Fumigant Toxicity of Essential Oils from Fifteen Plant Species against
Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae)
Kordali
*, S.; B. Emsen
**and E. Yildirim
**Atatürk University, Faculty of Agriculture, Department of Plant Protection, Erzurum, Turkey **Karamanoğlu Mehmetbey University, Kamil Özdağ, Fac. of Science, Dept. of Biology, Karaman, Turkey
(Received: June 10, 2013 and Accepted: August 13, 2013)
ABSTRACT
Essential oils obtained from fifteen different plant species (Achillea biserrata M. Bieb., Achillea coarctata Poir., Achillea gypsicola Hub-Mor., Achillea wilhemsii C. Koch, Artemisia santonicum L., Hypericum perforatum L., Melilotus officinalis (L.) Lam., Origanum acutidens (Hand.-Mazz), Origanum onites L., Origanum rotundifolium L., Origanum syriacum L., Satureja hortensis L., Satureja spicigera (C. Koch)., Salvia nemorosa L., and Tanacetum agrophyllum (L.) C. Koch were tested against adults of Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). Obtained results showed that the tested essential oils isolated from all plant species, except that from S. nemorosa had significant insecticidal effects against S. zeamais adults, compared with the control. Mortality rate of S. zeamais adults increased significantly (p < 0.01), as the concentration (µl/L air) level and/or exposure time increased. Treatments with the essential oils of A. coarctata, A. gypsicola, O. onites, S. hortensis, and S. spicigera showed highest percentages of adult mortalities, when they were applied at the high concentrations (10 & 20 µl/L air) and at all the post treatment periods or at a moderate concentration (5 µl/L air) with long exposure periods (48 or 96 hs). The essential oil of S. nemorosa had lowest effect. After 96 h of exposure, at the maximum concentration (20 µl/L air essential oil) of A. biserrata, A. coarctata, A. gypsicola, A. santonicum, H. perforatum, M. officinalis, O. onites, O. rotundifolium, S. hortensis, S. spicigera, T. agrophyllum recorded 100% mortality, while O. syriacum, O. acutidens, A. wilhemsii, and S. nemorosa attained 99-76.77 mortality. After 96 h of treatment, 100% mortality was achieved at the concentration of 5 µl/L air essential oil of A. gypsicola. Essential oils of M. officinalis, H. perforatum, and O. rotundifolium possessed the most effective fumigant toxicity against S. zeamais adults, with LC50 values of 0.289, 0.526, and 0.573 µl/L air,
respectively.
Key words: Essential oils, Plant species, Fumigant toxicity, Sitophilus zeamais, Turkey.
INTRODUCTION
The maize weevil, Sitophilus zeamais
Motschulsky (Coleoptera: Curculionidae) is a stored grain pest that causes yield losses in storage products in Turkey (Yildirim, 2012). It occurs in corn storage and sometimes destroys the entire corn product. It attacks equity portion of the corn and makes the product useless. Several insecticides have been tried for years to prevent its damages. Chemical/ synthetic insecticides constitute the first part of the trials but many negative results have occurred; such as toxicity to non-target animals, residue problems and insecticide resistance (Isman, 2006). These side effects of the synthetic pesticides have created a viewpoint for the introduction of alternative compounds. Hence, in recent years researches have showed that natural products can be preferred by farmers to protect their stored grains from insects’ invasion (Tapondjou et al., 2005). Among the natural products, essential oils have acted as botanical pesticides. One of the most important features of botanical pesticides is that they provide strange manners of action against some insect pests and risk of cross-resistance of these insect species reduces (Isman, 2006).
Essential oils are secondary metabolites that are not related to plant metabolism. They constitute the main feature of many plants and retain the plant
from bacteria, fungi and fluid loss and thereby some
essential oils have bactericidal, fungicidal,
antiparasitical and insecticidal properties (Bakkali et
al., 2008). At the same time, they give rise to
fumigant and topical toxicity as well as antifeedant and repellent effects (Regnault-Roger, 1997 and Shaaya et al., 1997). Fumigation is a potential technique in insect pest elimination in stored products. Fumigation penetrates homogenously to endpoints due to its diffusion height and it can be applied to a large amount in a short time (Zettler and Arthur, 2000). Contact and fumigant insecticidal activities of plant essential oils have been well demonstrated against stored product pests (Huang et
al., 1997; Tripathi et al., 2000; Lee et al., 2003;
Tapondjou et al., 2005; Yildirim et al., 2005 a, b; Kordali et al., 2008; Egwurube et al., 2010; Yildirim
et al., 2011 and Kordali et al., 2012).
