Contents lists available atScienceDirect
Archives of Oral Biology
journal homepage:www.elsevier.com/locate/archoralbio
Immune responses in women with periodontitis and preterm low birth
weight: Levels of CD4+ and CD8+ T cells in gingival granulation tissue
Nezahat Arzu Kayar
a,⁎,
İlhami Çelik
b, Nilgün Özlem Alptekin
caAkdeniz University Faculty of Dentistry, Department of Periodontology, Antalya, 07058, Turkey bSelcuk University Faculty of Veterinary Medicine, Department of Biochemistry, Konya, Turkey cBaşkent University Faculty of Dentistry, Department of Periodontology, Ankara, Turkey
A R T I C L E I N F O Keywords:
Preterm birth Low birth weight CD4+ CD8+
Gingival granulation tissue
A B S T R A C T
Objective: Preterm Low-Birth-Weight (PLBW) is frequently associated with periodontal disease. However, the mechanism is still unknown. The present study was performed to examine the possible link between periodontal infections and PLBW in post-partum women utilizing clinical parameters and CD4+ and CD8 + T lymphocytes ratio in gingival granulation tissue.
Materials: The tissues used in this study consisted of 35 gingival granulation tissue biopsies from 35 mothers of healthy infants (HTBW), 35 biopsies of gingival granulation tissue from 35 mothers of PLBW within one month postpartum and gingival tissue biopsies from 7 control individual with no periodontal disease (HC). CD4+ and CD8 + T lymphocyte ratios in a unit area of the gingival granulation tissue were determined by hystometrically. Statistical analysis was performed by using Kruskal-Wallis and Mann-Whitney U tests.
Results: CD8 + T lymphocytes were more prevalent in the PLBW group than in the HTBW and HC group (P < 0.05). The CD4+/CD8+ ratio in the PLBW group was lower than those of the other groups (p < 0.05). There were no statistically significant differences in CD4 + T lymphocytes counts between all groups (P > 0.05).
Conclusion: Within the limits of this study it can be concluded that CD8 + T lymphocytes in gingival tissue may play important roles in the pathogenesis of periodontitis and PLBW.
1. Introduction
Periodontitis mainly results from dental plaque, however other contributory factors are necessary (Kurgan & Kantarci, 2018). The bacterial dental plaque persuades an inflammatory infiltrate in peri-odontal tissue, in which the effective immune cell populations may diverge from person to person. The magnitude of tissue destruction in response to biofilm is largely determined by the response of the host.
Adaptive immune responses through activated CD4 + T helper/in-ducer (Th/i) cells and secretion of immunoglobulin by B cells also play a
part in containment of the biofilm, however, paradoxically, may also be drivers of disease progression (Baker et al., 1999; Baker, Howe, Garneau, & Roopenian, 2002). CD4 + Th/i lymphocytes facilitate
dif-ferentiation of B cells to plasma cells producing specific antibodies, whereas CD8+ cytotoxic/suppressor T lymphocytes (Tc/s) kill virally
infected cells. There is a fact that the healthy gingiva contains a CD4+/ CD8+ (Th/i /Tc/s) ratio comparable to that of the peripheral blood
(Okada, Kida, & Yamagami, 1982;Stoufi, Taubman, Ebersole, Smith, & Stashenko, 1987). However, a reduced CD4+/CD8+ ratio has been described in periodontal lesions, than that of the peripheral blood (Okada et al., 1982;Taubman, Stoufi, Ebersole, & Smith, 1984).
Pregnancy already imposes an immunological challenge on the host, since direct contact of circulating and uterine immune cells with pla-cental tissue requires adaptations by the maternal immune system to maintain tolerance to the fetus (Kieffer, Laskewitz, Scherjon, Faas, & Prins, 2019; Veenstra van Nieuwenhoven, Heineman, & Faas, 2003). Yet, the role of T lymphocytes in the pathogenesis of preterm birth (PTB) associated with intrauterine infection and inflammation remains relatively understudied. It is known that the immune system is com-promised during a healthy pregnancy. Memory-like CD4 + Th/i
lym-phocytes are also abundant at the human maternal–fetal interface during spontaneous labor at term (Gomez-Lopez et al., 2013;Kieffer et al., 2019). Pregnancy-driven CD4 + Th/i lymphocytes can exhibit
effector or regulatory functions (Gomez-Lopez, StLouis, Lehr,
Sanchez-https://doi.org/10.1016/j.archoralbio.2019.104551
Received 28 May 2019; Received in revised form 9 August 2019; Accepted 8 September 2019 Abbreviations: PLBW, Preterm Low Birth-Weight; HTBW, Healthy Term Infants; HC, Healty Control
⁎Corresponding author.
E-mail address:narzu@hotmail.com(N.A. Kayar).
