Contents lists available atScienceDirect
Industrial Crops & Products
journal homepage:www.elsevier.com/locate/indcropSyzgium coriaceum Bosser & J. Guého
—An endemic plant potentiates
conventional antibiotics, inhibits clinical enzymes and induces apoptosis in
breast cancer cells
Mohamad Fawzi Mahomoodally
a,*
, Asli Ugurlu
b, Eulogio J. Llorent-Martínez
c,
Meenathee Nagamootoo
a, Marie Carene Nancy Picot-Allain
a, Mehmet Cengiz Baloglu
d,
Yasemin Celik Altunoglu
d, Muzzammil Hosenally
e, Gokhan Zengin
faDepartment of Health Sciences, Faculty of Science, University of Mauritius, 230 Réduit, Mauritius bDepartment of Biology, Faculty of Science and Arts, Kastamonu University, Kastamonu, Turkey
cDepartment of Physical and Analytical Chemistry, University of Jaén, Campus Las Lagunillas S/N, E-23071, Jaén, Spain dDepartment of Genetics and Bioengineering, Faculty of Engineering and Architecture, Kastamonu University, Kastamonu, Turkey eDepartment of Economics and Statistics, Faculty of Social Sciences & Humanities, University of Mauritius, Réduit, Mauritius fDepartment of Biology, Faculty of Science, Selcuk University, Turkey
A R T I C L E I N F O Keywords: Phytochemical Apoptosis Antioxidant Enzyme Checkerboard Cytotoxicity A B S T R A C T
Syzygium species are renowned for being important reservoirs of phytochemicals with pharmaceutical and biomedical potential. However, no attempt has been made to delineate the pharmacological potential and phytochemical profile of Syzgium coriaceum Bosser & J. Guého, an endemic plant to Mauritius. The present study aimed to determine the antibacterial, antioxidant, cytotoxicity, enzyme inhibitory and phytochemical profile of the ethyl acetate and methanol extracts of S. coriaceum. Preliminary qualitative phytochemical study of the extracts showed the presence of phenol, tannins, and alkaloids. Chemical characterisation showed the presence of derivatives of tannins, gallic acids, quercetin, and kaempferol. Potentiating activity between S. coriaceum extracts and antibiotics (ampicillin and streptomycin) using the checkerboard method showed additive inter-action. The extracts showed potent 2,2-diphenyl-1-picrylhydrazyl (DPPH) (2.95 and 2.93 mmol trolox equivalent (TE)/g sample for ethyl acetate and methanol extracts, respectively) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid (ABTS) (4.09 and 3.83 mmol TE/g sample for ethyl acetate and methanol extracts, respectively) scavenging abilities. Syzygium coriaceum extracts were active inhibitors ofα-glucosidase (about 47 mmol acar-bose equivalent/g sample for ethyl acetate and methanol extract). S. coriaceum methanol extract caused max-imum inhibition against human breast adenocarcinoma (MDA-MB-231) cancer cells after 48 h treatment with the IC50value of 53.41μg/mL. Expression of anti-apoptotic Bcl2 and BIRC5 genes were down-regulated. It can be
concluded that S. coriaceum extracts lead to MDA-MB-231 cells apoptosis. This investigation has provided a comprehensive report of the biological and chemical profile of S. coriaceum. Collected scientific evidences can open new avenues for research and contributes towards establishing primary data on Syzygium species endemic to Mauritius for bioprospection of novel phytopharmaceuticals.
1. Introduction
In a recent WHO publication (WHO, 2019), traditional remedy has been advocated to be an important and often underestimated health resource with a panoply of applications, especially in the prevention and management of both lifestyle-related chronic and communicable diseases. Indeed, the therapeutic potential of herbal products for the management of human ailments has been sustained through traditional
medicinal systems. It is believed that about 60 % of existing plants on the earth have medicinal and health promoting virtues (Hao and Xiao, 2018), yet thisfigure could have been underestimated. Over the past decades, significant advances have been made in the medical field, providing better healthcare treatment. Nevertheless, a large proportion of the global population still relies on natural products, particularly botanical products to alleviate sufferings. Additionally, the side effects of currently used drugs have urged scientific communities to show a
https://doi.org/10.1016/j.indcrop.2019.111948
Received 7 June 2019; Received in revised form 4 November 2019; Accepted 5 November 2019
⁎Corresponding author.
E-mail address:f.mahomoodally@uom.ac.mu(M.F. Mahomoodally).
Available online 13 November 2019
0926-6690/ © 2019 Elsevier B.V. All rights reserved.
renewed interest towards naturally derived molecules for the develop-ment of new, effective, and safe drugs.
Plants from the Syzygium Gaertn. genus occur generally as evergreen trees and shrubs in tropical or subtropical regions. Syzygium has also been reported as the largest woody genus of flowering plants in the world (Ahmad et al., 2016). The reported therapeutic uses of the species from thus genus include the treatment of chronic diarrhoea, diabetes, throat inflammation, and toothache (Pulikottil and Nath, 2015). For instance, Syzygium aromaticum, commonly known as clove, is widely cultivated as a spice but also for its therapeutic virtues. In traditional medicine, S. aromaticum is used as a painkiller to manage toothache and as a carminative. A systematic review published byCortes-Rojas et al. (2014) reported the antioxidant, antimicrobial, antiviral, and cyto-toxicity activities of S. aromaticum. Syzygium cumini, also known as jambolan or java plum, is famous in folk medicine, where a decoction of S. cumini fruits and leaves is used to treat diarrhoea, diseases of the mouth, and diabetes. Syzygium cumini has demonstrated biological ac-tivities such as antioxidant, inflammatory, gastroprotective, anti-microbial, and anti-diabetic activities (Ayyanar and Subash-Babu, 2012). However, there is still a lack of scientific knowledge and a pressing need for comprehensive studies focusing on Syzygium plants, particularly endemic species.
Interestingly, the tropical island of Mauritius, located in the Indian Ocean, is considered a biodiversity hotspot. About 691 plant species have been identified in Mauritius, including 273 species endemic to the island (Baider et al., 2010). In addition, the local population of Maur-itius has a long and rich history of use of medicinal plants for primary health care needs. However, most of the medicinal plants used in the Mauritian traditional medicine are exotic and have been introduced by immigrants and hence only a small percentage of endemic species are used in traditional medicine (Mahomoodally et al., 2019a). Syzygium coriaceum Bosser & J.Guého, a member from the Syzygium genus, is an endemic plant to Mauritius. As far as the scientific literature search could ascertain, no study has been done yet to establish the compre-hensive chemical and biological profile of S. coriaceum. Therefore, the main objective of this study is to establish the chemical profile and biological propensities of S. coriaceum extracts. To obtain a compre-hensive biological profile, several biological assays to assess the anti-microbial, antibiotic potentiating, antioxidant, and anticancer proper-ties were undertaken. In addition, key enzyme inhibitory properproper-ties involved in common pathologies such as diabetes (amylase and gluco-sidase), neurodegenerative diseases (cholinesterases), and hy-perpigmentation (tyrosinase) were assayed. It is anticipated that data gathered from this study will open new avenues for research and con-tribute towards establishing primary data on Syzygium species endemic to Mauritius for the bioprospection of novel phytopharmaceuticals.
2. Material and methods
2.1. Collection of plant samples
The plant samples were collected at Pétrin, Mauritius through the months of October and November 2017. The sample was identified by the botanist of the Mauritius Herbarium and an accession number (MAU0018403) was provided.
2.2. Sample preparation
Freshly collected leaves were weighed, thoroughly washed and patted dry before allowed to air dry until a constant mass was reached. The dried leaves were then ground to afine powder and stored in clean McCartney vials at 4 °C. To prepare solvent extracts, 50 g of leaf powder was added to an Erlenmeyerflask followed by 500 ml of solvent (me-thanol or ethyl acetate). The samples were allowed to macerate in the solvents forfive days on a magnetic stirrer with constant mixing and then filtrated using Whatman (Grade 1) paper. The extracts were
concentrated under vacuum at 40 °C using a Rotary Vacuum Evaporator (Stuart RE100) and stored in clean vials at 4 °C.
2.3. Determination of antioxidant and enzyme inhibitory potential
The ability of the extracts to inhibit α-amylase, α-glucosidase, cholinesterases, and tyrosinase was assessed in vitro (Uysal et al., 2017). Results are expressed as equivalents of appropriate standards (Uysal et al., 2017).
Antioxidant capacity of the tested extracts was measured using ferric reducing antioxidant power (FRAP), cupric reducing antioxidant capacity (CUPRAC), DPPH, ABTS, phosphomolybdenum, and metal chelating assays. Details of the protocols are as reported in previous studies (Uysal et al., 2017).
2.4. Antimicrobial assays
The cultures were of the American Type Culture Collection (ATCC); Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853), Staphylococcus aureus (ATCC 25923), Staphylococcus epidermidis (ATCC 14990), and Bacillus cereus (ATCC 10876).
Disc diffusion method as described byArullappan et al. (2009)was performed as a preliminary assay to determine the antibacterial effect of the tested extracts. Stock solutions of the samples were prepared in 5 % dimethyl sulfoxide (DMSO). Discs with only 5 % DMSO were used as negative controls while pre-dosed antibiotic discs of ampicillin and streptomycin (10μg/disc) were used as positive controls.