The present research was therefore undertaken to investigate the fumigant toxicity of some essential oils against S. zeamais adults, the important pest of maize crop products during storage in Turkey.
MATERIALS AND METHODS Insect rearing
S. zeamais was obtained from storage house in
Tokat region, Turkey. Corn grains were purchased
The corn was washed by tap water, dried and heated to prevent pre-infestation and before using for the experiments. S. zeamais adults were reared on whole corn in the laboratory at 12-13% moisture content in plastic box (diameter 25 cm, height 30 cm) at 64±5 R.H, 25±1 °C and L: D = 12 h:12 h at the
Department of Plant Protection, Faculty of
Agriculture, Ataturk University, Erzurum- Turkey. Obtained adults from laboratory culture were stored in separate insect cages provided with corn. Different experiments were carried out under the same laboratory conditions.
Determination of adults’ age
Four to six day-old S. zeamais adults were used as test material. In order to get adults at the same age, few grains of corn that included larvae and pupae were placed separately in Petri dishes. After adult emergence, adults of the same age were collected and used.
Isolation of essential oils
Achillea biserrata M. Bieb., Achillea coarctata
Poir., Achillea gypsicola Hub-Mor., Achillea
wilhemsii C. Koch, Artemisia santonicum L., Hypericum perforatum L., Melilotus officinalis (L.)
Lam., Origanum acutidens (Hand.-Mazz),
Origanum onites L., Origanum rotundifolium L., Origanum syriacum L., Satureja hortensis L., Satureja spicigera (C. Koch)., Salvia nemorosa L.,
and Tanacetum agrophyllum (L.) C. Koch were collected at the flowering stage in Turkey in 2009-10. Collected plant materials were dried in shadow and ground in a grinder. The dried plant samples (500 g) were subjected to hydrodistillation (plant material in boiling water) using a Clevenger-type apparatus for 4 hours. Hydrodistillation of the abovementioned plants yielded 0.35, 0.21, 0.65, 0.85, 0.85, 0.24, 0.43, 0.60, 3.67, 0.87, 0.86, 2.30, 1.56, 0.38, and 0.19% (w/w) of essential oils, respectively. The yields were based on dry materials of plant samples.
Bioassays
In order to test the toxicity of the essential oils against S. zeamais adults, 33 individuals with 33 grains of corn were placed into Petri dishes (9 cm diameter). 5, 10, and 20 μl oil/L air was applied with an automatic pipette on a filter paper (2×2 cm) attached to the upside of the Petri dish. Mortality rate of the adults was determined after an exposure for 24, 48, and 96 hs. Petri dishes applied with only sterile water served as control. Three replicates were used/ concentration/ exposure time. Insecticidal action of oils was expressed as % mean mortality of the adults.
Statistical analysis
Differences among the fumigant toxicities of the essential oils tested were determined according to
analysis of variance (ANOVA) test by using SPSS 15.0 software package. Differences between means were tested through Duncan tests and values with p<
0.01 were considered significantly different. LC25,
LC50, and LC90 values at 96 h were calculated by
SPSS. Probit analysis of concentration-mortality
data was conducted to estimate the LC25, LC50, and
LC90 values and associated 95% confidence limits
for each treatment (EPA Probit Analysis).
RESULTS AND DISCUSSION
Toxicity effects of the essential oils extracted from the fifteen mentioned tested plant species; against adults of S. zeamais were estimated. The results showed that the essential oils of the different plant species studied had varying degrees of insecticidal activity against adults of S. zeamais, in comparison with the control, as the insecticidal activity increased with increasing concentration and exposure times. Also, the different toxicity ratios maybe originated by chemical constituents which were unique to them.
