ContentslistsavailableatScienceDirect
Journal
of
Pharmaceutical
and
Biomedical
Analysis
jou rn a l h om ep a g e :w w w . e l s e v i e r . c o m / l oc a t e / j p b a
Multidirectional
insights
into
the
biochemical
and
toxicological
properties
of
Bougainvillea
glabra
(Choisy.)
aerial
parts:
A
functional
approach
for
bioactive
compounds
Hammad
Saleem
a,b,
Gokhan
Zengin
c,
Irshad
Ahmad
d,
Joash
Tan
Ban
Lee
e,
Thet
Thet
Htar
a,
Fawzi
M.
Mahomoodally
f,
Rakesh
Naidu
g,
Nafees
Ahemad
a,h,i,∗aSchoolofPharmacy,MonashUniversityMalaysia,JalanLagoonSelatan,47500,BandarSunway,SelangorDarulEhsan,Malaysia bInstituteofPharmaceuticalSciences(IPS),UniversityofVeterinary&AnimalSciences(UVAS),Lahore,54000,Pakistan cDepartmentofBiology,FacultyofScience,SelcukUniversity,Campus/Konya,Turkey
dDepartmentofPharmacy,TheIslamiaUniversityofBahawalpur,Pakistan
eSchoolofScience,MonashUniversityMalaysia,JalanLagoonSelatan,47500,BandarSunway,SelangorDarulEhsan,Malaysia fDepartmentofHealthSciences,FacultyofScience,UniversityofMauritius,Mauritius
gJeffreyCheahSchoolofMedicineandHealthSciences,MonashUniversityMalaysia,JalanLagoonSelatan,47500,BandarSunway,SelangorDarulEhsan,
Malaysia
hTropicalMedicineandBiologyMultidisciplinaryPlatform,MonashUniversityMalaysia,JalanLagoonSelatan,47500,BandarSunway,SelangorDarul
Ehsan,Malaysia
iGlobalAsiainThe21stCentury(GA21)MultidisciplinaryResearchPlatform,MonashUniversity,Malaysia
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received11January2019
Receivedinrevisedform12March2019 Accepted13March2019
Availableonline16March2019
Keywords: Carcinomacelllines Antioxidant Cytotoxicity Enzymeinhibitor Bioactivecompounds
a
b
s
t
r
a
c
t
Thecurrentresearchworkwasconductedinordertoprobeintothebiochemicalandtoxicological
char-acterisationofmethanolanddichloromethane(DCM)extractsofBougainvilleaglabra(Choisy.)aerial
parts.Biologicalfingerprintswereassessedforinvitroantioxidant,keyenzymeinhibitoryand
cyto-toxicitypotential.Totalbioactivecontentsweredeterminedspectrophotometricallyandthesecondary
metabolitecomponentsofmethanolextractwasassessedbyUHPLCmassspectrometricanalysis.The
antioxidantcapabilitieswereevaluatedviasixdifferentinvitroantioxidantassaysnamelyDPPH,ABTS
(freeradicalscavenging),FRAP,CUPRAC (reducingantioxidantpower),phosphomolybdenum(total
antioxidantcapacity)andferrouschelatingactivity.Inhibitionpotentialagainstkeyenzymesurease,
␣-glucosidaseandcholinesteraseswerealsodetermined.Methanolextractexhibitedhigherphenolic
(24.01mgGAE/gextract)aswellasflavonoid(41.51mgQE/gextract)contents.Phytochemical
profil-ingofmethanolextractidentifiedatotaloftwentysecondarymetabolitesandthemajorcompounds
belongedtoflavonoids,phenolicsandalkaloidderivatives.Thefindingsofantioxidantassaysrevealedthe
methanolextracttoexhibitstrongerantioxidant(exceptphosphomolybdenum)activities.Similarly,the
methanolextractshowedhighestbutyrylcholinesteraseandureaseinhibition.TheDCMextractwasmost
activeforphosphomolybdenumand␣-glucosidaseinhibitionassays.Moreover,bothextractsexhibited
significantcytotoxicpotentialagainstfive(MCF-7,MDA-MB-231,CaSki,DU-145,andSW-480)human
carcinomacelllineswithhalfmaximalinhibitoryconcentrationvaluesof22.09to257.2g/mL.Results
fromthepresentstudyhighlightedthepotentialofB.glabraaerialextractstobefurtherexploredinan
endeavourtodiscovernovelphytotherapeuticsaswellasfunctionalingredients.
©2019ElsevierB.V.Allrightsreserved.