0003-9969/ © 2019 Published by Elsevier Ltd.
Rodriguez, & Arenas-Hernandez, 2014). T lymphocyte responses may also be impaired in the mechanisms that lead to PTB since CD8 + Th/i
lymphocytes are present at the maternal– fetal interface of women with chronic chorioamnionitis (Lee et al., 2011) which is a condition asso-ciated with spontaneous PTB (Kim et al., 2010).
Based on published datas, there is an association between period-ontal diseases and increased risk of preterm low birth-weight (PLBW) (Kayar, Alptekin, & Erdal, 2015;Konopka & Paradowska-Stolarz, 2012; Radochova et al., 2019), although the mechanisms for the various re-lationships remain largely unknown. CD4 + Th/i regulatory cells
manage the local microenvironment at the maternal–fetal interface through their anti-inflammatory functions. When a microbial infection is present, this regulation is disrupted and premature expulsion of the neonate occurs (Arenas-Hernandez et al., 2016). We thus hypothesized that persistent presence of periodontal pathogens in gingival tissue causes chronic inflammation and that a skewed immune response to-ward humoral immunity is a risk factor for preterm birth. This study aimed to evaluate the levels of CD4+ and CD8+ in gingival tissue of mothers with delivery PLBW infants, mothers with delivery healthy term infants (HTBW) infants and healthy control individuals (HC). 2. Materials and methods
2.1. Study population
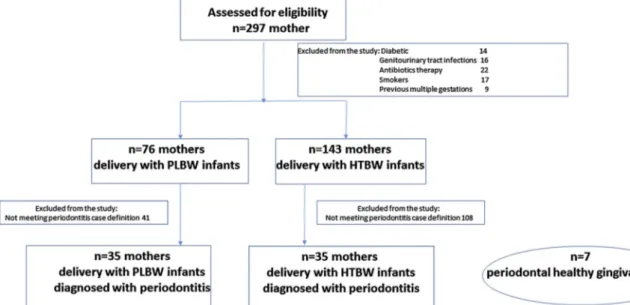
Two hundred ninety-seven mothers who gave birth at Dr. Faruk Sükan Maternity and Child Birth Hospital, Konya, Turkey were ex-amined during 7 months. Hospital was visited regularly 2 days a week. Information regarding birth weight, gestational period and delivery details were collected from patients’ medical records. The selection process of the patients is shown inFig. 1. The following exclusion cri-teria were then implemented to minimize the selection of women pre-senting risk factors to preterm/low-birth-weight: history of diseases including diabetes mellitus, genitourinary tract infections or HIV in-fection, previous multiple gestations, current/past tobacco user, pre-vious preterm birth/low-birth-weight infants, in vitro fertilization procedures, placental or cervical or uterine abnormalities, history of infertility, history of illegal drug use, history of human im-munodeficiencyvirus type I or hemoglobin levels less than 8 mg/dl following delivery and medical conditions that required antibiotics prophylaxis. Periodontal examinations were performed by profession-ally trained periodontists (NOA) in their hospital room within thefirst 24 h after delivery. Inclusion criteria for periodontitis patients were the
presence of at least two sites with≥5 mm probing depth (PD) and clinical attachment level (CAL) (excluding third molars and distal sur-faces of second molars), no history of initial periodontal therapy in-cluding scaling and root planing at least six weeks before the study. Mothers of 35 healthy term infants (≥37 weeks gestational period) (mean age 23.2 ± 2.9, range 18–31 years) and 35 preterm (< 37 weeks gestational period) low birth weight infants (≤2.500 g) (mean age 22.8 ± 3.3, range 18–30 years) were included in the study.
This study was approved by the Research Ethics Committee of Selcuk University Faculty of Dentistry (2008/1-1). Written informed consent was obtained from all participants. The study was registered at Thai Clinical Trials.gov (TCTR20190407001).
2.2. Clinical periodontal evaluation
Another professionally trained periodontist (NAK), masked to the patient’s group affiliation, performed all periodontal measurements as well as granulation tissue biopsies within thefirst 48 h after delivery. The following periodontal clinical parameters were recorded: plaque index (Loe, 1967), gingival index (Loe & Silness, 1963), bleeding on probing (Ainamo & Bay, 1975), probing pocket depth and clinical at-tachment level.
2.3. Granulation tissue biopsy
Following the clinical periodontal evaluation, granulation tissue biopsies (3 × 3 mm in size) from the periodontal interdental sulcus region with gingival index≥2, pocket depth ≥5 mm and showing BOP, including gingival epithelium and ligament, were obtained during subgingival curettage. The gingival biopsies were divided into two parts. One part was placed in 10% formal-saline and the other part in formol sucrose.