The microdilution broth susceptibility assay described byBaker and Tenover (1996)was employed to determine the minimum inhibitory concentration (MIC) of each extract against the microbial strains. Cul-tures (24 h) were diluted with MHB until an absorbance of 0.4-0.6 was read at 600 nm. Stock solutions of antibiotic were prepared in sterile water and used as a positive control while 5 % DMSO and MHB were used as negative controls. A volume of 50μL of adjusted inoculum was transferred to a sterile 96-well microtiter plate containing plant samples and the plate was incubated for 24 h at 37 °C and 48 h at 25 °C, for bacterial and fungal strains, respectively. After incubation, 40μL of the (2-(4-iodophenyl)-3-(4–nitrophenyl)-5-phenyltetrazolium chloride) (INT) (0.2 mg/mL) dye was added to each well of the microtiter plates. A reddish-pink colour indicated viable microorganisms. The lowest concentration of plant extract showing no colour development was recorded as the MIC. Experiments were performed in triplicate.
2.5. Checkerboard assay
The checkerboard assay, described byYap et al. (2013), was per-formed to evaluate synergistic activity by combining the samples ex-tracts and antibiotic at different concentrations. Antibiotic and extract/ essential oil as well as negative controls were included with 50μl of standardised inoculum at 37 °C for 24 h. After incubation, 40μL of 0.2 mg/ml INT was added to each well of the plate. The well showing no colour development was recorded as the MIC for the tested sample and antibiotic. The assay was performed in duplicates for each bac-terium and all combinations.
2.6. Fractional inhibitory concentration index (FICI)
Using MIC obtained from the combination of antibiotics and sam-ples extracts, the fractional inhibitory concentration index (FICI) was determined. The effect of the combinations and degree of synergism can be determined by the FICI value as described byXu et al. (2018). The individual FIC was calculated for the sample and antibiotic and the sum of the two FIC values was determined to be FICI.
FIC(sample/antibiotic)= MIC of sample or antibiotic in combination/MIC
FICI = FIC of sample + FIC of antibiotics.
FICI values calculated were then interpreted as described byPillai et al. (2005), i.e., FICI≤ 0.5, synergistic; 0.5 ≤ FICI < 1, additive ef-fect; 1≤ FICI≤4.0, no interaction; FICI > 4.0, antagonistic.
2.7. Profile of bioactive compounds
2.7.1. Quantitative analysis and determination of total phenolic and the other components
Test tubes tests analysis involving different reagents as described by Harborne (1973) were performed to determine presence the anti-microbial phytochemicals including phenols, coumarins, tannins, fla-vonoids, terpenoids, and alkaloids in the prepared samples. The total phenolic andflavonoid contents were determined using the Folin-Cio-calteu and AlCl3assays, respectively (Uysal et al., 2017). Results were
expressed as gallic acid (GAE) (mg GAE/g extract) and rutin equivalents (RE) (mg RE/g extract) for respective assays.
2.8. Instrumentation and reagents
An Agilent Series 1100 HPLC system with a G1315B diode array detector (Agilent Technologies) connected to an ion trap mass spec-trometer (Esquire 6000, Bruker Daltonics) with an electrospray inter-face was used. Separation was performed in a Luna Omega Polar C18
analytical column (150 × 3.0 mm; 5μm particle size) with a Polar C18
Security Guard cartridge (4 × 3.0 mm), both purchased from Phenomenex. Detailed chromatographic conditions are available in (Llorent-Martínez et al., 2018). LC–MS grade acetonitrile, LC–MS grade methanol, gallic acid, quercetin, and kaempferol were purchased from Sigma-Aldrich (Madrid, Spain). Ultrapure water (Milli-Q Waters pur-ification system; Millipore; Milford, MA, USA) was also used. 2.9. Determination of cytotoxic activity on cancer cells
2.9.1. Plant extracts
Methanol (MeOH) extract of S. coriaceum was analyzed for cytotoxic activity. Briefly, 0.1 % DMSO was used for solubilization of extracts, which were thenfiltered by 0.22 μm filter for sterilization and stored at −20 °C.
2.9.2. Cancer cell culture and MTT assay
MDA-MB-231 (human breast adenocarcinoma) cells were pro-liferated in Dulbecco's Modified Eagle Medium (DMEM) including 10 % FBS, 0.1 mg/ml human insulin, 1 % non-essential amino acids (NEAA) and 0.1 % penicillin/streptomycin at 37 °C under 5 % CO2. Cytotoxic
activity of extracts was evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) analysis. According to the MTT analysis protocol, 104MDA-MB-231 cells were incubated with 0.1, 1,
10,100 and 1000μg/mL of MeOH extracts for 24 h and 48 h (Mahomoodally et al., 2019b). After application of extracts, cells were treated with 100μl MTT (0.5 mg/mL) at 37 °C for 4 h. Then, 3 % sodium dodecyl sulfate (SDS) was added to the cells and incubated by shaking
for 5 min. It was waited for 15 min to dissolve MTT-formazan crystals after adding of 40 mM HCl/isopropanol (Locatelli et al., 2018). Mul-tiskan Go (Thermo Scientific, USA) was utilized to measure the absor-bance values of the test at 570 nm wavelength. A logarithmic dose-re-sponse curve was constructed by using GraphPad Prism 7.04 program with the data to calculate the IC50value.
2.9.3. Quantitative real-time PCR (qRT-PCR) analysis for apoptotic and autophagy marker genes
RNA isolation from cancer cells which treated with MeOH extract of S. coriaceum was performed by GeneJET RNA Purification Kit (Thermo Scientific, USA) according to manufacturers’ protocol. After isolation, DNase treatment was applied to destroy DNA residues. cDNA synthesis was carried out by RevertAid First Strand cDNA synthesis kit (Thermo Scientific, USA). Primers of apoptotic marker, autophagy marker (Jiang et al., 2012) and human telomerase genes were listed inTable 1. Gly-ceraldehyde-3-Phosphate Dehydrogenase (GAPDH) gene was used as a reference. Gene expressions were analyzed by Rotor Gene-Q (Qiagen, Germany) by quantitative real time PCR (qRT-PCR). Briefly, the reac-tion mixture (20μl) including cDNA samples, primers and SYBR Green solution (BioRad, USA) were incubated at 95 °C for 5 min forfirst de-naturation. Then, denaturation at 95 °C for 10 s, annealing and exten-sion cycle at 57 °C for 30 s were repeated for 40 cycles. Data analysis was performed by CT method (ΔΔCt) in order to determine expression levels of studied genes (Livak and Schmittgen, 2001).
2.10. Statistical analysis
All experiments and analysis were performed in triplicate. The re-sults were expressed as mean values with a standard error of the mean (SEM). The diff ;erences between the extract and control were analyzed using one-way analysis of variance (ANOVA) with Minitab v.17 pro-gram and p < 0.05 indicated significant diff ;erence.
3. Results and discussion
3.1. Phytochemical screening
Preliminary qualitative phytochemical screening of the methanol and ethyl acetate extracts of S. coriaceum revealed the presence of phenol, tannins, and alkaloids while coumarins were not detected Table 1
Primer sequences used for qRT-PCR analysis.
Gene Forward primer sequence (5’-3’) Reverse primer sequence (5’-3’)
GAPDH GGAAGGTGAAGGTCGGAGTC AACATGTAAACCATGTAGTTGAGGT
Bax CCCGAGAGGTCTTTTTCCGAG CCAGCCCATGATGGTTCTGAT
Bcl-2 GGTGGGGTCATGTGTGTGG CGGTTCAGGTACTCAGTCATCC
Bak1 ATGGTCACCTTACCTCTGCAA TCATAGCGTCGGTTGATGTCG
Birc5 AGGACCACCGCATCTCTACAT AAGTCTGGCTCGTTCTCAGTG
Beclin GGCTGAGAGACTGGATCAGG CTGCGTCTGGGCATAACG
LC3 GAGAAGCAGCTTCCTGTTCTGG GTGTCCGTTCACCAACAGGAAG
TERT CGGAAGAGTGTCTGGAGCAA GGATGAAGCGGAGTCTGGA
Table 2
Qualitative phytochemical determination of S. coriaceum extracts.
Phytochemicals Methanol extract Ethyl acetate extract
Phenol ++ ++ Coumarins – – Flavonoids ++ ++ Tannins ++ ++ Terpenes + + Alkaloids ++ ++
(Table 2). The identified compounds are known to possess bioactive properties, such as antioxidant, antibacterial, anti-inflammatory, anti-hyperglycaemic, and anti-cancer activities amongst others (Al-Saleem et al., 2018;Graça et al., 2016;Kanlayavattanakul et al., 2018;Majouli et al., 2017). Quantitative determination of phenolics andflavonoids was carried out using well-known, rapid spectrophotometric methods, namely the Folin-Ciocalteu and aluminum chloride assays, respectively. As presented inTable 3, the methanol (117.58 mg GAE/g extract) and ethyl acetate (116.79 mg GAE/g extract) extracts of S. coriaceum pos-sessed high phenolic contents. Besides, the phytochemical profiles of the different extracts of S. coriaceum were characterised by HPLC-ESI-MSnusing the negative ion mode. To the best of knowledge, this is the
first report of the phytochemical profile of S. coriaceum. The chemical characterization is shown inTable 4.
3.1.1. Tannins and gallic acid derivatives
First of all, gallic acid (compound7) was identified by comparison with an analytical standard (typical 169→125 fragmentation). Compound 12 was a methyl-gallate (Santos et al., 2012), whereas compound16 was tentatively characterized as a gallic acid derivative due to the fragment ions at m/z 169 and 125.