1. Introduction
Cancer,cardiovasculardiseases,chronicrespiratory disorders anddiabetes,categorizedasnoncontagiousdiseases(NCD’s),are
∗ Correspondingauthorat:SchoolofPharmacy,MonashUniversityMalaysia,Jalan LagoonSelatan,47500,BandarSunway,SelangorDarulEhsan,Malaysia
E-mailaddress:nafees.ahemad@monash.edu(N.Ahemad).
theprimarycauseofdeathsglobally.Thesedisordersare account-ableforapproximately40milliondeathsperyearwhichisabout 70%ofalldeathsworldwide[1].NCD’saremostlylinkedto ele-vatedlevelsofoxidativestress,whichinturnisduetoanimbalance betweenexcessivefreeradicalproductionandtheantioxidant lev-elsinthebody.Asapartofnormalbodyfunction,thefreeradicals areproducedallthetimesinthecellsandarebalancedbyeither internalantioxidantdefencesystemorbyexternallyintheform offood.Thesefreeradicalscanbeeitheroxygenderivedor nitro-https://doi.org/10.1016/j.jpba.2019.03.027
orbioactivephytochemicalsareregardedasprospective materi-als.
Bougainvillea glabra (Nyctaginaceae), commonly known as “Glory of the Garden”, is native to Southern America and has been used traditionally for various medicinal purposes such asinsecticidal,anti-inflammatory[5],anti-diarrhoeal,anti-ulcer, anti-microbial[6]and anti-hyperglycaemicagent[7].Inspiteof the considerable traditional importance, there have been only limited attempts to explore the chemical and pharmacological propertiesofthisspeciesinrelationwithitsmedicinaluses.Thus, we aimed to evaluate, in this paper, B. glabra aerial parts for their chemical composition (total phenolic and flavonoid con-tentsandUHPLC-MSsecondarymetabolitesprofile),antioxidant capabilities (DPPH, ABTS, FRAP, CUPRAC, phosphomolybdenum andmetalchelation)andkeyenzymeinhibitionpotentialagainst urease,␣-glucosidase, acetylcholinesterase (AChE) and butyryl-cholinesterase (BChE). Moreover, cytotoxic activities were also evaluatedusingMTTassayagainsthumanbreast,cervix,prostate andcoloncarcinomacelllines.Theobservedfindingwouldprovide newintuitionsforB.glabraplantspecies.
2. Materialandmethods 2.1. Plantmaterialsandextraction
Aerial parts of B. glabra plant were collected from district, MuzaffarGarh(Punjab),PakistanandidentifiedbyDr.Abdul Mun-sif,DepartmentofBotany,S.E.College,Bahawalpur.Furthermore, a voucher representative number (BG-AP-01-16-111) was also depositedintheherbariumofDepartmentofPharmacyand Alter-nativeMedicine,TheIslamiaUniversityofBahawalpur,Pakistan.
Aftershade drying and grindingofthe aerialparts ofplant, itspowderwasextractedbymaceration(72h)successivelywith dichloromethane(DCM)andmethanol.Thepooledextractswere thenfilteredanddriedundervacuumat40◦C.Theextractswere abbreviatedas;BM(B.glabraaerialmethanolextract)andBD(B. glabraaerialDCMextract).
2.2. Totalbioactivecontentsandsecondarymetaboliteprofiling Totalphenolic contents were determined using well-known standardFolin–Ciocalteumethod[8]usinggallicacidasastandard. Theamountoftotal phenolicswasdeterminedasmilligramsof gallicacidequivalentspergram(mgGAE/gextract).Moreover,the amountofflavonoidsinalltheextractswereassessedutilizing stan-dardaluminiumchloridecalorimetricmethod[8].Totalflavonoids werenotedasmgQE/gextract(milligrams ofquercetin equiva-lents)usingquercetinasstandard.Similarly,thephytochemical composition of the methanol extract was evaluated by UHPLC Accurate-MassQ-TOF(Agilent1290InfinityLCsystemcoupledto Agilent6520)massspectrometerwithdualESIsourceasdescribed previously[8].
asgallicacidequivalent(mgGAE/gextract).TheresultsofABTS andCUPRACassayswereexpressedastroloxequivalents,whilefor metalchelatingassay,EDTAwasusedasreferencestandard. 2.4. Enzymeinhibitionassays
Cholinesterases(AChE:acetylcholinesteraseandBChE: butyryl-cholinesterase), ␣-glucosidase and urease inhibitory potential wasevaluatedutilizingpreviouslyreportedstandardmethodsas reportedpreviously[8].EserinewasusedascontrolforAChEand BChE,acarbosefor␣-glucosidaseandkojicacidforurease. Inhi-bitionpercentageoftheplantextractsatdifferentconcentrations wascalculatedas:
Inhibition(%)=ControlControl−Test×100
2.5. Cytotoxicityassay
ThecytotoxicitywastestedusingMTTassayasreportedearlier [8] againstfive differenthumancarcinomacell linesi.e., MDA-MB-231,MCF-7(breastcancer),CaSki (cervicalcancer),DU-145 (prostatecancer),andSW-480(coloncancer).Thepercentagecell viability(%)wasdeterminedbyfollowingformula:
Cellviability(%)=Abss–Absc×100
2.6. Statisticalanalysis
All the assays were performed in triplicates in order to determine the means and these values are reported as the mean±standarddeviation(SD).Moreover,theresultswere ana-lysedviaOnewayanalysisofvariance(ANOVA)followedbyTukey’s testfortheposthoctreatmentusingStatisticalPackageforSocial Science(SPSS24.0forwindows).TheIC50valuesweredetermined
utilizingGraphPadPrismsoftware. 3. Resultsanddiscussion 3.1. Phytochemicalcomposition
MethanolandDCMsolventswereusedfortheextractionofB. glabraaerialparts,andthepercentageextractionyieldwasalso calculated.Boththeextractswerescreenedfortotalbioactive com-positiontodeterminetheirtotalphenolicandflavonoidcontents andthevaluesarepresentedinTable1.Totalphenoliccontentsof methanolextractwassignificantlyhigherthanDCM.Similarly,the highestflavonoidcontentswerealsoobtainedfromthemethanol extract.