Analysis of variances demonstrated that the effects of these essential oils on the mortality rate of
S. zeamais were highly significant on the basis of
both concentration rate and exposure time (p < 0.01) (Figs. 1 and 2). High concentrations and long exposure times scored maximum toxicities on S.
zeamais. Treatments with the essential oils of A. biserrata, A. gypsicola, A. coarctata, A. santonicum, H. perforatum, M. officinalis, O. onites, O. rotundifolium, S. hortensis, S. spicigera, and T. agrophyllum showed higher mortality rates, while
the lowest one was found in case of S. nemorosa (Table 1). On the other hand, mortality percentages, after 96 h of treatments, with the maximum concentration (20 µl/L air) of the essential oils of A.
biserrata, A. gypsicola, A. coarctata, A. santonicum, H. perforatum, M. officinalis, O. onites, O. rotundifolium, S. hortensis, S. spicigera, T. agrophyllum, O. syriacum, O. acutidens, A. wilhemsii, and S. nemorosa, gave 100, 100, 100,
100, 100, 100, 100, 100, 100, 100, 100, 98.99, 97.98, 94.95, and 76.77%, respectively (Table 1, Figs. 1 and 2). No mortality was recorded in the control.
Higher levels of mortality percentages were achieved after 96 h of treatments at the concentration of both 10 and 20 µl/L air of the essential oils of A. coarctata, O. onites, S. hortensis, and S. spicigera (100%), 5, 10, and 20 µl/L air of the essential oils of A. gypsicola (100%), 20 µl/L air of the essential oils of O. syriacum (98.99%) (Table 1 and Fig. 2).
The most effective one among the fifteen plant
Fig. ( 1) : Mo rt al it y ra tes of Si to phi lus ze am a is a dul ts e xpose d to fi ft een e ss en ti al o il s at di ff er en t co nce n tr at io ns dos es . Fig. ( 2) : Mo rt al it y ra tes of Si to phi lus ze am a is t re at ed w it h es sent ia l o il s ext ra ct ed f ro m f if te en p lan t spe ci es a t d if fe re n t tr ea tm en ts .
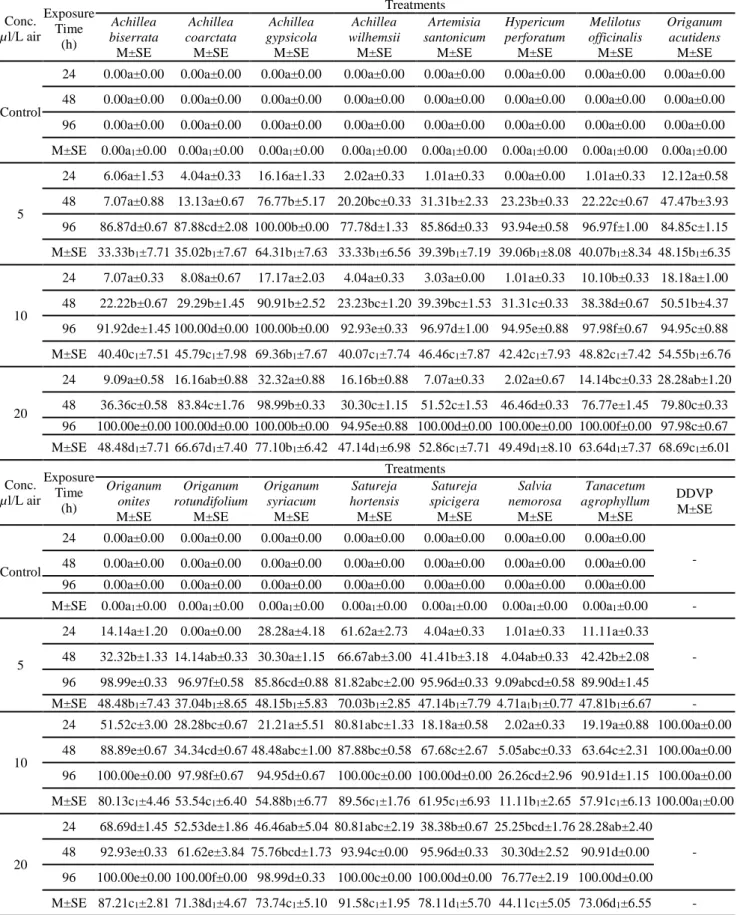
Table (1): Mortality percent of essential oils extracted from different plants and DDVP against Sitophilus
zeamais adults under laboratory conditions
Conc. µl/L air Exposure Time (h) Treatments Achillea biserrata M±SE Achillea coarctata M±SE Achillea gypsicola M±SE Achillea wilhemsii M±SE Artemisia santonicum M±SE Hypericum perforatum M±SE Melilotus officinalis M±SE Origanum acutidens M±SE Control
24 0.00a±0.00 0.00a±0.00 0.00a±0.00 0.00a±0.00 0.00a±0.00 0.00a±0.00 0.00a±0.00 0.00a±0.00
48 0.00a±0.00 0.00a±0.00 0.00a±0.00 0.00a±0.00 0.00a±0.00 0.00a±0.00 0.00a±0.00 0.00a±0.00 96 0.00a±0.00 0.00a±0.00 0.00a±0.00 0.00a±0.00 0.00a±0.00 0.00a±0.00 0.00a±0.00 0.00a±0.00