Secondly, the most abundant compounds in S. coriaceum were gal-lotannins (galloyl hexosides). All these compounds were identified based on the neutral losses of 152 Da (galloyl moiety) and 162 Da (hexoside moiety). Compound4 suffered the neutral loss of a hexoside moiety to yield gallic acid at m/z 169, which indicated a galloyl-hexoside. Compounds3, 6, and 9 had an additional galloyl moiety and were characterized as digalloyl-hexoside isomers. In the same way, compounds 10, 13, and 14 were characterized as trigalloyl-hexoside isomers, compounds 17, 18, and 19 as tetragalloyl hexoside isomers and compound 20 as a pentagalloyl hexoside. Finally, compound 11 was tentatively characterized as a gallotannin based on bibliographic information (Romani et al., 2012).
Finally, compound5 exhibited the deprotonated molecular ion at m/z 481 and base peak at m/z 301, characteristic of hexahydrox-ydiphenyl-hexoside (Fernandes et al., 2011). Compounds2 and 8, with [M−H]- at m/z 633, were tentatively characterized as hexahydrox-ydiphenyl-galloyl-hexoside isomers, whereas compound 15 was char-acterized as hexahydroxydiphenyl-digalloyl-hexoside (Fernandes et al., 2011).
3.1.2. Flavonoids
Several derivatives of theflavonols quercetin and kaempferol were characterised in the analysed extracts.
Quercetin aglycone (compound31) was identified by the deproto-nated molecular ion at m/z 301 and fragment ions at m/z 179 and 151 (comparison with an analytical standard). Compounds21, 22, 23, 26, 27, 29, and 30 were all quercetin O-glycosides. Quercetin aglycone and its main fragment ions were observed in all cases. The attached moieties were characterized due to the neutral losses of 152 Da (galloyl), 146 Da (deoxyhexoside), and 132 Da (pentoside).
Compounds24 and 25 were characterized as kaempferol-O-pento-side-deoxyhexoside and kaempferol-O-deoxyhexoside, respectively based on the same neutral losses mentioned before. The aglycone kaempferol was observed at m/z 285 (comparison with an analytical
standard).
3.1.3. Other compounds
Compound1, with [M−H]- at m/z 341, was identified as a dis-accharide due to the fragment ion at m/z 179 (hexoside) and the hexoside fragments at m/z 161, 119, 113, and 101 (Verardo et al., 2009).
3.2. Quantification of phenolic compounds by HPLC-DAD
Calibration curves were constructed using six concentrations (0.3–100 mg/l) in MeOH, plotting peak area versus concentration, ob-taining r≥0.995 in all cases. The following standards were used: gallic acid, kaempferol, and quercetin. Chromatograms were recorded at 280 nm for gallic acid and 350 nm for kaempferol and quercetin. Considering the absence of specific analytical standards for each com-pound, gallic acid was used for the semi-quantification of its deriva-tives, kaempferol for compounds24 and 25, and quercetin for all its glycosides). Repeatability (same day, n = 3) was lower than 4 %.
Tannins and gallic acid derivatives (40.7 mg/g DE) accounted for 65 % of the total phenolics, whereas flavonoids (21.5 mg/g DE) re-presented 35 % of total phenolics.
Compounds 21 (quercetin-O-pentoside-deoxyhexoside) and 22 (quercetin-O-deoxyhexoside) were the most abundant phenolics in the analyzed extract, accounting for 32 % of total phenolics. On the other hand, the most abundant gallotannins were tetragalloyl hexosides (16.8 mg g-1), followed by trigalloyl hexosides (8.1 mg/g DE) (Table 5). There is very scarce information available in the scientific biblio-graphy regarding individual phenolic contents of Syzygium species, so there is no easy comparison with the present results. In general, the presence of tannins and quercetin glycosides has been reported in Syzygium species, in agreement with the results, but without quanti fi-cation data. As an example, in S. aromaticum (Wojdyło et al., 2007) and S. jambus (Hossain et al., 2016), quercetin values of 0.7–1.55 mg/g DE have been reported, much lower than the ones here observed for quercetin and derivatives. Regarding gallotannins, the levels here ob-served are similar to those quantified in other plant leaves, such as in Quercus species, which are also rich in gallotannins (García-Villalba et al., 2017;Molina-García et al., 2018). Due to the beneficial health effects of gallotannins, the analysed leaves can be considered a poten-tial source of these bioactive compounds.
3.3. Antibacterial activity
Global prevalence of infectious diseases, particularly bacterial in-fections, coupled with antibiotic resistance caused in prolonged illness, disability, and death, is a major public health burden (Aumeeruddy et al., 2019). In this context, the extracts of S. coriaceum were screened for antibacterial activity against Gram-positive and negative bacteria. The extracts of S. coriaceum showed varying degree of antibacterial potential due to different phytochemical composition.
Two techniques were employed to assess bacterial growth inhibi-tion, namely the disc diffusion and microdilution broth susceptibility assays. Results of the antibacterial activity of the tested samples are presented inTables 6 and 7. The zone of inhibition (ZOI) obtained from the disc diffusion assay are summarized in Table 6. Staphylococcus aureus was more susceptible to the ethyl acetate (ZOI 18.13 mm) and methanol extracts (ZOI 17.17 mm). It was found that the tested extracts (ZOI 16.57-16.50 and 14.27-13.53 mm for B. cereus and P. aeruginosa) effectively prevented B. cereus and P. aeruginosa proliferation compared to the control ampicillin (ZOI 12.27 and 6.17 mm for B. cereus and P. aeruginosa). E. coli was the least susceptible strain against the extracts (ZOI 12.87 and 9.10 mm for ethyl acetate and methanol extracts, re-spectively). In fact, E. coli has an outer membrane made up mainly of phospholipids, preventing diffusion of molecules across the cell, and proteins, further restricting movement of foreign substances into the Table 3
Total phenolic andflavonoid contents of S. coriaceum extracts. Samples Total phenolic
(mg GAE/g extract)
Totalflavonoid (mg RE/g extract)
Ethyl acetate extract 117.58 ± 2.13 25.24 ± 0.27 Methanol extract 116.79 ± 0.84 24.57 ± 0.29
Values expressed are means ± S.D. of three parallel measurements. GAE: Gallic acid equivalent; RE: Rutin equivalent.
cell. Pseudomonas aeruginosa strain used in the present study was re-sistant to the antibiotic ampicillin (ZOI 6.17 mm), but was susceptible to S. coriaceum extracts (ZOI 14.27 and 13.53 mm for ethyl acetate and methanol extracts, respectively).
Results of the micro-dilution susceptibility assay are presented in Table 7. It was noted that B. cereus was the most susceptible bacteria as its growth was inhibited by extracts at relatively low MICs (1.56–3.13 mg/mL). Phytochemical analysis of the ethyl acetate extract Table 4
Characterisation of the compounds found in the S. coriaceum methanol extract. No. tR
(min)
[M-H]-m/z
m/z (% base peak) Assigned identification
1 1.7 341 MS2[341]: 179 (100), 161 (33), 131 (45), 119 (17), 113 (19), 101 (39) Disaccharide 2 1.9 633 MS2[633]: 301 (78), 275 (18) MS3[633→301]: 301 (100), 257 (15), 229 (28) HHDP-galloyl-hexoside 3 1.9 483 MS2[483]: 331 (100), 313 (33), 271 (9), 211 (6), 169 (48) Digalloyl-hexoside 4 2.6 331 MS2[331]: 271 (32), 211 (15), 169 (100), 125 (11) MS3[331→169]: 125 (100) Galloyl-hexoside 5 2.6 481 MS2[481]: 301 (100), 275 (9) HHDP-hexoside 6 3.0 483 MS2[483]: 331 (100), 313 (35), 169 (78) MS3[483→331]: 271 (30), 211 (27), 193 (83), 169 (100), 125 (9) Digalloyl-hexoside 7 3.2 169 MS2[169]: 125 (100) Gallic acid 8 3.6 633 MS2[633]: 301 (100), 275 (14) HHDP-galloyl-hexoside 9 3.6 483 MS2[483]: 331 (100), 313 (22), 193 (7), 169 (44) MS3[483→331]: 271 (20), 211 (15), 193 (45), 169 (100) MS4[483→331→169]: 125 (100) Digalloyl-hexoside 10 4.3 635 MS2[635]: 483 (100), 301 (16) MS3[635→483]: 331 (100), 313 (56), 271 (68), 193 (64), 169 (81) Trigalloyl-hexoside 11 6.3 801 MS2[801]: 757 (100), 633 (7) MS3[801→757]: 633 (100) Gallotannin 12 8.6 183 MS2[183]: 168 (100) MS3[183→168]: 124 (100) Methyl-gallate 13 8.8 635 MS2[635]: 483 (100), 465 (95), 313 (11) MS3[635→483]: 331 (25), 313 (12), 271 (47), 193 (20), 169 (100) Trigalloyl-hexoside 14 10.7 635 MS2[635]: 483 (100), 465 (24), 313 (10) MS3[635→483]: 423 (100), 331 (44), 313 (11), 271 (44), 193 (14), 169 (71) Trigalloyl-hexoside 15 11.4 785 MS2[785]: 785 (100), 635 (56), 483 (29), 301 (21) MS3[785→483]: 331 (49), 313 (100), 193 (43), 169 (93) MS4[785→483→169]: 125 (100) HHDP-digalloyl-hexoside 16 11.7 467 MS2[467]: 423 (100), 315 (30), 169 (7) MS3[467→423]: 313 (50), 169 (100) MS4[467→423→169]: 125 (100)
Gallic acid derivative
17 14.7 787 MS2[787]: 635 (100), 617 (38), 465 (13) MS3[787→635]: 483 (100), 465 (45) MS4[787→635→483]: 465 (46), 331 (35), 271 (57), 193 (100), 169 (59) Tetragalloyl hexoside 18 18.1 787 MS2[787]: 635 (100), 617 (46), 465 (12) MS3[787→635]: 483 (100), 465 (42), 423 (51) MS4[787→635→483]: 465 (24), 331 (17), 313 (39)(, 271 (23), 193 (100), 169 (37) Tetragalloyl hexoside 19 19.0 787 MS2[787]: 635 (18), 617 (18) MS3[787→617]: 617 (100), 573 (60), 465 (33) MS4[787→617→465]: 313 (100), 169 52) Tetragalloyl hexoside 20 22.7 939 MS2[939]: 787 (100), 769 (20) MS3[939→787]: 635 (100), 617 (24), 465 (20) Pentagalloyl hexoside 21 24.6 579 MS2[579]: 301 (100), 271 (19) MS3[579→301]: 271 (100), 255 (38), 179 (8), 151 (9) Quercetin-O-pentoside-deoxyhexoside 22 24.6 447 MS2[447]: 301 (100) MS3[447→301]: 179 (100), 151 (69) Quercetin-O-deoxyhexoside 23 27.6 731 MS2[731]: 579 (100) MS3[731→579]: 301 (100) MS4[731→579→301]: 271 (100), 255 (55), 179 (13), 151 (14) Quercetin-O-galloyl-pentoside-deoxyhexoside 24 28.1 563 MS2[563]: 285 (74), 284 (100) MS3[563→284]: 255 (100) Kaempferol-O-pentoside-deoxyhexoside 25 28.6 431 MS2[431]: 285 (100) MS3[431→285]: 255 (100), 241 (25), 229 (18) Kaempferol-O-deoxyhexoside 26 29.2 731 MS2[731]: 579 (100) MS3[731→579]: 301 (100) MS4[731→579→301]: 271 (100), 255 (26), 179 (7), 151 (11) Quercetin-O-galloyl-pentoside-deoxyhexoside 27 29.9 599 MS2[599]: 447 (100) MS3[599→447]: 301 (100) MS4[599→447→301]: 271 (100), 255 (63), 179 (95), 151 (42) Quercetin-O-galloyl- deoxyhexoside 28 31.0 567 MS2[567]: 521 (15), 413 (91), 293 (100) MS3[567→293]: 149 (100), 131 (74), 125 (55), 113 (54) Unknown 29 31.5 731 MS2[731]: 579 (100) MS3[731→579]: 301 (100), 271 (10) MS4[731→579→301]: 271 (100), 255 (83), 179 (8), 151 (5) Quercetin-O-galloyl-pentoside-deoxyhexoside 30 33.1 599 MS2[599]: 301 (100) MS3[599→301]: 179 (39), 151 (100) Quercetin-O-galloyl- deoxyhexoside 31 35.7 301 MS2[301]: 179 (100), 151 (85) Quercetin HHDP= Hexahydroxydiphenyl.