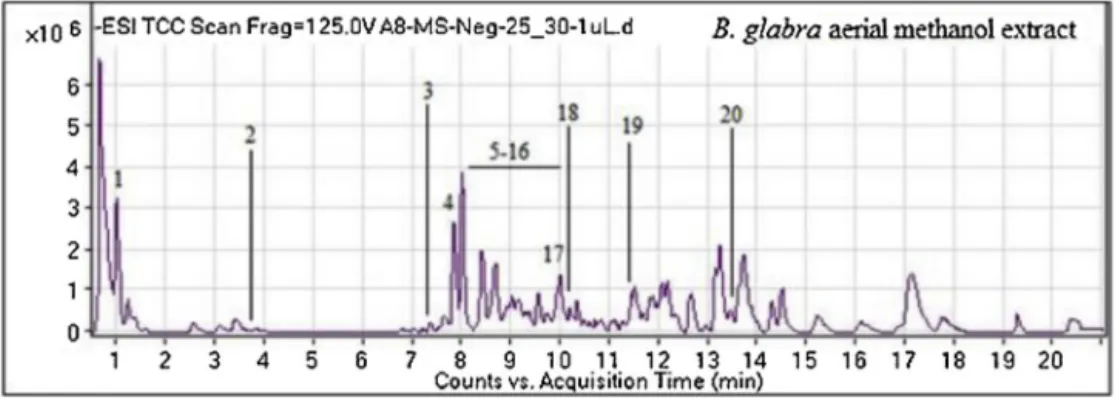
Secondary metabolites profiling of the methanol extract was determined using UHPLC-MS in negative ionization mode and its total ion chromatogram (TIC) is shown in Fig. 1. The base peak analysis of the UHPLC–MS chromatogram identified 20 different secondary metabolite compounds and
Fig.1.Totalionchromatograms(TIC)ofB.glabraaerialmethanolextract.
Table1
ExtractionyieldandtotalbioactivecontentsofB.glabraaerialextracts. PlantCode Yield(%) Totalphenolic
content(mgGAE/g)
Totalflavonoid content(mgQE/g) BM 6.4 24.01±2.09a 41.51±0.80a
BD 2.8 15.49±2.26b 18.68±0.74b
Datafromthreerepetitions,withmean±standarddeviation;meanswithdifferent superscriptlettersinthesamecolumnaresignificantly(p<0.05)different.GAE: gal-licacidequivalent;QE:quercetinequivalent;BM:B.glabraaerialmethanolextract, BD:B.glabraaerialDCMextract.
are presented in Table 2. Most of these compounds were
flavonoidsderivativesincludingneoeriocitri,robinin,isorhamnetin 3-glucosyl-(1->6)-galactoside,kaempferol 3-neohesperidoside-7-(2”-ferulylglucoside), quercetin 3-(2”’-feruloylsophoroside) and isovitexin 7-(6”’-sinapoylglucoside) 4’-glucoside. Polyphenolics presentwere3,4-dihydroxybenzoicacidandp-salicylicacid.Two terpenoids(oleosidedimethylesterandgoyaglycosideh)andone alkaloid(calysteginB2)werealsoidentified.Overall,theB.glabra methanolextractwasobservedtohavemoreflavonoidsandthis greateramountofflavonoidcompoundsinthisextractisin accor-dancetoitshighertotalflavonoidcontentsaspresentedinTable1. 3.2. Antioxidantproperties
3.2.1. Radicalscavengingandtotalantioxidantcapacity
ThefreeradicalscavengingactivityofB.glabraextractswere determined using DPPH and ABTS assays and the results are
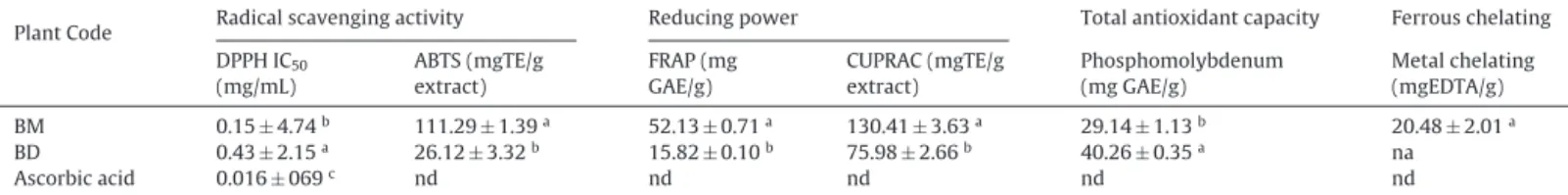
assembled in Table 3. The DPPH assay demonstrated that, BM exhibited the highest activity with IC50 values of 0.15mg/mL.