Periodontal healthy gingival tissue (HC) was used as healthy re-ference tissue. For this purpose, the gums around the teeth of seven female patients (mean age 23 ± 1.5, range 21–25 years) who under-went tooth extraction due to the orthodontic reasons. All biopsies were from clinically healthy sites defined by the following criteria: no loss of attachment (CAL < 3 mm), PPD≤ 3 mm and BOP negative.
2.4. Tissue sampling, processing, and histologic evaluation
The gingival tissue samples were divided into two pieces. One of the pieces wasfixed in formal-sucrose and kept in Holt’s solution for for
Fig. 1. Flow chart of the inclusion and exclusion of participants.
additional 22 h. Frozen sections in 12μm thickness were taken with cryostat (Slee, London,UK), and a T-lymphocyte specific enzyme, alpha-naphthyl acetate esterase (ANAE) was demonstrated in the frozen sec-tions (Knowles & Holck, 1978). For this purpose a reaction medium was prepared by mixing 40 ml of 0.067 M phosphate buffer (pH 5.0), 2.4 ml of hexazotized pararosaniline (Sigma, St. Louis, USA, C.I.N. 42500) and 10 mg of alpha-naphthyl acetate (Sigma, N-8505) in 0.4 ml acetone. The pH of thefinal medium was adjusted to 5.8 using 2 N NaOH. The frozen sections were incubated in the reaction solution for controlled periods. Following incubation, the sections were washed in distilled water and counterstained with 1% methyl green (Merck, Darmstadt, Germany, C.I.N. 2585) in 0.1 M acetate buffer pH 4.2 for 10 min. For specific determination of macrophages and T lymphocytes, sodium fluoride inhibition was also performed by adding 0.1% sodium fluoride into the incubation medium. Following dehydration in increasing concentrations of ethanol, the slides were cleaned in xylene and mounted with synthetic mounting medium (Entellan, Merck, Darm-stadt, Germany). The lymphocytes showing 1–4 reddish-brown reaction products in the cytoplasm were scored as ANAE + . Macrophages dis-played a diffuse and fine granular cytoplasmic staining pattern. In each specimen, ANAE-positive cells were counted in unit area (283490.27 square micrometers) and the mean values of the groups were expressed as cell count/unit area (CC/UA).
The second half of each gingival sample was fixed in buffered formal-saline (0.1 M, pH 7.4) and immersed in paraffin wax using routine histological methods. From each tissue block, four sections in 6μm thickness were taken, and one section was stained with a modified Giemsa stain, the second one with trichrome stain (Culling et al., 1985), and the remaining two sections were used for immunohistochemical demonstration of either CD4+ or CD8 + T lymphocytes. For this pur-pose, specific anti-human CD4 and CD8 primary antibodies
(Monoclonal Mouse anti-human CD8, Clone no C8/144 B (Dako); Monoclonal Mouse anti-human CD4 Ab-8, Clone no 4B12 (Lab Vision/ NeoMarkers, California, USA) and Dako cytomation LSAB 2 System-HRP (Biotinylated link streptavidin-System-HRP Ref. K0675) Detection Kit (Agilent Dako, California, USA) was used. Briefly, tissue sections mounted on poly-L-lysine coated glass slides were deparaffinized, de-hydrated in graded alcohols and rede-hydrated in distilled water. Endogenous peroxidase was inhibited by immersing the sections for 1/ 2 h in 3% hydrogen peroxide in phosphate buffer (0.1 M, pH 7.4). Following, the sections were incubated for 20 min in 3% (v/v) bovine serum albumin (BSA) prepared with phosphate buffer, and then stirred 3 times in phosphate buffer. Producer’s recommendations for primary and secondary monoclonal antibody dilutions, antigen retrieval condi-tions in the microwave oven, and incubation periods were strictly fol-lowed. Horseradish peroxidase (HRP) activity was detected by immer-sing the sections into the incubating solution prepared by mixing 1 mg/ ml diaminobenzidine (DAB), 1 ml of 50 mM Tris HCL and 20μl of 3% H2O2. Thefinal pH of the incubating solution was adjusted to 7.3 and
the sections were treated with this solution 20 min. Nuclei were stained with Harris’s haematoxylin. The specimens were observed at high magnification (X1000) and the cells having lymphocyte morphology and displaying brownish membrane HRP positivity were considered as positive for the primary antibody (CD4+ or CD8+) according to what antibody was used. In each specimen, both CD4+ and CD8+ cells were counted in unit area (283490.27 square micrometer) and the mean values of the groups were expressed as cell count/unit area (CC/UA). 2.5. Statistical analysis
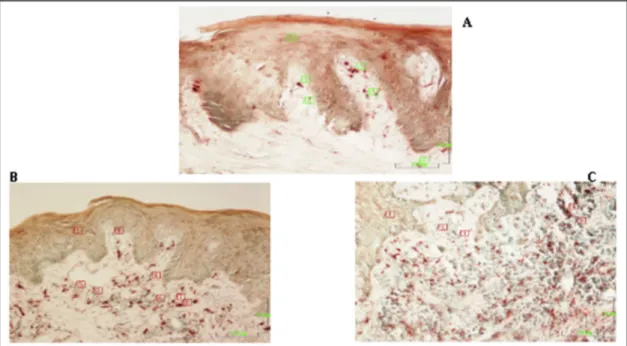
The descriptive analysis of the data was performed to characterize the participants of this study and then comparisons were done between Fig. 2. A: A section of gingival tissue from a healthy group. Keratinized stratified squamous epithelium covers the surface. Keratin (1) is relatively thick and epithelium contains cell layers. Sections of dermal papillae (3) are frequent in the epithelium. Basal epithelial cells (5) are polymorphic cells. Lamina propria is mainly occupied with thick collagenfibers (5). Trichrome staining. Magnification bar: 100 μm.