revealed the presence of phenolics and alkaloids. It has been reported that phenolic antimicrobial compounds from plants could induce membrane damage through leakage of cellular contents, including the release of potassium ion (Lambert and Hammond, 1973). On the other hand, alkaloids were reported to inhibit important microbial enzymes required for the synthesis of nucleic acids (Cushnie et al., 2014). P. aeruginosa strain was resistant to the positive control ampicillin; how-ever, its growth was inhibited by the extracts (MIC ranging 3.13–6.25 mg/mL).
The synergistic activity between S. coriaceum extracts and conven-tional antibiotics, ampicillin and streptomycin, was assessed using the checkerboard method. MIC values of the combination of antibiotics and extracts against the different bacteria are summarized inTable 8. In order to grade the effect of antibiotics used in combination with ex-tracts as synergistic or additive, FICI was computed (Table 8). Most combinations showed additive interaction. Combination of ethyl acetate extract with ampicillin lowered the MIC of the antibiotic from 0.78 mg/ml to 0.39 mg/ml against S. aureus, presenting an additive relationship. The ethyl acetate extract reduced MIC of ampicillin from 1.56 to 0.39 mg/ml against E.coli, suggesting a synergistic interaction. The extracts were also very effective when combined with ampicillin against P. aeruginosa. The MIC of ampicillin against P. aeruginosa was lowered from 25 to 6.25 mg/ml when combined with methanol extract and to 3.13 mg/ml when combined with ethyl acetate extract.
Streptomycin was not potentiated by S. coriaceum extracts (FICI > 1). Additive effects were noted when all four samples were combined with streptomycin against Gram-positive S. aureus and Gram-negative E. coli. Different combinations of methanol extract with streptomycin reduced MIC of the antibiotic from 0.78 to 0.39 mg/ml against S. aureus and E. coli, respectively. Gallotannins, identified from the S. coriaceum me-thanol extract, have been reported to exhibit inhibitory activity against bacteria, including S. aureus and E. coli (Engels et al., 2011). Additive relationship was also observed when methanol extract was combined with streptomycin against S. epidermidis and B. cereus, respectively. No synergism was noted when combining S. coriaceum extracts and the antibiotics. However, some samples that were found to possess mod-erate antimicrobial activities on their own, were able to interact posi-tively when combined with the antibiotics.
3.4. Antioxidant activity
The antioxidant activity of the different extracts of S. coriaceum in terms of their scavenging activity against ABTS and DPPH, reducing power on Cu (II), Fe (III) and Mo (VI), and metal chelating activity are shown inTable 9. Both S. coriaceum extracts showed potent DPPH (2.95 and 2.93 mmol TE/g sample for ethyl acetate and methanol extracts, respectively) and ABTS (4.09 and 3.83 mmol TE/g sample for ethyl acetate and methanol extracts, respectively) scavenging abilities. In-deed, these methods have been widely used for the estimation of radical scavenging properties of herbal extracts. FRAP, CUPRAC, and phos-phomolybdenum assays were used to assess the reducing potential of S. coriaceum extracts. As seen inTable 9, the reducing potential of both extracts was equivalent. Besides, other Syzygium species endemic to Mauritius, namely, S. commersonii, S. venosum, S. glomeratum, and S. mauritianum were reported to exhibit antioxidant activities (Neergheen et al., 2006). According to HPLC-DAD results, quercetin was present in high amounts in the extracts. Indeed, comprehensive reviews reported that quercetin possessed powerful antioxidant activity. However, quercetin might not be the only phytochemical responsible for the observed antioxidant activity. Phenolic compounds might act in sy-nergism with other phytochemicals thereby exhibiting potent anti-oxidant activities.
3.5. Enzyme inhibitory activity
Inhibition of key enzymes relevant to clinical conditions is a fun-damental approach in drug development for the management of chronic non-communicable diseases. In the present study, the extracts Table 5
Quantification of the main compounds detected in the S. coriaceum methanol extract.
Nº Assigned identification mg/g DE (dry
extract) Tannins and gallic acid derivatives
6 Digalloyl-hexoside 1.4 ± 0.1 7 Gallic acid 4.3 ± 0.2 8 + 9 HHDP-galloyl-hexoside + digalloyl-hexoside 2.1 ± 0.1 10 Trigalloyl-hexoside 0.38 ± 0.03 12 Methyl-gallate 3.2 ± 0.2 13 Trigalloyl-hexoside 1.4 ± 0.1 14 Trigalloyl-hexoside 6.7 ± 0.3
15 + 16 HHDP-digalloyl-hexoside + gallic acid derivative 4.4 ± 0.3 17 Tetragalloyl hexoside 1.6 ± 0.1 18 Tetragalloyl hexoside 7.7 ± 0.3 19 Tetragalloyl hexoside 1.3 ± 0.1 20 Pentagalloyl hexoside 6.2 ± 0.2 Total 40.7 ± 0.7 Flavonoids 21 + 22 Quercetin glycosides 19.9 ± 0.8 23 Quercetin-O-galloyl-pentoside-deoxyhexoside 0.30 ± 0.02 24 Kaempferol-O-pentoside-deoxyhexoside 0.35 ± 0.03 25 Kaempferol-O-deoxyhexoside 0.16 ± 0.01 26 Quercetin-O-galloyl-pentoside-deoxyhexoside 0.18 ± 0.01 27 Quercetin-O-galloyl- deoxyhexoside 0.111 ± 0.008 29 Quercetin-O-galloyl-pentoside-deoxyhexoside 0.18 ± 0.01 30 Quercetin-O-galloyl- deoxyhexoside 0.13 ± 0.01 31 Quercetin 0.21 ± 0.01 Total 21.5 ± 0.80 TIPC 62 ± 1
TIPC = Total individual phenolic content; defined as the sum of all the quan-tified compounds.
Table 6
Antibacterial activity (zone of inhibition in mm) of S. coriaceum extracts using the disc diffusion assay.
Ethyl acetate extract Methanol extract Ampicillin Streptomycin
S. aureus 18.13 ± 0.21 17.17 ± 0.29 27.87 ± 0.23 17.83 ± 0.29 S. epidemidis 15.87 ± 0.35 15.80 ± 0.30 14.5 ± 0.40 20.47 ± 0.45 B. cereus 16.57 ± 0.49 16.50 ± 0.44 12.27 ± 0.46 22.17 ± 0.29 E. coli 12.87 ± 0.32 9.10 ± 0.17 18.33 ± 0.38 24.20 ± 0.35 P. aeruginosa 14.27 ± 0.46 13.53 ± 0.47 6.17 ± 0.29 16.03 ± 0.06 Table 7
Antibacterial activity (MICs (mg/mL)) of S. coriaceum extracts using the micro-dilution susceptibility assay.