M±SE 0.00a1±0.00 0.00a1±0.00 0.00a1±0.00 0.00a1±0.00 0.00a1±0.00 0.00a1±0.00 0.00a1±0.00 0.00a1±0.00
5
24 6.06a±1.53 4.04a±0.33 16.16a±1.33 2.02a±0.33 1.01a±0.33 0.00a±0.00 1.01a±0.33 12.12a±0.58
48 7.07a±0.88 13.13a±0.67 76.77b±5.17 20.20bc±0.33 31.31b±2.33 23.23b±0.33 22.22c±0.67 47.47b±3.93 96 86.87d±0.67 87.88cd±2.08 100.00b±0.00 77.78d±1.33 85.86d±0.33 93.94e±0.58 96.97f±1.00 84.85c±1.15
M±SE 33.33b1±7.71 35.02b1±7.67 64.31b1±7.63 33.33b1±6.56 39.39b1±7.19 39.06b1±8.08 40.07b1±8.34 48.15b1±6.35
10
24 7.07a±0.33 8.08a±0.67 17.17a±2.03 4.04a±0.33 3.03a±0.00 1.01a±0.33 10.10b±0.33 18.18a±1.00
48 22.22b±0.67 29.29b±1.45 90.91b±2.52 23.23bc±1.20 39.39bc±1.53 31.31c±0.33 38.38d±0.67 50.51b±4.37 96 91.92de±1.45 100.00d±0.00 100.00b±0.00 92.93e±0.33 96.97d±1.00 94.95e±0.88 97.98f±0.67 94.95c±0.88
M±SE 40.40c1±7.51 45.79c1±7.98 69.36b1±7.67 40.07c1±7.74 46.46c1±7.87 42.42c1±7.93 48.82c1±7.42 54.55b1±6.76
20
24 9.09a±0.58 16.16ab±0.88 32.32a±0.88 16.16b±0.88 7.07a±0.33 2.02a±0.67 14.14bc±0.33 28.28ab±1.20
48 36.36c±0.58 83.84c±1.76 98.99b±0.33 30.30c±1.15 51.52c±1.53 46.46d±0.33 76.77e±1.45 79.80c±0.33 96 100.00e±0.00 100.00d±0.00 100.00b±0.00 94.95e±0.88 100.00d±0.00 100.00e±0.00 100.00f±0.00 97.98c±0.67 M±SE 48.48d1±7.71 66.67d1±7.40 77.10b1±6.42 47.14d1±6.98 52.86c1±7.71 49.49d1±8.10 63.64d1±7.37 68.69c1±6.01 Conc. µl/L air Exposure Time (h) Treatments Origanum onites M±SE Origanum rotundifolium M±SE Origanum syriacum M±SE Satureja hortensis M±SE Satureja spicigera M±SE Salvia nemorosa M±SE Tanacetum agrophyllum M±SE DDVP M±SE Control
24 0.00a±0.00 0.00a±0.00 0.00a±0.00 0.00a±0.00 0.00a±0.00 0.00a±0.00 0.00a±0.00
- 48 0.00a±0.00 0.00a±0.00 0.00a±0.00 0.00a±0.00 0.00a±0.00 0.00a±0.00 0.00a±0.00
96 0.00a±0.00 0.00a±0.00 0.00a±0.00 0.00a±0.00 0.00a±0.00 0.00a±0.00 0.00a±0.00
M±SE 0.00a1±0.00 0.00a1±0.00 0.00a1±0.00 0.00a1±0.00 0.00a1±0.00 0.00a1±0.00 0.00a1±0.00 -
5
24 14.14a±1.20 0.00a±0.00 28.28a±4.18 61.62a±2.73 4.04a±0.33 1.01a±0.33 11.11a±0.33
- 48 32.32b±1.33 14.14ab±0.33 30.30a±1.15 66.67ab±3.00 41.41b±3.18 4.04ab±0.33 42.42b±2.08 96 98.99e±0.33 96.97f±0.58 85.86cd±0.88 81.82abc±2.00 95.96d±0.33 9.09abcd±0.58 89.90d±1.45 M±SE 48.48b1±7.43 37.04b1±8.65 48.15b1±5.83 70.03b1±2.85 47.14b1±7.79 4.71a1b1±0.77 47.81b1±6.67 -
10
24 51.52c±3.00 28.28bc±0.67 21.21a±5.51 80.81abc±1.33 18.18a±0.58 2.02a±0.33 19.19a±0.88 100.00a±0.00
48 88.89e±0.67 34.34cd±0.67 48.48abc±1.00 87.88bc±0.58 67.68c±2.67 5.05abc±0.33 63.64c±2.31 100.00a±0.00 96 100.00e±0.00 97.98f±0.67 94.95d±0.67 100.00c±0.00 100.00d±0.00 26.26cd±2.96 90.91d±1.15 100.00a±0.00 M±SE 80.13c1±4.46 53.54c1±6.40 54.88b1±6.77 89.56c1±1.76 61.95c1±6.93 11.11b1±2.65 57.91c1±6.13 100.00a1±0.00