B: A section of gingival tissue from the HTBW group with periodontitis. Keratin (1) and stratified squamous epithelium (2) relatively thin, dermal papillae (3,4) are enlarged. Lamina propria is edematous and both the epithelium and connective tissue the is infiltrated with mononuclear cells evidencing weak inflammatory changes. Trichrome staining. Magnification bar: 100 μm.
C: A section from PLBW group with periodontitis. The gingival epithelium (1) is immature as evidenced by the lack of the keratin layer. Mild edema, increased vascularization, infiltration of both epithelium and connective tissue infiltrated with mononuclear cells indicate moderate inflammatory changes. Trichrome satining. Magnification bar: 100 μm.
their mean CD4+ and CD8 + T lymphocyte levels. The Kruskal-Wallis test was used for the comparison of the mean levels of CD4+ and CD8 + T lymphocytes, CD4+/CD8+ ratio among all groups. The Mann-Whitney U test was used for comparisons between pairs. The Bonferroni test was performed for multiple comparison correction P values≤ 0.016 were considered statistically significant.
3. Results
In the gingival tissue of the healthy group, keratinized strati-fiedsquamous epithelium covered the surface. Keratin was re-lativelythick and epithelium contained cell layers. Sections of dermal papillae were frequent in the epithelium. Basal epithelial cells had polymorphic shapes. Lamina propria was mainly occupied with thick collagenfibers (Fig. 2A).Gingival tissue of the HTBW group with peri-odontitis had relatively thinner stratified squamous epithelium with a thin keratin layer, dermal papillae were enlarged. Lamina propria was edematous and infiltrated with mononuclear cells indicating weak in-flammatory changes (Fig. 2B). In the sections of PLBW group with periodontitis, the gingival epithelium was immature as evidenced by the lack of the keratin layer. Connective tissue displayed moderate in-flammatory changes evidenced by mild edema, increased vasculariza-tion and infiltravasculariza-tion of both epithelium and connective tissue infiltrated with mononuclear cells (Fig. 2C).
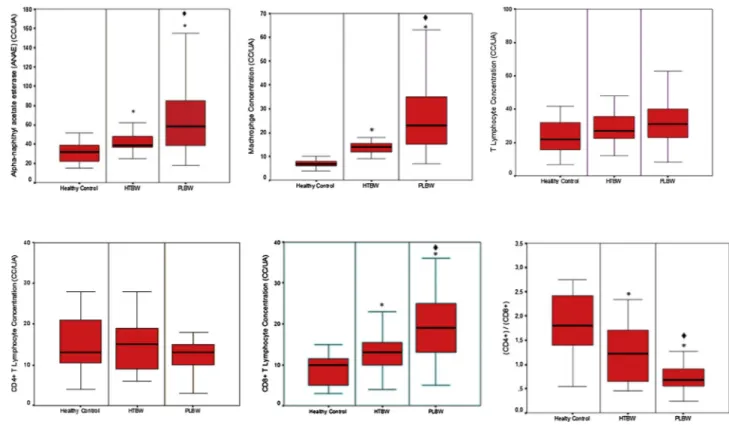
ANAE positive cells were found to be heavily involved in the con-nective tissue. ANAE staining results with sodiumfluoride inhibition of the individuals of HTBW and ND groups were shown inFig. 3B and C respectively. In the HTBW and HTBW groups, intense infiltration tissue was observed in the deeper regions of epithelium and connevtive tis-sues. The rate of ANAE positive lymphocytes in the HTBW group was higher than that of the HTBW group (p < 0.05, Fig. 4). The lowest ANAE positivity level was in healthy controls (p < 0.05) (Fig. 4).