Ethyl acetate Methanol Ampicillin Streptomycin
S. aureus 3.13 3.13 0.78 0.78
S. epidemidis 6.25 6.25 1.56 0.78
B. cereus 1.56 3.13 0.78 0.39
E. coli 6.25 12.50 1.56 0.78
P. aeruginosa 3.13 6.25 25.00 3.13
of S. coriaceum were screened for their cholinesterase (acetyl and bu-tyryl cholinesterase), tyrosinase, α-amylase, and α-glucosidase in-hibitory activities. These enzymes have been targeted for the front-line management of Alzheimer’s disease, epidermal hyperpigmentation problems, and diabetes. In general, both extracts inhibited the tested enzymes showing almost similar activity. The upregulation of acetyl and butyryl cholinesterase has been associated with the increased hy-drolysis of acetylcholine, a neurotransmitter, consequently causing a decline in cognitive function and memory, the hallmark of Alzheimer’s disease (Mishra et al., 2019). Therefore, the inhibition of acetyl and butyryl cholinesterase offers new therapeutic possibilities for the management of Alzheimer’s disease. Nonetheless, the low efficacy of currently used therapies has fuelled the need for new cholinesterase inhibitors. Recently, much research has sought compounds from natural such as plants that could function as cholinesterase inhibitors. S. ar-omaticum, used in folk medicine for its neuroprotective properties, de-monstrated inhibition against acetyl and butyryl cholinesterase (Dalai et al., 2014). A group of researchers reported thatflavonoids isolated from S. samarangense showed remarkable inhibition against acetyl cholinesterase (Amor et al., 2005). Results of the inhibitory activity of the extracts of S. coriaceum on tyrosinase are displayed in Table 10. Both the ethyl acetate and methanol extracts showed similar inhibitory activity against tyrosinase. Tyrosinase plays a key role in the bio-synthesis of melanin. Melanin, a pigment present in the skin, shields the skin by absorbing UV rays. However, excessive production of melanin causes hyperpigmentation disorders such as melasma, freckles, age spots, and lentigines. Recently, there has been a renewed interest in the application of natural products for the development of cosmetic pro-ducts. Several Syzygium species have been studied for the management of skin related complications (Sharma et al., 2013). Interestingly, in the present study, the tyrosinase inhibitory action of S. coriaceum is re-ported for thefirst time. Besides, quercetin, identified in the extracts, was reported to bind with tyrosinase by hydrophobic interaction in-ducing conformational changes. Additionally, the catechol structure of quercetin chelated copper at the active site of tyrosinase, thereby ex-erting an inhibitory property (Fan et al., 2017). In addition,
gallotannins identified from S. coriaceum methanol extract have been previously found to inhibit tyrosinase activity (Chen et al., 2009). The ability of S. coriaceum extract to inhibit enzymes targeted for the management of diabetes has been also investigated. As depicted in Table 10, the extracts showed low inhibition againstα-amylase (0.92 and 0.85 mmol acarbose equivalent (ACAE)/g sample for ethyl acetate and methanol extracts, respectively) and pronounced inhibition against α-glucosidase (46.96 and 46.53 mmol ACAE/g sample for ethyl acetate and methanol extract, respectively). This pattern of inhibition is in line with several studies advocating that markedα-amylase inhibition was associated with gastrointestinal disturbances caused by undigested food.
3.6. Cytotoxic effects of S. coriaceum
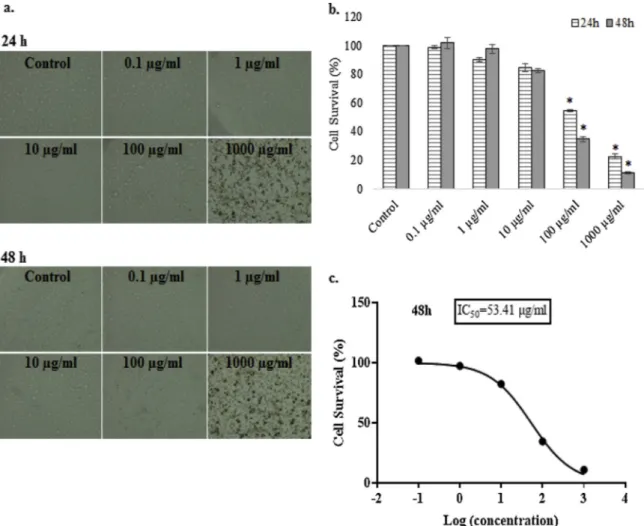
The toxic effects of conventional drugs used in cancer treatment on normal cells have prompted investigators to probe into alternative therapies against cancer. Plant extracts have showed promising results on variable cancer cells by different ways, such as stimulating the im-mune response, leading cancer cells to apoptosis or suppression of an-giogenesis (Baloglu et al., 2019;Mahomoodally et al., 2019b;Marwan Almosnid et al., 2018). The cytotoxic activity of S. coriaceum methanol extract on human breast adenocarcinoma cells (MDA-MB-231) has been evaluated in the current study. S. coriaceum methanol extract caused maximum inhibition against MDA-MB-231 cell line after 48 h treatment with the IC50value of 53.41μg/mL (Fig. 1). Inhibition of the cells was
induced by the dose and time increment according to the results. It can be argued that the effect of S. coriaceum methanol extracts on MDA-MB-231 cell line is dose and time-dependent.
Gallic acid (3,4,5-trihydroxybenzoic acid) and its derivatives were the main compounds found in S. coriaceum extract based on the HPLC analysis. In addition, high concentration of quercetin was recorded. Gallic acid, which is mainly found in plants and foods, has numerous pharmacological activities (Shahrzad et al., 2001;Wang et al., 2016). Among these pharmacological activities, the anticancer activity of gallic acid has been determined in variable cell types including cervical Table 8
Antibiotic potentiating activity of S. coriaceum extracts.
Bacteria MIC (mg/mL) FICI 1 FICI 2
Ampicillin Streptomycin Extract Ampicillin + extract Streptomycin + extract
S. aureus 0.78 0.78 ME 0.39 0.39 0.75 (A) 1.00 (A)
0.78 0.78 EA 0.39 0.39 1.00 (A) 0.62 (A)
S. epidermidis 1.56 0.78 ME 1.56 0.39 1.50 (NI) 1.50 (NI)
1.56 0.78 EA 0.78 0.39 1.00 (A) 1.00 (A)
B. cereus 0.78 0.39 ME 0.39 0.39 0.62 (A) 1.12 (NI)
0.78 0.39 EA 0.39 0.39 0.75 (A) 1.25 (NI)
E. coli 1.56 0.78 ME 0.78 0.39 1.00 (A) 1.00 (A)
1.56 0.78 EA 0.39 0.39 0.50 (S) 0.75 (A)
P. aeruginosa 25.00 3.13 ME 6.25 1.56 0.75 (A) 1.00 (A)
25.00 3.13 EA 3.13 0.39 0.62 (A) 0.75 (A)
MIC: minimum inhibitory concentration; ME: methanol extract; EA: ethyl acetate extract; FICI 1: FICI value for ampicillin and extract; FICI 2: FICI value for streptomycin and extract; A: Additive; S: Synergistic; NI: No interaction.
Table 9
Antioxidant activity of S. coriaceum extracts. Samples Phosphomolybdenum (mmol TE/g
sample) DPPH (mmol TE/g sample) ABTS (mmol TE/g sample)
CUPRAC (mmol TE/g sample)
FRAP (mmol TE/g sample)
Metal chelating (mg EDTAE/g sample)
Ethyl acetate 5.40 ± 0.07 2.95 ± 0.01 4.09 ± 0.10 5.85 ± 0.06 3.11 ± 0.05 17.23 ± 0.97
Methanol 4.57 ± 0.08 2.93 ± 0.01 3.83 ± 0.08 5.56 ± 0.33 2.81 ± 0.09 18.15 ± 0.87
Values expressed are means ± S.D. of three parallel measurements. DPPH: 2,2-diphenyl-1-picrylhydrazyl; ABTS: 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid; CUPRAC: Cupric reducing antioxidant capacity; FRAP: Ferric reducing antioxidant power; TE: Trolox equivalent; EDTAE: Ethylenediaminetetraacetic equivalent.
(Shi et al., 2016), colon (Subramanian et al., 2016), lung (You et al., 2011) and hepatocellular carcinoma cells (Sun et al., 2016). Moreover, quercetin is aflavonoid found in variable vegetables and fruits and its cytotoxic activities have been detected on different cancer cells (Del Follo-Martinez et al., 2013;Gates et al., 2009; Liu et al., 2017;Song et al., 2014). Cytotoxic activity of S. coriaceum extracts, rich in gallic acid derivatives and quercetin, on breast cancer cell line was consistent with the above studies that demonstrated anticancer effects of gallic acid and quercetin. Additionally, gallotannins, identified from S. cor-iaceum methanol extract, showed antiproliferative effects on MDA-MB-231 cell.