20
24 68.69d±1.45 52.53de±1.86 46.46ab±5.04 80.81abc±2.19 38.38b±0.67 25.25bcd±1.76 28.28ab±2.40 - 48 92.93e±0.33 61.62e±3.84 75.76bcd±1.73 93.94c±0.00 95.96d±0.33 30.30d±2.52 90.91d±0.00
96 100.00e±0.00 100.00f±0.00 98.99d±0.33 100.00c±0.00 100.00d±0.00 76.77e±2.19 100.00d±0.00
M±SE 87.21c1±2.81 71.38d1±4.67 73.74c1±5.10 91.58c1±1.95 78.11d1±5.70 44.11c1±5.05 73.06d1±6.55 -
M±SE : mean ± SE of three replicates, each set-up with 33 adults a, b, c, d, e, f : in the same column the exposure times differ (p < 0.01) a1, b1, c1, d1 : in the same column the doses differ (p < 0.01)
Table (2): LC25, LC50, and LC90 values (µl/L air) of essential oils extracted from fifteen plant species against
Sitophilus zeamais adults after 96 h under laboratory conditions
Treatments LC25 (Limits) LC50 (Limits) LC90 (Limits) Slope ± SE
Achillea biserrata 0.691 (0.30-1.663) 1.506 (0.182-2.766) 6.611 (4.544-8.810) 1.994 ± 0.579 Achillea coarctata 1.616 (0.117-2.716) 2.431 (0.411-3.496) 5.279 (3.914-6.498) 3.805 ± 1.317 Achillea gypsicola a a a 0.000 ± 0.000 Achillea wilhemsii 0.566 (0.027-1.469) 1.516 (0.215-2.839) 9.845 (7.374-15.282) 1.577 ± 0.428 Artemisia santonicum 1.324 (0.210-2.331) 2.220 (0.639-3.298) 5.933 (4.586-7.388) 3.003 ± 0.828 Hypericum perforatum 0.185 (0.000-1.082) 0.526 (0.000-1.902) 3.833 (0.016-6.096) 1.485 ± 0.673 Melilotus officinalis 0.100 ( b ) 0.289 ( b ) 2.169 ( b ) 1.464 ± 0.925 Origanum acutidens 0.532 (0.013-1.447) 1.280 (0.106-2.556) 6.790(4.447-9.311) 1.768 ± 0.523 Origanum onites 0.390 ( b ) 0.690 ( b ) 1.997 ( b ) 2.778 ± 3.753 Origanum rotundifolium 0.260 ( b ) 0.573 ( b ) 2.565 ( b ) 1.968 ± 1.196 Origanum syriacum 0.682 (0.032-1.637) 1.476 (0.184-2.718) 6.402 (4.325-8.496) 2.011 ± 0.578 Satureja hortensis 2.803 (0.596-3.659) 3.587 (1.408-4.258) 5.728 (5.168-7.913) 6.304 ± 2.303 Satureja spicigera 1.260 ( b ) 1.850 ( b ) 3.835 ( b ) 4.043 ± 2.756 Salvia nemorosa 8.609 (7.496-9.639) 13.316 (11.952-15.049) 30.493 (25.045-40.621) 3.561 ± 0.380 Tanacetum agrophyllum 0.357 (0.000-1.273) 0.944 (0.010-2.295) 5.998 (2.782-8.442) 1.596 ± 0.558
a For this essential oil no LC values are computed because the ratios of response counts to subject counts are the
same, i.e. the slope is zero. b Narrow limit
LC25, LC50, and LC90 values, at 96 h belong to
M. officinalis were 0.100, 0.289, and 2.169,
respectively. The least effective one after 96 h
was S. nemorosa, where the LC25, LC50, and LC90
values were 8.609, 13.316, and 30.493, respectively (Table 2).