Similarly, in the ABTS assay, BM (111.29mg TE/g extract) had the highest radical scavenging activity. It is noted that, the totalbioactivecontentsofthetestedextractsfollowedthesame pattern as the radicals scavenging capacities. As phenolic and flavonoid compounds are excellent electron donors [10], they mightbeaccountablefortheobservedDPPHandABTSscavenging potential.
Total antioxidant activitywas determined via the phospho-molybenum assay based on reduction of molybdenum (VI) to molybdenum(V)bythecompoundshavingantioxidantcapacity andtheresultantformationofmolybdenum(V)complex(green incolor) [11]and theresultsare depictedin Table3.TheDCM extract(40.26mgGAE/gextract)exhibitedthehighertotal antiox-idantpotential compared tomethanol extract (29.14mg GAE/g extract).Thisassayalsoknownastotalantioxidantcapacityassay, measures the antioxidant potential of both phenolic and non-phenolic compounds presentin the plantextracts.The present findingsfortheDCMextractbythismethodmightbecorrelated totheexistenceofsomenon-phenoliccompounds,asvitaminC ortocopherolasexamples.Thisisalsoinlinewiththeprevious researches[12] whichhad presented theDCMextractstohave highertotalantioxidantcapacities.Moreover,ourresultsare fur-thersupportedbysomeotherreportspresentingaweakcorrelation betweenphosphomolybdenumassayandtotalbioactivecontents [8].
Table2
UHPLC-MSanalysisofB.glabraaerialmethanolextract(negativeionizationmode).
S.No Rt(min) B.peak(m/z) Proposedcompounds Compoundclass Mol.formula Mol.mass DiffDB(ppm)
1 1.109 174.07 CalysteginB2 Alkaloid C7H13NO4 175.08 0.13
2 3.839 417.14 3,4-Dihydroxybenzoicacid Phenol C7H6O4 154.02 −3.14
3 7.511 417.14 Oleosidedimethylester Terpene C18H26O11 418.14 −2.35
4 7.841 595.16 Neoeriocitrin Flavonoid C27H32O15 596.17 −1.49
5 8.389 755.20 Kaempferol3-(2G-glucosylrutinoside) Flavonoid C33H40O20 756.21 0.48 6 8.429 785.21 Isorhamnetin3-glucosyl-(1->2)-[rhamnosyl-(1->6)-galactoside] Flavonoid C34H42O21 786.22 −3.57
7 8.614 739.20 Robinin Flavonoid C33H40O19 740.21 0.89
8 8.655 769.219 Kaempferol4’-methylether3-(2Glc-glucosylrutinoside) Flavonoid C34H42O20 770.22 0.29 9 8.655 609.14 Robinetin3-rutinoside Flavonoid C27H30O16 610.15 −2.06 10 8.689 639.15 Isorhamnetin3-glucosyl-(1->6)-galactoside Flavonoid C28H32O17 640.16 −4.81 11 9.752 931.253 Kaempferol3-neohesperidoside-7-(2”-ferulylglucoside) Flavonoid C43H48O23 932.26 −2.42 12 9.808 801.18 Quercetin3-(2”’-feruloylsophoroside) Flavonoid C37H38O20 802.19 −2.68 13 9.901 901.24 Isovitexin2”-O-(6”’-(E)-p-coumaroyl)glucoside4’-O-glucoside Flavonoid C42H46O22 902.25 −4.43 14 9.904 931.25 Kaempferol3-[2Gal-(6”’-feruloylglucosyl)-robinobioside] Flavonoid C43H48O23 932.26 −2.96
15 9.96 961.26 p-Salicylicacid Phenol C7H6O3 138.03 −4.43
16 9.99 901.24 Isovitexin7-(6”’-sinapoylglucoside)4’-glucoside Flavonoid C44H50O24 962.27 −2.95 17 10.10 915.25 Kaempferol3-rhamnoside-7-[6”’-ferulyglucosyl-(1->3)-rhamnoside] Flavonoid C43H48O22 916.26 −3.75 18 10.19 785.19 Kaempferol3-(6”-(E)-feruloylglucosyl)-(1->2)-galactoside Flavonoid C37H38O19 786.20 −4.36
19 11.47 813.46 Goyaglycosideh Terpenoid C42H70O15 814.47 −1.73
20 13.48 723.39 AlliospirosideC Steroid C38H60O13 724.40 0.24
3.2.2. Reducingpowerandmetalchelatingactivity
Ferricreducingantioxidantpower(FRAP)andcupricreducing
antioxidantcapacity(CUPRAC)methodswereutilizedtomeasure
thereductioncapabilityofthestudiedplantsamplesandtheresults
are shown in Table 3. Both assays measure the plant extracts
potentialforreducingferrictoferrousandcuprictocuprousions, respectively[13]. Themethanol extract(FRAP: 52.13mgGAE/g extract and CUPRAC: 130.41mg TE/g extract) exhibited higher reducingpowercapacityascomparedtoDCMextract.Similarto radicalscavengingresults,phenolicandflavonoidrichBMextract wasthemostactivecompoundsinbothreducingpowerassays. Phenolicandflavonoidsareregardedtobethemostactive anti-oxidativeplantcomponentsbecauseoftheirabilitytoquenchfree radicalsandreactiveoxygenspecies[14].Someresearchershave alreadyreportedastrongpositiveassociationbetweenradical scav-engingpotential,reducingcapacitiesandtotalbioactivecontents ofdifferentplantextracts[15].Moreover,theferrousion chelat-ingcapacityofbothextractsweredeterminedbymetalchelating ferrozineassay(Table4).Interestingly,asobservedinother antiox-idantassayresults,BMwasfoundtobeactiveforferrouschelating activitywithvalueof20.48mgEDTA/gextract,whiletheBDextract wasinactive.