Besides, the effects of S. coriaceum methanol extracts on the ex-pression levels of autophagy-related genes Beclin1 and LC3, pro-apop-totic Bax and Bak1 genes, anti-apoppro-apop-totic Bcl2 and BIRC5 genes and TERT gene were analyzed in this study. Autophagy pathway is the di-rection of long-lived proteins and organelles to lysosomes for de-gradation. This process is related with tumor formation as well as tumor
inhibition. Degradation of oncogenic substrates, harmful proteins, in-tracellular pathogens or disrupted organelles by autophagy inhibits tumor formation. Besides, recycling of compounds for mitochondrial functions by autophagy mechanism results in tumor promotion (Schmitz et al., 2016). LC3 (microtubule-associated protein 1 light chain 3) is an ubiquitin-like protein and related with auto-phagosomal membranes. It is a well-known autophagy marker and involved in auto-phagosomal membrane expansion and fusion to select the cargo and direct to lysosome for degradation (Kabeya et al., 2000;Schläfli et al., 2015). Beclin is a member of Class III PI3K complex, which directs autophagy proteins to pre-autophagosomal membrane (Kihara et al., 2001). In a present study, gene expression levels of LC3 and Beclin were significantly decreased in MDA-MB-231 cells after 48 h treatment of S. coriaceum methanol extracts with IC50concentrations when compared
to the untreated control cells (Fig. 2). In addition, treatment of cancer cells with S. coriaceum extracts caused downregulation of telomerase (TERT) gene expression levels in MDA-MB-231 cells (Fig. 2). Table 10
Enzyme inhibitory activity of S. coriaceum extracts. Samples AChE inhibition (mg
GALAE/g sample)
BChE inhibition (mg GALAE/g sample)
Tyrosinase inhibition (mg KAE/ g sample)
Amylase inhibition (mmol ACAE/g sample)
Glucosidase inhibition (mmol ACAE/g sample)
Ethyl acetate 2.40 ± 0.01 2.33 ± 0.08 108.29 ± 0.12 0.92 ± 0.03 46.96 ± 0.01
Methanol 2.38 ± 0.01 2.60 ± 0.10 108.06 ± 0.48 0.85 ± 0.03 46.53 ± 0.04
Values expressed are means ± S.D. of three parallel measurements. AChE: Acetylcholinesterase; BChE: Butyrylcholinesterase; GALAE: Galatamine equivalent; KAE: Kojic acid equivalent; ACAE: Acarbose equivalent.
Fig. 1. a.The cellular morphology of human breast adenocarcinoma (MDA-MB-231) cells after S. coriaceum treatment. b. The graphical representation of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) analysis results. Data represents the means ± SEM (n = 3). *, p < 0.05.c. IC50value for 48 h of S.
Telomerase is an enzyme which adds DNA to telomere regions of chromosomes (Greider and Blackburn, 1985). In mammalian cells, telomeric regions contain TTAGGG nucleotide repeats which are shorten every cell division. Telomeric shortening causes chromosomal instability. Telomeric DNA regions are significant for the survival of the cancer cells. Telomerase enzyme is expressed in most human tumors (85–90 %) and maintains the telomere length whereas it is absent in normal cells (Jafri et al., 2016;Kim et al., 1994;Shay, 2016). It can be concluded that S. coriaceum extracts lead to a decrease in TERT gene expression level in MDA-MB-231 cells in which telomerase activity was probably diminished. So, it can be suggested that the down-regulation of TERT gene in cancer cells by S. coriaceum extracts may affect the survival rate of cancer cells.
On the other hand, pro-apoptotoic (Bax and Bak1) and anti-apop-totic (Bcl2 and BIRC5) gene expression levels were evaluated in the MDA-MB-231 cells treated with S. coriaceum extracts in the study. Although there are no significant differences in the expression levels of pro-apoptotic Bax and Bak1 genes in control and plant extract treated MDA-MB-231 cells, a small increment on Bax and Bak1 gene expression compared to control. However, expression levels of anti-apoptotic Bcl2 and BIRC5 genes were significantly down-regulated in S. coriaceum extract treated MDA-MB-231 cells when compared to the non-treated control cells (Fig. 3). According to these findings, up-regulated pro-apoptotic/anti-apoptotic ratio by S. coriaceum extract which might lead to the induction of apoptosis in cancer cells. Apoptosis is one of the programmed cell death mechanisms that conserve tissue homeostasis. The extrinsic pathway (death receptor-mediated pathway) and the in-trinsic pathway (mitochondrial-mediated pathway), which leads the activation of caspases, are apoptotic mechanisms in mammalian cells (Hassen et al., 2012). The absence of growth factors, cytokines or hormones can induce apoptosis and also mitochondrial-mediated pathway is activated by these inducers. B-cell lymphoma 2 protein (Bcl-2) family members have key roles in mitochondrial pathway. These family members include anti-apoptotic such as 2, X, XL, Bcl-XS, BclW and Bag and pro-apoptotic proteins such as Bcl-10, Bax, Bak, Bid, Bad, Bim, Bik, and Blk. Cell viability depends on the balance among these anti-apoptotic and pro-apoptotic proteins (Goldar et al., 2015;Slattery et al., 2018). Bax and Bak proteins are recently classified in the subgroup of proapoptotic proteins as multi- Bcl-2 homology (BH) domain effector proteins. Other proapoptotic members of Bcl-2 family induce Bax/Bak activation whereas inhibition occurs on Bax/Bak acti-vation by anti-apoptotic protein members (Youle and Strasser, 2008). After activation of Bax/Bak proteins, apoptogenic proteins are released from mitochondrial membrane to cytoplasm, which causes apoptosome complex formation and caspase activation (Jiang, 2004;O’Neill et al.,
2016). Both over expression of anti-apoptotic proteins and inhibition of pro-apoptotic Bcl2 family members cause defects in apoptosis me-chanism in cancer (Fulda, 2009). Poor prognosis, lack of response to cancer therapeutics or recurrence of the cancer is especially correlated with inducement of anti-apoptotic Bcl2 family members ( Wuilleme-Toumi et al., 2005). In addition, inhibitor of apoptosis proteins (IAPs), which is including Baculoviral IAP Repeat Containing (BIRC) proteins, can bind to caspases to inhibit apoptosis pathway (Chen et al., 2016). BIRC5 which is also known as survivin is one of the proteins in this group and acts as a caspase inhibitor (Su et al., 2015). Based on these knowledge, inhibition of anti-apoptotic gene expression by S. coriaceum extract in MDA-MB-231 cells might direct the cells to apoptosis which can be the mode of action of S. coriaceum extracts.
Gallic acid and quercetin, which were mostly found compounds in S. coriaceum extract based on the current study, have been reported to possess cytotoxic effects on variable cancer cell types and induce apoptosis by effecting gene expression levels. It was reported that the viability of H44 g lung cancer cells was decreased to 65 % after 24 h by the 3μg/mL concentrations of gallic acid stimulation. In addition, Gallic acid treatment has changed the cellular morphology, inhibited cell growth and lead H446 cells to apoptosis via inducing generation of reactive oxygen species (ROS), defecting mitochondrial membrane potential (MMP), inducing Bax, Apaf-1, DIABLO and p53 gene expres-sions (Wang et al., 2016). Besides, colon cancer cell (HCT15) growth was inhibited by gallic acid treatment. In the same study, early apop-tosis events like lipid layer destruction and a decrement in mitochon-drial membrane potential were demonstrated using flow cytometry analysis (Subramanian et al., 2016). Moreover, it was reported that quercetin stimulated apoptosis in bladder cancer cells by the induce-ment of AMP-activated protein kinase (AMPK) signalling pathway (Su et al., 2016). In a study conducted byChien et al. (2009), quercetin treatment caused up-regulation of the pro-apoptotic Bax protein whereas down-regulation the anti-apoptotic Bcl-2 protein in MDA-MB-231 cells. In addition, quercetin induced the caspase-3, -8 and -9 apoptosis-inducing factor (AIF) release from mitochondria in MDA-MB-231 cells. They suggested that these results can be explained by apop-tosis occurs via mitochondrial and caspase-3-dependent pathways after treatment with quercetin in MDA-MB-231 cells (Chien et al., 2009). In another study, it was reported that quercetin treatment had inhibitory effects on breast cancer cell line (MCF7). The expression of genes in-cluding Twist, p16 and p21, which stimulated apoptosis, were regulated by quercetin in MCF7 cells. However, in the same study, quercetin treatment showed no effect on MDA-MB-231 cells, which had different hormone receptors than MCF7 cells, even at higher doses of quercetin (Ranganathan et al., 2015). The cytotoxic activity of S. coriaceum Fig. 2. Gene expression profiles of autophagy markers and human telomerase genes. human breast adenocarcinoma (MDA-MB-231) cells were treated with S. coriaceum extract at IC50concentration for 48 h. Control cells were treated with 0.1 % dimethyl sulfoxide (DMSO). Transcript levels of the marker genes were
analyzed in total RNA by by quantitative real-time PCR (qRT-PCR). Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) was used as an internal control. *, p < 0.05.
extracts and their direction of the cells to the apoptosis based on the gene expression analysis can be explained by the gallic acid and its derivatives which are main compounds in the extracts in the current study. The present findings are consistent with the previous studies based on the cytotoxic effects of gallic acid on different cancer cells. 4. Conclusion
This is thefirst report of the antioxidant, antibacterial, and enzyme inhibitory properties of S. coriaceum, a species of the Syzygium genus endemic to Mauritius. This study has provided baseline data regarding the biological activities of this Syzygium species, which may be regarded as a potential ingredient for the development of novel pharmaceutical, nutraceutical, and/or cosmetic agents. S. coriaceum might also be con-sidered as a natural source of phytochemicals that can be used for the prevention and/or management of infectious diseases of bacterial origin, oxidative stress-related disorders, diabetes, skin hy-perpigmentation complications, and neurodegenerative complications. The mechanism behind cell growth inhibition of MDA-MB-231 cells after application of S. coriaceum methanol extracts was also studied. Gene expression and MTT results can shed light on the
phytopharmacological potential of S. coriaceum extracts on breast cancer line, MDA-MB-231 cells. Forthcoming investigations should focus on the determination of the biological activity of isolated phy-tochemicals from S. coriaceum.