Macrophage levels in the biopsy samples taken from HTBW groups were higher than the healthy control group and HTBW group (p < 0.05,Fig. 5A–C). T lymphocytes and CD4 + T lymphocyte counts did not significantly differ (p > 0.05) between the groups (Figs. 4B, C,
5A). CD8 + T lymphocyte counts in the HTBW group was significantly higher (p < 0.05) than those of the HTBW and healthy controls (Figs. 6A–C, 4). The highest CD8+ ratio was found in the healthy control group (p < 0.05) (Fig. 4). The CD4+/CD8+ ratio in the HTBW group was lower than those of the other groups (p < 0.05).
4. Discussion
In the present study, the levels of CD4+ and CD8 + T lymphocytes and CD4+/CD8+ ratio in the gingival tissues of mothers with peri-odontitis who delivered PLBW and HTBW infants. The majorfindings of this study were the observation of the highest CD8 + T lymphocyte levels and consequently the lowest CD4+/CD8+ ratio in the PLBW group diagnosed with periodontitis. An important feature of the in-flammatory response is T lymphocyte activation and it causes an im-balance between innate and adaptive immune cells at the ma-ternal–fetal interface is therefore critical. In this study, we also compared the immune response in the periodontal tissues of PLBW, HTBW and HC groups. The number of CD8 + T lymphocytes, ANAE + cells and macrophages significantly increased in both mothers of HTBW and PLBW diagnosed with periodontitis in comparison to the HC individuals. According to these results, it can be suggested that the changes in the levels of T lymphocyte subgroups, especially in CD8 + T lymphocyte levels may play an important role both in delivery with PLBW and periodontitis. Besides, alterations in the CD4 + T lympho-cyte level in gingival biopsies from healthy sites in the HC group were not found to be significantly different than those in gingival biopsies from periodontitis sites of HTBW and PLBW groups.
It was considered that distortions in the Th1/ Th2cell-ratio in
gin-gival tissues accompany the development and pathogenesis of period-ontitis (Stoufi et al., 1987). CD4 + T lymphocytes and cytokines play important roles in the adaptive immune responses and the pathogenesis of the periodontal disease (Baker et al., 1999;Wassenaar, Reinhardus, Abraham-Inpijn, Snijders, & Kievits, 1998). In a previous study, the researchers (Stoufi et al., 1987) reported that CD4 + T lymphocyte le-vels decreased (consequently CD4+/CD8 + T-cell ratio reduced),
Fig. 3. A: A section from healthy gingiva. Epithelium (1), lamina propria (2). ANAE positive cells (3,4,5) are mainly located in the connective tissue. (ANAE) staining. Magnification bar: 100 μm.
B: A section from inflamed gingiva from the HTBW group with periodontitis. The epithelium (1) covers the mildly inflamed edematous connective tissue (2), which is populated with ANAE positive cells (3–7). (ANAE) staining. Magnification bar: 100 μm.
C: A section from inflamed gingiva from the PLBWgroup with periodontitis. The connective tissue is highly populated with ANAE positive cells (3,4) and an ANAE-negative (5) cell is also seen. (ANAE) staining. Magnification bar: 100 μm.
whereas CD8 + T lymphocytes increased in inflammatory gingival tis-sues and/or peripheral blood. Nevertheless,Suarez, Ocampo, Duenas, and Rodriguez (2004) found that the decline in CD4+/CD8 + T
lymphocyte ratio directly correlates with the decrease of CD4 + T lymphocytes in gingival tissues (Suarez et al., 2004), while others have shown that this decrease has arisen from the increase of CD8 + T Fig. 4. Frequency and differences of T-cell populations of the Healty Control, HTBW and PLBW groups.
* p < 0016; Mann-Whitney U test with Bonferroni Correction; Comparison with healthy control. ♦ * p < 0016; Mann-Whitney U test with Bonferroni Correction; Comparison with HTBW.
Fig. 5. A: CD4+ cells in a section from gingiva of a healthy group. (3,4) Gingival epithelium (1), lamina propria (2), CD4+ cells (3,4). Immunohistochemical staining. Magnification bar: 100 μm.
B: CD4+ cells in the gingival section from the HTBW group with gingivitis. The epithelium (1) and connective tissue (2) are seen. CD4+ lymphoid cells are seen especially between basal epithelial cells (4) and in the connective tissue (5). Immunohistochemical staining. Magnification bar: 100 μm.