Some of the previous studies demonstrated that in general, the toxicity of essential oils isolated from plant samples against stored pests was mainly related to their major components (Tripathi et al., 2000; Lee
et al., 2003; Tapondjou et al., 2005; Yildirim et al.,
2005 a, b; Egwurube et al., 2010; Yildirim et al., 2011, 2012 and Emsen et al., 2012a, b).
Increasing use of natural insecticides will help to decrease the negative effects like toxicity to non-target animals, residue problems (environmental pollution) and insecticidal resistance of synthetic or
chemical insecticides. In this context,
bio-insecticides may be also effective, bio-degradable, selective and associated with little advancement of resistance in the insect population and as a result, more safe to the environment. In the present study, 96 h of exposure, at the maximum concentration (20
µl/L air) of essential oils of A. biserrata, A. gypsicola, A. coarctata, A. santonicum, H. perforatum, M. officinalis, O. onites, O. rotundifolium, S. hortensis, S. spicigera, and T. agrophyllum, was determined to cause the highest
mortality rates in S. zeamais adults. The compounds isolated from the essential oils of these plants may be suggested to be potential insecticidal agents for controlling the adults of S. zeamais in stored food products.
Obtained results and those reported earlier clearly indicated the variations in the effects of essential oils
in regard to the stage, the species of insect and plant origin of the essential oil. Otherwise, DDVP (2, 2-dichlorovinyl dimethyl phosphate) which is an effective chemical pesticide was used in this study and 10 µl/L air DDVP with a standard of pesticide applications showed 100% insecticidal activity on S.
zeamais. However, in recent years negative effects
of chemical-containing pesticides as DDVP were investigated. Excessive usage of such pesticides causes environmental pollution (Tortelli et al., 2006 and Kopecka-Pilarczyk, 2010). Moreover, Van Maele-Fabry and Willems (2004) and Koutros et al. (2008) reported that using of DDVP increased the human cancer risk. Therefore, tested essential oils proved to be promising as control alternatives against stored product insects especially, S. zeamais. However, further studies are also needed to evaluate the cost, efficacy and safety of the active insecticidal ingredients and essential oils of those plants, on a wide range of pests and beneficial arthropods prevailing in the commercial stores.
REFERENCES
Bakkali, F., S. Averbeck, D. Averbeck and M. Idaomar 2008. Biological effects of essential oils e a review. Food Chem. Toxicol., 46: 446-475.
Egwurube, E., B. T. Magaji and Z. Lawal 2010.
Laboratory evaluation of neem (Azadirachta
indica) seed and leaf powders for the control of
khapra beetle, Trogoderma granarium
(Coleoptera: Dermestidae) Infesting Groundnut. Int. J. Agric. Biol. 12: 638–640.
Emsen, B., Y. Bulak, E. Yildirim, A. Aslan and S. Ercisli 2012a. Activities of two major lichen compounds, diffractaic acid and usnic acid against Leptinotarsa decemlineata Say, 1824
(Coleoptera: Chrysomelidae). Egypt. J. Biol. Pest Cont., 22(1): 5-10.
Emsen, B., E. Yildirim, A. Aslan, M. Anar and S. Ercisli 2012b. Insecticidal effect of the extracts of Cladonia foliacea (Huds.) Willd. and
Flavoparmelia caperata (L.) Hale against adults
of the grain weevil, Sitophilus granarius (L.) (Coleoptera: Curculionidae). Egypt. J. Biol. Pest Cont. 22(2): 145-149.
Huang, Y., J. M. Kini, R. M. Tan and S. H. Ho 1997. Toxic and antifeedant action of nutmeg oil against Tribolium casteneum (Herbst) and
Sitophilus zeamais Motsch. J. Stored Prod. Res.,
33: 289-298.