Declaration of Competing Interest
The authors wish to confirm that there are no known conflicts of interest associated with this publication and there has been no sig-nificant financial support for this work that could have influenced its outcome.
Acknowledgments
The authors wish to thank the Mauritius Herbarium for Identification of sample and Mr Puchooa for any technical and/or non-technical help.
References
Ahmad, B., Baider, C., Bernardini, B., Biffin, E., Brambach, F., Burslem, D., Byng, J.W., Christenhusz, M., Florens, F.V., Lucas, E., 2016. Syzygium (Myrtaceae):
Fig. 3. Gene expression profiles of apoptotic marker genes. Human breast adenocarcinoma (MDA-MB-231) cells were treated with S. coriaceum extract at IC50
concentration for 48 h. Control cells were treated with 0.1 % dimethyl sulfoxide (DMSO). Transcript levels of the marker genes were analyzed in total RNA by quantitative real-time PCR (qRT-PCR). Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) was used as an internal control. *, p < 0.05.
monographing a taxonomic giant via 22 coordinated regional revisions. PeerJ Prepr.
Al-Saleem, M.S., Awaad, A.S., Alothman, M.R., Alqasoumi, S.I., 2018. Phytochemical standardization and biological activities of certain desert plants growing in Saudi Arabia. S. Saudi Pharm. J. 26, 198–204.
Amor, E.C., Villaseñor, I.M., Nawaz, S.A., Hussain, M.S., Choudhar, I., 2005. A dihy-drochalcone from Syzygium samarangense with anticholinesterase activity. Philipp. J. Sci. 134, 105.
Arullappan, S., Zakaria, Z., Basri, D.F., 2009. Preliminary screening of antibacterial ac-tivity using crude extracts of Hibiscus rosa sinensis. Trop. Life Sci. Res. 20, 109–118.
Aumeeruddy, M.Z., Aumeeruddy-Elalfi, Z., Neetoo, H., Zengin, G., Blom van Staden, A., Fibrich, B., Lambrechts, I.A., Rademan, S., Szuman, K.M., Lall, N., Mahomoodally, F., 2019. Pharmacological activities, chemical profile, and physicochemical properties of raw and commercial honey. Biocatal. Agric. Biotechnol. 18, 101005.
Ayyanar, M., Subash-Babu, P., 2012. Syzygium cumini (L.) skeels: a review of its phyto-chemical constituents and traditional uses. Asian Pac. J. Trop. Biomed. 2, 240–246.
Baider, C., Florens, F.V., Baret, S., Beaver, K., Matatiken, D., Strasberg, D., Kueffer, C., 2010. Status of plant conservation in oceanic islands of the Western Indian Ocean. Proceedings of the 4th Global Botanic Gardens Congress.
Baker, C.N., Tenover, F.C., 1996. Evaluation of Alamar colorimetric broth microdilution susceptibility testing method for Staphylococci and Enterococci. J. Clin. Microbiol. 34, 2654–2659.
Baloglu, M.C., Llorent-Martínez, E.J., Aumeeruddy, M.Z., Mahomoodally, M.F., Altunoglu, Y.C., Ustaoglu, B., Ocal, M., Gürel, S., Bene, K., Sinan, K.I., Zengin, G., 2019. Multidirectional insights on Chrysophyllum perpulchrum leaves and stem bark extracts: HPLC-ESI-MSn profiles, antioxidant, enzyme inhibitory, antimicrobial and cytotoxic properties. Ind. Crops Prod. 134, 33–42.
Chen, L.G., Chang, W.L., Lee, C.J., Lee, L.T., Shih, C.M., Wang, C.C., 2009. Melanogenesis inhibition by gallotannins from Chinese galls in B16 mouse melanoma cells. Biol. Pharm. Bull. 32, 1447–1452.
Chen, X., Duan, N., Zhang, C., Zhang, W., 2016. Survivin and tumorigenesis: molecular mechanisms and therapeutic strategies. J. Cancer 7, 314.
Chien, Y., Wu, Y.-C., Chung, J.-G., Yang, J.-S., Lu, H.-F., Tsou, M.-F., Wood, W., Kuo, S.-J., Chen, D.-R., 2009. Quercetin-induced apoptosis acts through mitochondrial-and caspase-3-dependent pathways in human breast cancer MDA-MB-231 cells. Hum. Exp. Toxicol. 28, 493–503.
Cortes-Rojas, D.F., de Souza, C.R., Oliveira, W.P., 2014. Clove (Syzygium aromaticum): a precious spice. Asian Pac. J. Trop. Biomed. 4, 90–96.
Cushnie, T.P.T., Cushnie, B., Lamb, A.J., 2014. Alkaloids: an overview of their anti-bacterial, antibiotic-enhancing and antivirulence activities. Int. J. Antimicrob. Agents 44, 377–386.
Dalai, M.K., Bhadra, S., Chaudhary, S.K., Bandyopadhyay, A., Mukherjee, P.K., 2014. Anti-cholinesterase activity of the standardized extract of Syzygium aromaticum L. Pharmacogn. Mag. 10, S276–282.
Del Follo-Martinez, A., Banerjee, N., Li, X., Safe, S., Mertens-Talcott, S., 2013. Resveratrol and quercetin in combination have anticancer activity in colon cancer cells and re-press oncogenic microRNA-27a. Nutr. Cancer 65, 494–504.
Engels, C., Schieber, A., Gänzle, M.G., 2011. Inhibitory spectra and modes of anti-microbial action of gallotannins from mango kernels (Mangifera indica L.). Appl. Environ. Microbiol. 77, 2215–2223.
Fan, M., Zhang, G., Hu, X., Xu, X., Gong, D., 2017. Quercetin as a tyrosinase inhibitor: inhibitory activity, conformational change and mechanism. Food Res. Int. 100, 226–233.
Fernandes, A., Sousa, A., Mateus, N., Cabral, M., de Freitas, V., 2011. Analysis of phenolic compounds in cork from Quercus suber L. by HPLC–DAD/ESI–MS. Food Chem. 125, 1398–1405.
Fulda, S., 2009. Tumor resistance to apoptosis. Int. J. Cancer 124, 511–515.
García-Villalba, R., Espín, J.C., Tomás-Barberán, F.A., Rocha-Guzmán, N.E., 2017. Comprehensive characterization by LC-DAD-MS/MS of the phenolic composition of seven Quercus leaf teas. J. Food Anal. 63, 38–46.
Gates, M.A., Vitonis, A.F., Tworoger, S.S., Rosner, B., Titus‐Ernstoff, L., Hankinson, S.E., Cramer, D.W., 2009. Flavonoid intake and ovarian cancer risk in a population‐based case‐control study. Int. J. Cancer 124, 1918–1925.
Goldar, S., Khaniani, M.S., Derakhshan, S.M., Baradaran, B., 2015. Molecular mechanisms of apoptosis and roles in cancer development and treatment. Asian Pac. J. Cancer Prev. 16, 2129–2144.
Graça, V.C., Ferreira, I.C.F.R., Santos, P.F., 2016. Phytochemical composition and bio-logical activities of Geranium robertianum L.: a review. Ind. Crops Prod 87, 363–378.
Greider, C.W., Blackburn, E.H., 1985. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell 43, 405–413.
Hao, D.-C., Xiao, P.-G., 2018. Deep in shadows: epigenetic and epigenomic regulations of medicinal plants. Chin. Herb. Med. 10, 239–248.
Harborne, A., 1973. Phytochemical Methods A Guide To Modern Techniques of Plant Analysis. Chapman and Hall Ltd, London, pp. 279.
Hassen, S., Ali, N., Chowdhury, P., 2012. Molecular signaling mechanisms of apoptosis in hereditary non-polyposis colorectal cancer. World J. Gastrointest. Pathophysiol. 3, 71.
Hossain, H., Rahman, S.E., Akbar, P.N., Khan, T.A., Rahman, M.M., Jahan, I.A., 2016. HPLC profiling, antioxidant and in vivo anti-inflammatory activity of the ethanol extract of Syzygium jambos available in Bangladesh. BMC Res. Notes 9, 191.
Jafri, M.A., Ansari, S.A., Alqahtani, M.H., Shay, J.W., 2016. Roles of telomeres and tel-omerase in cancer, and advances in teltel-omerase-targeted therapies. Genome Med. 8, 69.
Jiang, X., 2004. Wang X. Cytochrome-c mediated apoptosis. Annu. Rev. Biochem. 73, 87–106.
Jiang, Z.-F., Shao, L.-J., Wang, W.-M., Yan, X.-B., Liu, R.-Y., 2012. Decreased expression of Beclin-1 and LC3 in human lung cancer. Mol. Biol. Rep. 39, 259–267.
Kabeya, Y., Mizushima, N., Ueno, T., Yamamoto, A., Kirisako, T., Noda, T., Kominami, E., Ohsumi, Y., Yoshimori, T., 2000. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 19, 5720–5728.
Kanlayavattanakul, M., Lourith, N., Chaikul, P., 2018. Biological activity and phyto-chemical profiles of Dendrobium: a new source for specialty cosmetic materials. Ind. Crops Prod. 120, 61–70.
Kihara, A., Kabeya, Y., Ohsumi, Y., Yoshimori, T., 2001. Beclin–phosphatidylinositol 3‐kinase complex functions at the trans‐Golgi network. EMBO Rep. 2, 330–335.
Kim, N.W., Piatyszek, M.A., Prowse, K.R., Harley, C.B., West, M.D., Ho, Pd.L., Coviello, G.M., Wright, W.E., Weinrich, S.L., Shay, J.W., 1994. Specific association of human telomerase activity with immortal cells and cancer. Science 266, 2011–2015.