C: CD4+ cells in a low power magnification of the gingival section from the PLBW group with gingivitis. The deeper regions of the epithelium (1) and connective tissue (2) are also seen. Intensive mononuclear inflammatory cell infiltration is evident. CD4+ cells are especially located between basal epithelial cells (3) and in the connective tissue (5). Immunohistochemical staining. Magnification bar: 100 μm.
lymphocytes in the crevice of patients (Gemmell, Carter, & Seymour, 2001;Sigusch, Wutzler, Nietzsch, & Glockmann, 2006). Interestingly, incompatible with all,Petit et al. (2001)showed that peripheral blood CD8 + T lymphocyte counts and CD4+/CD8+ ratio were similar in both severe periodontitis and gingivitis groups. The reason for these contradictory results may be the differences in the periodontal tissue health of the individuals included in the study and the difference be-tween the tissue samples examined.
Based on the hypothesis that, among the several suspected period-ontal pathogens, Porphyromonas gingivalis (P. gingivalis) may be effective in the production of proinflammatory cytokines.Choi, Kang, Park, Kim, and Ki, (2004)andChung et al. (2003) showed the presence of heat shock protein (HSP) in the gingival connective tissue of individuals diagnosed with periodontitis and isolated P. gingivalis. In the light findings above, it has been suggested that P. gingivalis may cause in-filtration of peripheral blood T lymphocytes into the gingival tissues and thus the gingivalfibroblasts would create a cross-T-cell response to HSP. In this study, the observation of the highest CD8 + T lymphocyte counts in both HTBW and PLBW groups with periodontal disease, may be related to the higher incidence of P. gingivalis in infected gum tissues. Therefore, susceptibility traits might not be found locally in the peri-odontal lesion.
A role for regulatory T lymphocytes in the maintenance of foetal tolerance and pregnancy has also been defined, with particular im-portance placed on a T helper 2 cytokine response (Saito, Nakashima, Shima, & Ito, 2010). Yet, the role of T lymphocytes in the pathogenesis of preterm birth associated with intrauterine infection and in flamma-tion remains not understood in detail. Endotoxin administraflamma-tion to the pregnant mice did not alter functions of effector CD4 + T lymphocytes at the maternal-fetal interface. However, it caused an expansion of CD8+ effector T lymphocytes, which participate in the maternal pro-inflammatory response that leads to PTB (Arenas-Hernandez et al.,
2016). An increase in both systemic and peripheral cytotoxic CD8 + T lymphocyte activity has been described in pregnant women with chronic chorioamnionitis and this rise increased systemic cytotoxic capacity as a manifestation of maternal antifetal rejection (Xu et al., 2012). Theoretically, intrauterine malnutrition that often affects fetuses in preterm birth pregnancies, maybe a factor delaying or inhibiting the maturation of immune cell types. In a previous research, (Jacques & Qureshi, 1998) immunohistochemically showed that CD8+ cells pre-sent in moderate numbers, whereas CD4+ cells in smaller numbers in areas of chronic chorioamnionitis. We found no differences in the T cell and CD4 + T lymphocyte counts among HTBW and PLBW groups. Ac-cording to the results of the present study, it can be argued that CD8 + T lymphocyte level which was higher in the PLBW group may cause low "regulated on activation normal T-cell expressed and se-creted" (RANTES) level and consequently weaken the host immunity in HTBW group. However, previous studies have demonstrated that CD8 + T cells are implicated in the timing of term parturition ( Gomez-Lopez, Olson, & Robertson, 2016) and endotoxin-induced preterm labor/birth (Arenas-Hernandez et al., 2016;Gomez-Lopez et al., 2017). Altogether, these data suggest that a reduction in the number of these cells may be associated with preterm labor/birth cause of periodontal disease.
There were some limitations to this study. First, the study was performed in only maternal gingival tissues, although the foetal circu-lating lymphocytes can be adversely affected by pregnancy outcome. Second, investigations of other immune cells and inflammatory cyto-kines also will be needed to clarify the mechanisms of immune re-sponses required to maintain healty pregnancy and periodontal healthy. 5. Conclusions
An important feature of the inflammatory response is T lymphocyte Fig. 6. A: CD8+ cells in the gingival section from a healthy group. Epithelium (1) and connective tissue and CD8+ cells (2,3) are seen. Immunohistochemical staining. Magnification bar: 100 μm.
B: CD8+ cells in the gingival section from the HTBW group with periodontitis. Epithelium (1) and the inflamed connective tissue (2) are clearly seen. CD8+ lymphoid cells are located mainly between basal epithelial cells (1) and in the connective tissue (2). Immunohistochemical staining. Magnification bar: 100 μm. C: CD8+ cells in the gingival section from the PLBW group with periodontitis. Intensive mononuclear inflammatory cell infiltration is evident. CD8+ positive lymphoid cells with brownish membrane staining are seen in the connective tissue. Immunohistochemical staining. Magnification bar: 100 μm.