3.3. Enzymeinhibitionactivities
There is an extravagant progress in the prevalence of Alzheimer’sdiseaseanddiabetesmellitusandaccordinglythese aretheburningchallengesforpublichealth.Likewise,according torecentreports,Alzheimer’sdiseasehadaffectedmorethan50 millionpopulations,anduntil2050,thisstatisticsispresumedto beincreasedbyaboutthreetimesmore[16,17].Similarly,urease isone ofthemajorresponsible enzymefor killingHelicobacter pyloriwhich exist in stomachand is the maincause for many gastrointestinaldiseases,e.g.,gastriccancer,peptic,duodenaland gastritis ulcer, amongst others [18]. Accordingly, new effective treatmentstrategiestomanagetheseimportanthealthproblems haveesteemedattentions[19].Amidofthemostefficaciousoptions toovercomethesedisorders,thetheorytoinhibitthekeyenzymes involvedinthesepathologiesistheone,wellrecognizedapproach [20].Takingintoconsiderationtheabovestatedparameters,the enzymeinhibitoryabilityofB.glabraaerialextractsweretested againstAChE,BChE,␣-glucosidaseandurease,andthefindingsof theseassaysareillustratedinTable4.Moreover,comparisonof per-centageenzymeinhibitionofbothextractsascomparedtostandard drugsispresentedinFig.2.
Bothtested extractsshowedleastactivityagainstAChE with IC50 of above 5mg/mL. Although, a considerable BChE
inhibi-tionactivitywasrecordedforBMwithIC50valueof0.28mg/mL
(Table 4). According to the photometric and chromatographic analyses, methanol extract was foundto have higher phenolic and flavonoids. Therefore, it can be argued that these bioac-tivemoleculesmayjustifytheobservedBChEinhibitoryactivity. Indeed,previousstudieshavereportedthatphytochemicals, par-ticularlyphenolicand flavonoids,mayexertpowerfuleffects on
Fig.2.Comparisonofpercentageenzymeinhibition(0.5mg/mL)ofB.glabraaerial extracts.EserinewascontrolforAChEandBChE,Acarbosefor␣-glucosidaseand Kojicacidforurease.
cognitivefunctions[21].Ourresultsarealsoconsistentwiththe findings of somepreviousreports [22], which alsopresented a linearassociationamongbioactivecontents andcholinesterases inhibition.
For␣-glucosidaseinhibition,BDpresentedthestrongest inhibi-tionpotentialhavingIC50valueof0.042g/mL.Ontheotherhand,
BMwasleastactive(Table4).Thishigher␣-glucosidaseactivity ofDCMextractmightbeduetoitssignificant phosphomolybde-numactivityandtheexistenceofsomenon-phenoliccompounds like ascorbic acid or tocopherols. Similarly, the observed ␣-glucosidase inhibition ofB. glabraextractscan becorrelated to itshigherflavonoidcontentsaspreviously,ithasbeenreported thatdisaccharidesaretargetsofflavonoidsintheregulationof glu-coseabsorptionandconsequentlyglucosehomeostasisandsome flavonoids,suchasluteolinandkampferolhavepreviouslybeen reportedfor␣-glucosidaseinhibitorypotentials[23].
The urease percentage inhibition of the studied extracts as showninTable4indicatethatBM(IC50;0.24mg/mL)extract
dis-playedthehigheranti-ureaseactivity.Thisobservedanti-urease activitymightbecorrelatedtohigheramountsofflavonoidsinthis extractaspreviously,Awlliaetal.[24]alsoreportedtheactivityof flavonoidsasnaturalinhibitorsofureaseenzyme.Thisworkisthe foremosttoinvestigateonsuchkeyenzymeinhibitionpotentialsof aerialpartsofB.glabra.Takentogether,thefindingsfromthe cur-rentstudycanbeastimulustodevelopnaturalenzymeinhibitors fromthisplantspeciesandcouldopennewhorizonstodesignnovel pharmaceuticals.
