on our findings, N. procumbens could be considered as highly active bio-logical agents including antioxidant, antidiabetic and anti-Alzheimer. However, further studies regarding its toxicity and bioavailability are need to provide full picture.
References
Abioye, A.V., Mohammed, Z., Ahmed, A., Ayeni, A.E., 2019. Evaluation of the analgesic potential of Basella alba (L.) leaves (Basellaceae). Trop. J. Nat. Prod. Res. 3, 22–25.
Abirami, A., Nagarani, G., Siddhuraju, P., 2014. In vitro antioxidant, anti-diabetic, cho-linesterase and tyrosinase inhibitory potential of fresh juice from Citrus hystrix and C.
Maxima fruits. Food Sci. Hum. Wellness. 3, 16–25.
Ajiboye, B.O., Ojo, O.A., Okesola, M.A., Akinyemi, A.J., Talabi, J.Y., Idowu, O.T., Fadaka, A.O., Boligon, A.A., Anraku de Campos, M.M., 2018. In vitro antioxidant activities and inhibitory effects of phenolic extract of Senecio biafrae (Oliv and Hiern) against key enzymes linked with type II diabetes mellitus and Alzheimer’s disease. Food Sci. Nutr. 6, 1803–1810.
Aktumsek, A., Zengin, G., Guler, G.O., Cakmak, Y.S., Duran, A., 2013. Antioxidant po-tentials and anticholinesterase activities of methanolic and aqueous extracts of three endemic Centaurea L. Species. Food Chem. Toxicol. 55, 290–296.
Ali, M., Abul Farah, M., Al-Hemaid, F., Abou-Tarboush, F., 2014. In vitro cytotoxicity screening of wild plant extracts from Saudi Arabia on human breast adenocarcinoma cells. Genet. Mol. Res. 13, 3981–3990.
Baessa, M., Rodrigues, M., Pereira, C., Santos, T., da Rosa Neng, N., Nogueira, J., Barreira, L., Varela, J., Ahmed, H., Asif, S., 2019. A comparative study of the in vitro enzyme inhibitory and antioxidant activities of Butea monosperma (Lam.) Taub. And Sesbania
grandiflora (L.) Poiret from Pakistan: new sources of natural products for public
health problems. S. Afr. J. Bot. 120, 146–156.
Boulos, L., 1999. Flora of Egypt, vol. 1. Al Hadara Publishing, Cairo, pp. 417.
Chen, H., Islam, M., Radhakrishnan, R., Wahab, S., Naji, M., 2004. Influence of aqueous extract from Neurada procumbens L. on blood pressure of rats. J. Ethnopharmacol. 90, 191–194.
Cvetanović, A., Zeković, Z., Švarc‐Gajić, J., Razić, S., Damjanović, A., Zengin, G., Delerue‐Matos, C., Moreira, M., 2018. A new source for developing multi‐functional products: biological and chemical perspectives on subcritical water extracts of
Sambucus ebulus L. J. Chem. Technol. Biotechnol. 93, 1097–1104.
Dai, J., Mumper, R.J., 2010. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules 15, 7313–7352.
Grochowski, D.M., Uysal, S., Aktumsek, A., Granica, S., Zengin, G., Ceylan, R., Locatelli,
M., Tomczyk, M., 2017. In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochem. Lett. 20, 365–372.
James, P.M., 2016. Flora of Eastern Saudi Arabia. Taylor Francis.
Jhong, C.H., Riyaphan, J., Lin, S.H., Chia, Y.C., Weng, C.F., 2015. Screening alpha‐glu-cosidase and alpha‐amylase inhibitors from natural compounds by molecular docking in silico. Biofactors 41, 242–251.
Khan, S., Nazir, M., Raiz, N., Saleem, M., Zengin, G., Fazal, G., Saleem, H., Mukhtar, M., Tousif, M.I., Tareen, R.B., 2019. Phytochemical profiling, in vitro biological prop-erties and in silico studies on Caragana ambigua stocks (Fabaceae): a comprehensive approach. Ind. Crop Prod. 131, 117–124.
Llorent-Martínez, E., Ortega-Barrales, P., Zengin, G., Mocan, A., Simirgiotis, M., Ceylan, R., Uysal, S., Aktumsek, A., 2017. Evaluation of antioxidant potential, enzyme in-hibition activity and phenolic profile of Lathyrus cicera and Lathyrus digitatus: po-tential sources of bioactive compounds for the food industry. Food Chem. Toxicol. 107, 609–619.
Locatelli, M., Zengin, G., Uysal, A., Carradori, S., De Luca, E., Bellagamba, G., Aktumsek, A., Lazarova, I., 2017. Multicomponent pattern and biological activities of seven
Asphodeline taxa: potential sources of natural-functional ingredients for bioactive
formulations. J. Enzyme Inhib. Med. Chem. 32, 60–67.