activation and the expression of early and late activation markers on T lymphocytes. (Sava & Toldi, 2016) Within the limitations of this study, significant differences were observed between the host immune re-sponses of the PLBW and HTBW groups diagnosed with periodontitis arising from the increase in CD8 + T lymphocyte level in gingival tissue and the decrease in the CD4+/CD8+ ratio, which was an important indicator of the alteration of the host defense system. A potential benefit derived from this study could be the early identification of the women at risk with periodontitis to apply appropriate periodontal treatment in need, and likely to avoid unexpected pregnancy outcomes. These findings raise the possibility of using pregnant woman’s oral health history in part of her obstetric care record aiming to prevent from undesired pregnancy outcome. Within the limits of this study, it can be concluded that CD8 + T lymphocytes in gingival tissue may play important roles in the pathogenesis of periodontitis and PLBW. In-vestigations of other immune cells and inflammatory cytokines also will be needed to clarify the mechanisms of periodontal health which is required to maintain perinatal health. Further studies with a more de-tailed immunological assessment of the periodontal tissue may also provide information on the prognostic role to yield a healthy delivery. Funding
This study was supported by grants from the Selcuk University Scientific Research Projects (2003065).
Declaration of Competing Interest
The authors declare that they have no conflict of interest. References
Ainamo, J., & Bay, I. (1975). Problems and proposals for recording gingivitis and plaque. International Dental Journal, 25(4), 229–235.
Arenas-Hernandez, M., Romero, R., St Louis, D., Hassan, S. S., Kaye, E. B., & Gomez-Lopez, N. (2016). An imbalance between innate and adaptive immune cells at the maternal-fetal interface occurs prior to endotoxin-induced preterm birth. Cellular & Molecular Immunolog, 13(4), 462–473.
Baker, P. J., Dixon, M., Evans, R. T., Dufour, L., Johnson, E., & Roopenian, D. C. (1999). CD4(+) T cells and the proinflammatory cytokines gamma interferon and inter-leukin-6 contribute to alveolar bone loss in mice. Infection and Immunity, 67(6), 2804–2809.
Baker, P. J., Howe, L., Garneau, J., & Roopenian, D. C. (2002). T cell knockout mice have diminished alveolar bone loss after oral infection with Porphyromonas gingivalis. FEMS Immunology and Medical Microbiology, 34(1), 45–50.
Choi, J. I., Kang, H. S., Park, Y. M., Kim, S. J., & Kim, U. S. (2004). Identification of T-cell epitopes of Porphyromonas gingivalis heat-shock-protein 60 in periodontitis. Oral Microbiology and Immunology, 19(1), 1–5.
Chung, S. W., Kang, H. S., Park, H. R., Kim, S. J., Kim, S. J., & Choi, J. I. (2003). Immune responses to heat shock protein in Porphyromonas gingivalis-infected periodontitis and atherosclerosis patients. Journal of Periodontal Research, 38(4), 388–393.
Gemmell, E., Carter, C. L., & Seymour, G. J. (2001). Chemokines in human periodontal disease tissues. Clinical and Experimental Immunology, 125(1), 134–141.
Gomez-Lopez, N., Olson, D. M., & Robertson, S. A. (2016). Interleukin-6 controls uterine Th9 cells and CD8(+) T regulatory cells to accelerate parturition in mice. Immunology and Cell Biology, 94(1), 79–89.
Gomez-Lopez, N., Romero, R., Arenas-Hernandez, M., Schwenkel, G., St Louis, D., Hassan, S. S., et al. (2017). In vivo activation of invariant natural killer T cells induces sys-temic and local alterations in T-cell subsets prior to preterm birth. Clinical and Experimental Immunology, 189(2), 211–225.
Gomez-Lopez, N., StLouis, D., Lehr, M. A., Sanchez-Rodriguez, E. N., & Arenas-Hernandez, M. (2014). Immune cells in term and preterm labor. Immunology and Cell
Biology, 11(6), 571–581.
Gomez-Lopez, N., Vega-Sanchez, R., Castillo-Castrejon, M., Romero, R., Cubeiro-Arreola, K., & Vadillo-Ortega, F. (2013). Evidence for a role for the adaptive immune response in human term parturition. American Journal of Reproductive Immunology, 69(3), 212–230.
Jacques, S. M., & Qureshi, F. (1998). Chronic chorioamnionitis: A clinicopathologic and immunohistochemical study. Human Pathology, 29(12), 1457–1461.
Kayar, N. A., Alptekin, N. O., & Erdal, M. E. (2015). Interleukin-1 receptor antagonist gene polymorphism, adverse pregnancy outcome and periodontitis in Turkish women. Archives of Oral Biology, 60(12), 1777–1783.
Kieffer, T. E. C., Laskewitz, A., Scherjon, S. A., Faas, M. M., & Prins, J. R. (2019). Memory t cells in pregnancy. Frontiers in Immunology, 10, 625.