3.4. Cytotoxicity
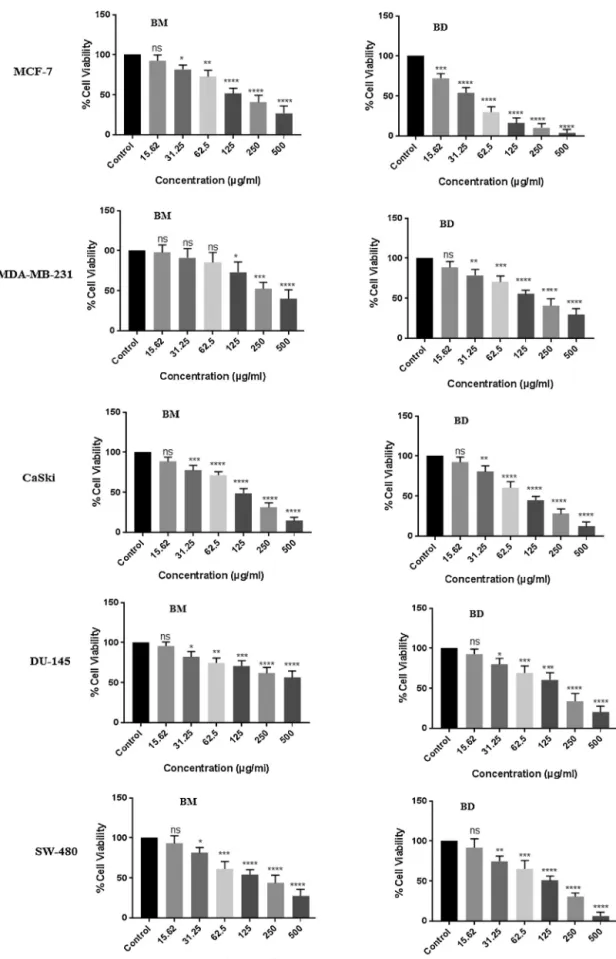
In thepresent work, thecytotoxicity of methanoland DCM extractsofB.glabraaerialpartswereassessedagainstfivedifferent humancancercelllinesi.e.,MCF-7,MDA-MB-231(breastcancer),
Fig.3.CytotoxicityofmethanolandDCMextractsofB.glabraaerialextracts.
Table5
Cytotoxicity(IC50;g/mL)ofB.glabraaerialextracts.
Celllines IC50(g/mL) BM BD MCF-7 109.1 22.09 MDA-MB-231 257.2 127.8 CaSki 176.7 91.88 DU-145 >500** 195.3 SW-480 65.59 214.7
IC50valuerepresentsconcentrationthatreducescellviabilityto50%.**TheIC50
valuewashigherthan500g/mL.
CaSki(cervixcancer),DU-145(prostatecancer),andSW-480(colon
cancer).Thepercentagecellviabilityattheconcentrationsranging
from500-15.625g/mLisdepictedinFig.3.Moreover,theIC50
val-ueswerealsodeterminedandexpressedasmeanoftriplicatesas giveninTable5.
Both theextracts exhibited considerable toxicity against all cell lines with IC50 values ranging from 22.09to 257.2g/mL.
BDwasmostactiveagainstMCF-7(IC50;22.09g/mL)andCaSki
(IC50; 91.88g/mL), whereas, BM extract showed high
anti-proliferativeactivityforSW-480(IC50;65.59g/mL)andMCF-7
(IC50; 109.1g/mL)celllines. Previously,theethanolextract of
B. glabra leaveshas been reported for cytotoxic effects in HT-29,AGS, and BL-13celllines [25].Anotherstudyconducted on differentextractsfrom B.glabra stemsand leavesreportedthe anti-proliferativeactivityagainstU373cells[26].Similarly,Joshny reportedtheanticancer activityofhydro-alcoholic extract ofB. glabraonHelacellswithIC50valueof47.11ug/mL[27].Arecent
studyreportedtheisolatedflavonesfromstembarkofB.spectabilis toshowcytotoxicityagainstfivecancercelllines(KB,HeLaS-3, MCF-7,HT-29,andHepG2)[28].Theobservedcytotoxicactivityof B.glabraextractsmightbelinkedtotheflavonoidsandterpenoids asidentifiedbytheUHPLC-MSanalysis(Table2),asthese com-poundsclassesarealreadyreportedforanticancerandapoptosis inducingcapabilities[29,30].Astoearlierreportscomparison,B. glabrarevealedstrongtomoderatetoxicitypotentialandasfaras literaturesearch,thisistheforemostoutlineregardingcytotoxicity ofB.glabraaerialextractsagainstthesecelllines.