Isman, M. B. 2006. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol., 51: 45–66.
Kopecka-Pilarczyk, J. 2010. The effect of pesticides and metals on acetylcholinesterase (AChE) in various tissues of blue mussel (Mytilus trossulus L.) in short-term in vivo exposures at different temperatures. J. Environ. Sci. Heal. B., 45: 336-346.
Kordali, S., A. Cakir, H. Ozer, R. Cakmakci, M.
Kesdek and E. Mete 2008. Antifungal.
phytotoxic and insecticidal properties of essential oil isolated from Turkish Origanum acutidens and its three components, carvacrol, thymol and p-cymene. Bioresource Technol., 99: 8788–8795. Kordali, S., E. Yildirim, G. Yazici, B. Emsen, G.
Kabaagac and S. Ercisli 2012. Fumigant toxicity of essential oils of nine plant species from Asteraceae and Clusiaceae against Sitophilus
granarius (L.) (Coleoptera: Curculionidae).
Egypt. J. Biol. Pest Cont. 22(1): 11-14.
Koutros, S., R. Mahajan, T. Zheng, J. A. Hoppin, X. Ma, C. F. Lynch, A. Blair and M. C. R. Alavanja 2008. Dichlorvos exposure and human cancer risk: results from the Agricultural Health Study. Cancer Cause. Control, 19: 59-65.
Lee, S., C. J. Peterson and J. R. Coats 2003. Fumigation toxicity of monoterpenoids to several stored product insects. J. Stored Prod. Res., 39: 77-85.
Regnault-Roger, C. 1997. The potential of botanical essential oils for insect pest control. Integ. Pest Man. Rev., 2: 25-34.
Shaaya, E., M. Kostjukovski, J. Eilberg and C.
Sukprakarn 1997. Plant oils as fumigant and contact insecticides for the control of stored product insects. J. Stored Prod. Res., 33: 7-15.
Tapondjou, A. L., C. Adler, D. A. Fontem, H. Bouda and C. Reichmuth 2005. Bioactivities of cymol and essential oils of Cupressus sempervirens and Eucalyptus saligna against Sitophilus
zeamais Motschulsky and Tribolium confusum
du Val. Journal of Stored Prod. Res., 41: 91-102. Tortelli, V., E. P. Colares, R. B. Robaldo, L. E. M.
Nery, G. L. L. Pinho, A. Bianchini and J. M. Monserrat 2006. Importance of cholinesterase kinetic parameters in environmental monitoring using estuarine fish. Chemosphere, 65: 560-566. Tripathi, A. K., V. Prajapati, K. K. Aggarwal, S.
P. S. Khanuja and S. Kumar 2000. Repellence and toxicity of oil from Artemisia annua to certain stored product beetles. J. Econ. Entomol., 93: 43-47.
Van Maele-Fabry, G. V. and J. L. Willems 2004. Prostate cancer among pesticide applicators: a meta-analysis. Int. Arch. Occ. Env. Hea., 77: 559-570.
Yildirim, E. 2012. Pests of stored products and their control methods. (3th ed.). Erzurum: Atatürk University, Agriculture Faculty Press, No: 191. Yildirim, E., M. Kesdek, I. Aslan, O. Calmasur and
F. Sahin 2005a. The effects of essential oils from eight plant species on two pests of stored product insects. Fresen. Environ. Bull., 14: 23-27.
Yildirim, E., M. Kesdek and S. Kordali
2005b. Effects of essential oils of three plant species on Tribolium confusum du Val and
Sitophilus granarius (L.) (Coleoptera:
Tenebrionidae and Curculionidae). Fresen.
Environ. Bull., 14: 574-578.
Yildirim, E., S. Kordali and G.Yazici 2011. Insecticidal effects of essential oils of eleven plant species from Lamiaceae on Sitophilus
granarius (L.) (Coleoptera: Curculionidae). Rom.
Biotech. Lett., 16: 6702-6709.
Yildirim, E., B. Emsen, A. Aslan, Y. Bulak and S. Ercisli 2012. Insecticidal activity of lichens against the maize weevil, Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). Egypt. J. Biol. Pest Cont. 22(2): 151-156.
Zettler, J. L. and F. H. Arthur 2000. Chemical control of stored product insects with fumigants and residual treatments. Crop Prot., 19: 577-582.
View publication stats View publication stats