Mamadalieva, N.Z., Böhmdorfer, S., Zengin, G., Bacher, M., Potthast, A., Akramov, D.K., Janibekov, A., Rosenau, T., 2019. Phytochemical and biological activities of Silene
viridiflora extractives. Development and validation of a HPTLC method for
quantifi-cation of 20-hydroxyecdysone. Ind. Crop Prod. 129, 542–548.
Marini, G., Graikou, K., Zengin, G., Karikas, G.A., Gupta, M.P., Chinou, I., 2018. Phytochemical analysis and biological evaluation of three selected Cordia species from Panama. Ind. Crop Prod. 120, 84–89.
Marzouk, M.M., Hussein, S.R., Ibrahim, L.F., Elkhateeb, A., Kawashty, S.A., Saleh, N.A., 2014. Flavonoids from Neurada procumbens L.(Neuradaceae) in Egypt. Biochem. Syst. Ecol. 57, 7e68.
Mocan, A., Zengin, G., Crişan, G., Mollica, A., 2016. Enzymatic assays and molecular modeling studies of Schisandra chinensis lignans and phenolics from fruit and leaf extracts. J. Enzyme Inhib. Med. Chem. 31, 200–210.
Moustafa, S., Menshawi, B., Wassel, G., Mahmoud, K., Mounier, M., 2014. Screening of some wild and cultivated Egyptian plants for their free radical scavenging activity. Int. J. Pharmtech. Res. 6, 1271–1278.
Rahman, M.J., de Camargo, A.C., Shahidi, F., 2018. Phenolic profiles and antioxidant activity of defatted camelina and sophia seeds. Food Chem. 240, 917–925.
Rajakumar, G., Gomathi, T., Thiruvengadam, M., Rajeswari, V.D., Kalpana, V., Chung, I.-M., 2017. Evaluation of anti-cholinesterase, antibacterial and cytotoxic activities of green synthesized silver nanoparticles using from Millettia pinnata flower extract. Microb. Pathog. 103, 123–128.
Saleem, H., Htar, T.T., Naidu, R., Ahmad, I., Zengin, G., Ahmad, M., Ahemad, N., 2019a. Investigations into the therapeutic effects of aerial and stem parts of Buxus papillosa CK Schneid.: in vitro chemical, biological and toxicological perspectives. J. Pharm. Biomed. Anal. 166, 128–138.
Saleem, H., Htar, T.T., Naidu, R., Nawawi, N.S., Ahmad, I., Ashraf, M., Ahemad, N., 2019b. Biological, chemical and toxicological perspectives on aerial and roots of
Filago germanica (L.) huds: functional approaches for novel phyto-pharmaceuticals.
Food Chem. Toxicol. 123, 363–373.
Saleem, H., Htar, T.T., Naidu, R., Zengin, G., Ahmad, I., Ahemad, N., 2018. Phytochemical profiling, antioxidant, enzyme inhibition and cytotoxic potential of
Bougainvillea glabra flowers. Nat. Prod. Res. 1–5.
Saleem, H., Zengin, G., Ahmad, I., Lee, J.T.B., Htar, T.T., Mahomoodally, F.M., Naidu, R., Ahemad, N., 2019c. Multidirectional insights into the biochemical and toxicological properties of Bougainvillea glabra (Choisy.) aerial parts: a functional approach for bioactive compounds. J. Pharm. Biomed. Anal. 170, 132–138.
Shahzad, M.I., Ashraf, H., Arshad, M., Parveen, S., Aslam, A., Naz, N., Kamran, Z., Khalid, S.G., Hameed, S., Ashfaq, M., 2019. Study of antiviral potential of Cholistani plants against new castle disease virus. Pak. J. Zool. 51, 395–398.
Zengin, G., Atasagun, B., Aumeeruddy, M.Z., Saleem, H., Mollica, A., Bahadori, M.B., Mahomoodally, M.F., 2019. Phenolic profiling and in vitro biological properties of two Lamiaceae species (Salvia modesta and Thymus argaeus): a comprehensive eva-luation. Ind. Crop Prod. 128, 308–314.
Zengin, G., Locatelli, M., Carradori, S., Mocan, A.M., Aktumsek, A., 2016. Total phenolics, flavonoids, condensed tannins content of eight Centaurea species and their broad inhibitory activities against cholinesterase, tyrosinase, α-amylase and α-glucosidase. Not. Bot. Horti Agrobot. Cluj-Napoca. 44, 195–200.
Zengin, G., Uysal, S., Ceylan, R., Aktumsek, A., 2015. Phenolic constituent, antioxidative and tyrosinase inhibitory activity of Ornithogalum narbonense L. from Turkey: a phytochemical study. Ind. Crop Prod. 70, 1–6.
Zengin, G., Zheleva-Dimitrova, D., Gevrenova, R., Nedialkov, P., Mocan, A., Ćirić, A., Glamočlija, J., Soković, M., Aktumsek, A., Mahomoodally, M.F., 2018. Identification of phenolic components via LC–MS analysis and biological activities of two Centaurea species: C. Drabifolia subsp. Drabifolia and C. Lycopifolia. J. Pharm. Biomed. Anal. 149, 436–441.