4. Conclusion
In thepresent research,we have presented thebiochemical profilesand cell-toxicitypotentialsof B.glabraaerialparts.Our findingsdemonstratedthemethanolextracttoberichin bioac-tivecompoundsand haveconsiderableantioxidantandenzyme inhibitorypotential.TheDCMextractshowedpotentactivityfor phosphomolybdenumand␣-glucosidaseinhibitionassays. More-over, both extracts showed varying cytotoxic potential against breast,cervical,prostateandcoloncancers.FromtheUHPLC-MS analysis,wefoundthat B.glabra containedmaximumnumbers offlavonoidcompounds.Thesesecondarymetabolitesmayjustify
theobservedbiologicalpotentials.Toconclude,thisplantcontain bioactiveantioxidantandenzymeinhibitorswhichcouldbefurther utilizedindrugdesigning,cosmeticapplicationsandasfood sup-plements.Nevertheless,isolationofpotentialbioactivemolecules fromthisplantsandtheirinvivostudiesarerequired.
Conflictofintereststatement Noconflictofinterest. References
[1]G.Zengin,A.Mollica,M.Z.Aumeeruddy,K.R.Rengasamy,M.F.Mahomoodally, PhenolicprofileandpharmacologicalpropensitiesofGynandririssisyrinchium throughinvitroandinsilicoperspectives,Ind.CropsProd.121(2018) 328–337.
[2]J.PatelChirag,S.Tyagi,N.Halligudi,J.Yadav,S.Pathak,S.P.Singh,A.Pandey, D.Singh,P.S.Kamboj,Antioxidantactivityofherbalplants:arecentreview,J. DrugDiscov.Ther.1(8)(2013)01–08.
[3]Z.R.Khan,F.Moni,S.Sharmin,M.A.Al-Mansur,A.Gafur,O.Rahman,F.Afroz, Isolationofbulkamountofpiperineasactivepharmaceuticalingredient(API) fromblackpepperandwhitepepper(PipernigrumL.),Pharmacol.Pharm.8 (07)(2017)253.
[4]G.Zengin,R.Ceylan,J.Katani ´c,A.Mollica,A.Aktumsek,T.Boroja,S.Mati ´c,V. Mihailovi ´c,S.Stani ´c,Z.Aumeeruddy-Elalfi,M.A.Yilmaz,M.F.Mahomoodally, Combininginvitro,invivoandinsilicoapproachestoevaluatenutraceutical potentialsandchemicalfingerprintsofMoltkiaaureaandMoltkiacoerulea, FoodChem.Toxicol.107(2017)540–553.
[5]S.Markandan,A.Abdullah,K.H.Musa,V.Subramaniam,K.Stockham, Determinationofantioxidantactivities,totalphenolicandflavanoidcontents inBougainvilleaglabrabractsatvariousmethanolconcentrationsin:AIP ConferenceProceedings,AIPPublishing,2016,030038.
[6]E.Edwin,E.Sheeja,E.Toppo,V.Tiwari,K.Dutt,Efectoantimicrobiano, antiulcerosoyantidiarreicodelashojasdebuganvilla(Bougainvilleaglabra Choisy),ArsPharm.48(2)(2007)135–144.
[7]E.Edwin,S.Edwin,A.Amalraj,R.Soni,G.Smita,V.Gupta,Antihyperglycemic activityofBougainvilleaglabra,Choisy,PlantaIndica2(3)(2006)25–26.
[8]H.Saleem,T.T.Htar,R.Naidu,N.S.Nawawi,I.Ahmad,M.Ashraf,N.Ahemad, Biological,chemicalandtoxicologicalperspectivesonaerialandrootsof Filagogermanica(L.)huds:Functionalapproachesfornovel
phyto-pharmaceuticals,FoodChem.Toxicol.123(2019)363–373.
[9]D.M.Grochowski,S.Uysal,A.Aktumsek,S.Granica,G.Zengin,R.Ceylan,M. Locatelli,M.Tomczyk,Invitroenzymeinhibitoryproperties,antioxidant activities,andphytochemicalprofileofPotentillathuringiaca,Phytochem.Lett. 20(2017)365–372.
[10]E.Bendary,R.Francis,H.Ali,M.Sarwat,S.ElHady,Antioxidantand structure–activityrelationships(SARs)ofsomephenolicandanilines compounds,Ann.Agric.Sci.58(2)(2013)173–181.
[11]P.Prieto,M.Pineda,M.Aguilar,Spectrophotometricquantitationof antioxidantcapacitythroughtheformationofaphosphomolybdenum complex:specificapplicationtothedeterminationofvitaminE,Anal. Biochem.269(2)(1999)337–341.
[12]E.J.Llorent-Martínez,G.Zengin,M.L.Fernández-deCórdova,O.Bender,A. Atalay,R.Ceylan,A.Mollica,A.Mocan,S.Uysal,G.O.Guler,Traditionallyused Lathyrusspecies:phytochemicalcomposition,antioxidantactivity,enzyme inhibitoryproperties,cytotoxiceffects,andinsilicostudiesofL.czeczottianus andL.nissolia,Front.Pharmacol.8(2017)83.
[13]G.Zengin,A.Mollica,A.Aktumsek,C.M.N.Picot,M.F.Mahomoodally,Invitro andinsilicoinsightsofCupressussempervirens,Artemisiaabsinthiumand Lippiatriphylla:bridgingtraditionalknowledgeandscientificvalidation,Eur. J.Integr.Med.12(2017)135–141.