Kim, C. J., Romero, R., Kusanovic, J. P., Yoo, W., Dong, Z., Topping, V., Gotsch, F., Yoon, B. H., Chi, J. G., & Kim, J. S. (2010). The frequency, clinical significance, and pa-thological features of chronic chorioamnionitis: A lesion associated with spontaneous preterm birth. Modern Pathology, 23(7), 1000–1011.
Knowles, D. M., 2nd, & Holck, S. (1978). Tissue localization of T-lymphocytes by the histochemical demonstration of acid alpha-naphthyl acetate esterase. Laboratory Investigation, 39(1), 70–76.
Konopka, T., & Paradowska-Stolarz, A. (2012). Periodontitis and risk of preterm birth and low birthweight–a meta-analysis. Ginekologia Polska, 83(6), 446–453.
Kurgan, S., & Kantarci, A. (2018). Molecular basis for immunohistochemical and in-flammatory changes during progression of gingivitis to periodontitis. Periodontology 2000, 76(1), 51–67.
Lee, J., Romero, R., Dong, Z., Xu, Y., Qureshi, F., Jacques, S., Yoo, W., Chaiworapongsa, T., Mittal, P., Hassan, S. S., & Kim, C. J. (2011). Unexplained fetal death has a bio-logical signature of maternal anti-fetal rejection: Chronic chorioamnionitis and al-loimmune anti-human leucocyte antigen antibodies. Histopathology, 59(5), 928–938.
Loe, H. (1967). The gingival index, the plaque index and the retention index systems. Journal of Periodontology, 38(6), 610–616.
Loe, H., & Silness, J. (1963). Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontologica Scandinavica, 21, 533–551.
Okada, H., Kida, T., & Yamagami, H. (1982). Characterization of the immunocompetent cells in human advanced periodontitis. Journal of Periodontal Research Supplement, 17(5), 472–473.
Petit, M. D., Hovenkamp, E., Hamann, D., Roos, M. T., van der Velden, U., Miedema, F., et al. (2001). Phenotypical and functional analysis of T cells in periodontitis. Journal of Periodontal Research, 36(4), 214–220.
Radochova, V., Stepan, M., Kacerovska Musilova, I., Slezak, R., Vescicik, P., Menon, R., Jacobsson, B., & Kacerovsky, M. (2019). Association between periodontal disease and preterm prelabour rupture of membranes. Journal of Clinical Periodontology, 46(2), 189–196.
Saito, S., Nakashima, A., Shima, T., & Ito, M. (2010). Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. American Journal of Reproductive Immunology, 63(6), 601–610.
Sava, F., & Toldi, G. (2016). Expression of lymphocyte activation markers of preterm neonates is associated with perinatal complications. BMC Immunology, 17(1), 19.
Sigusch, B. W., Wutzler, A., Nietzsch, T., & Glockmann, E. (2006). Evidence for a specific crevicular lymphocyte profile in aggressive periodontitis. Journal of Periodontal Research, 41(5), 391–396.
Stoufi, E. D., Taubman, M. A., Ebersole, J. L., Smith, D. J., & Stashenko, P. P. (1987). Phenotypic analyses of mononuclear cells recovered from healthy and diseased human periodontal tissues. Journal of Clinical Immunology, 7(3), 235–245.
Suarez, L. J., Ocampo, A. M., Duenas, R. E., & Rodriguez, A. (2004). Relative proportions of T-cell subpopulations and cytokines that mediate and regulate the adaptive im-mune response in patients with aggressive periodontitis. Journal of PeriodontologyJ, 75(9), 1209–1215.
Taubman, M. A., Stoufi, E. D., Ebersole, J. L., & Smith, D. J. (1984). Phenotypic studies of cells from periodontal disease tissues. Journal of Periodontal Research, 19(6), 587–590.
Veenstra van Nieuwenhoven, A. L., Heineman, M. J., & Faas, M. M. (2003). The im-munology of successful pregnancy. Human Reproduction Update, 9(4), 347–357.
Wassenaar, A., Reinhardus, C., Abraham-Inpijn, L., Snijders, A., & Kievits, F. (1998). Characteristics of Prevotella intermedia-specific CD4+ T cell clones from peripheral blood of a chronic adult periodontitis patient. Clinical and Experimental Immunology, 113(1), 105–110.
Xu, Y., Tarquini, F., Romero, R., Kim, C. J., Tarca, A. L., Bhatti, G., Lee, J., Sundell, I. B., Mittal, P., Kusanovic, J. P., Hassan, S. S., & Kim, J. S. (2012). Peripheral CD300a +CD8+ T lymphocytes with a distinct cytotoxic molecular signature increase in pregnant women with chronic chorioamnionitis. American Journal of Reproductive Immunology, 67(3), 184–197.