[14]S.Itagaki,T.Kurokawa,C.Nakata,Y.Saito,S.Oikawa,M.Kobayashi,T.Hirano, K.Iseki,Invitroandinvivoantioxidantpropertiesofferulicacid:a
comparativestudywithothernaturaloxidationinhibitors,FoodChem.114 (2)(2009)466–471.
[15]G.Zengin,M.Mahomoodally,C.Picot-Allain,Y.Cakmak,S.Uysal,A. Aktumsek,Invitrotyrosinaseinhibitoryandantioxidantpotentialof Consolidaorientalis,OnosmaisauricumandSpartiumjunceumfromTurkey,S. Afr.J.Bot.120(2018)119–123.
[16]M.J.Prince,WorldAlzheimerReport2015:theglobalimpactofdementia:an analysisofprevalence,incidence,costandtrends,Alzheimer’sDis.Int.(2015).
[17]M.C.Picot,G.Zengin,A.Mollica,A.Stefanucci,S.Carradori,M.Mahomoodally, InvitroandinsilicostudiesofmangiferinfromAphloiatheiformisonkey enzymeslinkedtodiabetestype2andassociatedcomplications,Med.Chem. (LosAngeles)13(7)(2017)633–640.
[18]S.Mahernia,K.Bagherzadeh,F.Mojab,M.Amanlou,Ureaseinhibitory activitiesofsomecommonlyconsumedherbalmedicines,Iran.J.Pharm.Res. 14(3)(2015)943.
[19]F.Mao,J.Li,H.Wei,L.Huang,X.Li,Tacrine–propargylaminederivativeswith improvedacetylcholinesteraseinhibitoryactivityandlowerhepatotoxicityas apotentialleadcompoundforthetreatmentofAlzheimer’sdisease,J. EenzymeInhib.Med.Chem.30(6)(2015)995–1001.
[20]A.Mocan,G.Zengin,G.Cris¸an,A.Mollica,Enzymaticassaysandmolecular modelingstudiesofSchisandrachinensislignansandphenolicsfromfruitand leafextracts,J.EenzymeInhib.Med.Chem.31(sup4)(2016)200–210.
[21]D.Vauzour,Effectofflavonoidsonlearning,memoryandneurocognitive performance:relevanceandpotentialimplicationsforAlzheimer’sdisease pathophysiology,J.Sci.FoodAgric.94(6)(2014)1042–1056.
[22]N.A.Mazlan,A.Mediani,F.Abas,S.Ahmad,K.Shaari,S.Khamis,N.Lajis, Antioxidant,antityrosinase,anticholinesterase,andnitricoxideinhibition activitiesofthreeMalaysianMacarangaspecies,Transfus.Apher.Sci.(2013).
[23]B.Na,P.-H.Nguyen,B.-T.Zhao,Q.-H.Vo,B.S.Min,M.H.Woo,Proteintyrosine phosphatase1B(PTP1B)inhibitoryactivityandglucosidaseinhibitoryactivity ofcompoundsisolatedfromAgrimoniapilosa,Pharm.Biol.54(3)(2016) 474–480.
[24]J.A.J.Awllia,M.Al-Ghamdi,E.Huwait,S.Javaid,S.Rasheed,M.Iqbal Choudhary,Flavonoidsasnaturalinhibitorsofjackbeanureaseenzyme,Lett. DrugDes.Discov.13(3)(2016)243–249.
[25]O.Kaisoon,I.Konczak,S.Siriamornpun,Potentialhealthenhancingproperties ofedibleflowersfromThailand,FoodRes.Int.46(2)(2012)563–571.
[26]A.Lamien-Meda,C.E.Lamien,M.M.Compaoré,R.N.Meda,M.Kiendrebeogo,B. Zeba,J.F.Millogo,O.G.Nacoulma,Polyphenolcontentandantioxidantactivity offourteenwildediblefruitsfromBurkinaFaso,Molecules13(3)(2008) 581–594.
[27]J.Joshny,R.Devi,V.Hari,Anti-cancerandAnti-microbialactivityofhydro alcoholicextractofBougainvilleaglabra,Int.J.Curr.Pharm.Rev.Res.3(4) (2013)79–85.
[28]L.T.Do,T.Aree,P.Siripong,N.T.Vo,T.T.Nguyen,P.K.Nguyen,S.Tip-pyang, CytotoxicflavonesfromthestembarkofBougainvilleaspectabilisWilld, PlantaMed.84(02)(2018)129–134.
[29]F.Kirollos,S.Elhawary,O.Salama,Y.Elkhawas,LC-ESI-MS/MSandcytotoxic activityofthreePistaciaspecies,Nat.Prod.Res.(2018)1–4.
[30]A.Choudhary,A.K.Mittal,M.Radhika,D.Tripathy,A.Chatterjee,U.C. Banerjee,I.P.Singh,Twonewstereoisomericantioxidanttriterpenesfrom Potentillafulgens,Fitoterapia91(2013)290–297.