Lambert, P., Hammond, S., 1973. Potassiumfluxes, first indications of membrane damage in micro-organisms. Biochem. Biophys. Res. Commun. 54, 796–799.
Liu, Y., Gong, W., Yang, Z., Zhou, X., Gong, C., Zhang, T., Wei, X., Ma, D., Ye, F., Gao, Q., 2017. Quercetin induces protective autophagy and apoptosis through ER stress via the p-STAT3/Bcl-2 axis in ovarian cancer. Apoptosis 22, 544–557.
Livak, K.J., Schmittgen, T.D., 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25, 402–408.
Llorent-Martínez, E.J., Zengin, G., Lobine, D., Molina-García, L., Mollica, A., Mahomoodally, M.F., 2018. Phytochemical characterization, in vitro and in silico approaches for three Hypericum species. New J. Chem. 42, 5204–5214.
Locatelli, M., Yerlikaya, S., Baloglu, M.C., Zengin, G., Altunoglu, Y.C., Cacciagrano, F., Campestre, C., Mahomoodally, M.F., Mollica, A., 2018. Investigations into the ther-apeutic potential of Asphodeline liburnica roots: in vitro and in silico biochemical and toxicological perspectives. Food Chem. Toxicol. 120, 172–182.
Mahomoodally, M.F., Protab, K., Aumeeruddy, M.Z., 2019a. Medicinal plants brought by Indian indentured immigrants: a comparative review of ethnopharmacological uses between Mauritius and India. J. Ethnopharmacol. 234, 245–289.
Mahomoodally, M.F., Yerlikaya, S., Llorent-Martínez, E.J., Uğurlu, A., Baloglu, M.C., Altunoglu, Y.C., Mollica, A., Dardenne, K.K., Aumeeruddy, M.Z., Puchooa, D., 2019b. Pharmacological and polyphenolic profiles of Phyllanthus phillyreifolius var. Commersonii Müll. Arg: an unexplored endemic species from Mauritius. Food Res. Int. 115, 425–438.
Majouli, K., Hamdi, A., Hlila, M.B., 2017. Phytochemical analysis and biological activities of Hertia cheirifolia L. roots extracts. Asian Pac. J. Trop. Med. 210, 1134–1139.
Marwan Almosnid, N., Zhou, X., Jiang, L., Ridings, A., Knott, D., Wang, S., Wei, F., Yuan, J., Altman, E., Gao, Y., Miao, J., 2018. Evaluation of extracts prepared from 16 plants used in Yao ethnomedicine as potential anticancer agents. J. Ethnopharmacol. 211, 224–234.
Mishra, P., Kumar, A., Panda, G., 2019. Anti-cholinesterase hybrids as multi-target-di-rected ligands against Alzheimer’s disease (1998-2018). Bioorg. Med. Chem. 27, 895–930.
Molina-García, L., Martínez-Expósito, R., Fernández-de Córdova, M., Llorent-Martínez, E., 2018. Determination of the phenolic profile and antioxidant activity of leaves and fruits of Spanish Quercus coccifera. J. Chem. 2018.
Neergheen, V.S., Soobrattee, M.A., Bahorun, T., Aruoma, O.I., 2006. Characterization of the phenolic constituents in Mauritian endemic plants as determinants of their an-tioxidant activities in vitro. J. Plant Physiol. 163, 787–799.
O’Neill, K.L., Huang, K., Zhang, J., Chen, Y., Luo, X., 2016. Inactivation of prosurvival Bcl-2 proteins activates Bax/Bak through the outer mitochondrial membrane. Genes Dev. 30, 973–988.
Pillai, S., Moellering, R., Eliopoulos, G., 2005. Antimicrobial combinations. In: Lorian, V. (Ed.), Antibiotics in Laboratory Medicine, 5th edition. the Lippincott Williams & Wilkins Co., Philadelphia, pp. 365–440.
Pulikottil, S., Nath, S., 2015. Potential of clove of Syzygium aromaticum in development of a therapeutic agent for periodontal disease: a review. S. Afr. Dent. J. 70, 108–115.
Ranganathan, S., Halagowder, D., Sivasithambaram, N.D., 2015. Quercetin suppresses twist to induce apoptosis in MCF-7 breast cancer cells. PLoS One 10, e0141370.
Romani, A., Campo, M., Pinelli, P., 2012. HPLC/DAD/ESI-MS analyses and anti-radical activity of hydrolyzable tannins from different vegetal species. Food Chem. 130, 214–221.
Santos, S.A.O., Villaverde, J.J., Freire, C.S.R., Domingues, M.R.M., Neto, C.P., Silvestre, A.J.D., 2012. Phenolic composition and antioxidant activity of Eucalyptus grandis, E. urograndis(E. grandis×E. urophylla) and E. maidenii bark extracts. Ind. Crops Prod 39, 120–127.
Schläfli, A., Berezowska, S., Adams, O., Langer, R., Tschan, M., 2015. Reliable LC3 and p62 autophagy marker detection in formalinfixed paraffin embedded human tissue by immunohistochemistry. Eur. J. Histochem. 59.
Schmitz, K.J., Ademi, C., Bertram, S., Schmid, K.W., Baba, H.A., 2016. Prognostic re-levance of autophagy-related markers LC3, p62/sequestosome 1, Beclin-1 and ULK1 in colorectal cancer patients with respect to KRAS mutational status. World J. Surg. Oncol. 14, 189.
Shahrzad, S., Aoyagi, K., Winter, A., Koyama, A., Bitsch, I., 2001. Pharmacokinetics of gallic acid and its relative bioavailability from tea in healthy humans. J. Nutr. 131, 1207–1210.
Sharma, R., Kishore, N., Hussein, A., Lall, N., 2013. Antibacterial and anti-inflammatory effects of Syzygium jambos L. (Alston) and isolated compounds on acne vulgaris. BMC Complement. Altern. Med. 13, 292.
Shay, J.W., 2016. Role of telomeres and telomerase in aging and cancer. Cancer Discov. 6, 584–593.
Shi, L., Lei, Y., Srivastava, R., Qin, W., Chen, J.J., 2016. Gallic acid induces apoptosis in human cervical epithelial cells containing human papillomavirus type 16 episomes. J. Med. Virol. 88, 127–134.
Slattery, M.L., Mullany, L.E., Sakoda, L.C., Wolff, R.K., Samowitz, W.S., Herrick, J.S., 2018. Dysregulated genes and miRNAs in the apoptosis pathway in colorectal cancer patients. Apoptosis 23, 237–250.
Song, N.R., Chung, M.-Y., Kang, N.J., Seo, S.G., Jang, T.S., Lee, H.J., Lee, K.W., 2014. Quercetin suppresses invasion and migration of H-Ras-transformed MCF10A human epithelial cells by inhibiting phosphatidylinositol 3-kinase. Food Chem. 142, 66–71.
Su, Q., Peng, M., Zhang, Y., Xu, W., Darko, K.O., Tao, T., Huang, Y., Tao, X., Yang, X., 2016. Quercetin induces bladder cancer cells apoptosis by activation of AMPK sig-naling pathway. Am. J. Cancer Res. 6, 498.
Su, Z., Yang, Z., Xu, Y., Chen, Y., Yu, Q., 2015. Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol. Cancer 14, 48.
Subramanian, A.P., Jaganathan, S.K., Mandal, M., Supriyanto, E., Muhamad, I.I., 2016. Gallic acid induced apoptotic events in HCT-15 colon cancer cells. World J. Gastroenterol. 22, 3952.
Sun, G., Zhang, S., Xie, Y., Zhang, Z., Zhao, W., 2016. Gallic acid as a selective anticancer agent that induces apoptosis in SMMC-7721 human hepatocellular carcinoma cells. Oncol. Lett. 11, 150–158.
Uysal, S., Zengin, G., Locatelli, M., Bahadori, M.B., Mocan, A., Bellagamba, G., De Luca, E., Mollica, A., Aktumsek, A., 2017. Cytotoxic and enzyme inhibitory potential of two Potentilla species (P. Speciosa L. And P. Reptans Willd.) and their chemical composi-tion. Front. Pharmacol. 8, 290.
Verardo, G., Duse, I., Callea, A., 2009. Analysis of underivatized oligosaccharides by li-quid chromatography/electrospray ionization tandem mass spectrometry with post‐column addition of formic acid. Rapid Commun. Mass Spectrom. 23, 1607–1618.
Wang, R., Ma, L., Weng, D., Yao, J., Liu, X., Jin, F., 2016. Gallic acid induces apoptosis and enhances the anticancer effects of cisplatin in human small cell lung cancer H446 cell line via the ROS-dependent mitochondrial apoptotic pathway. Oncol. Rep. 35, 3075–3083.
WHO, 2019. WHO Global Report on Traditional and Complementary Medicine 2019. ISBN 978-92-4-151543-6..
Wojdyło, A., Oszmiański, J., Czemerys, R., 2007. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 105, 940–949.
Wuilleme-Toumi, S., Robillard, N., Gomez, P., Moreau, P., Le Gouill, S., Avet-Loiseau, H., Harousseau, J., Amiot, M., Bataille, R., 2005. Mcl-1 is overexpressed in multiple myeloma and associated with relapse and shorter survival. Leukemia 19, 1248.
Xu, X., Xu, L., Yuan, G., Wang, Y., Qu, Y., Zhou, M., 2018. Synergistic combination of two antimicrobial agents closing each other’s mutant selection windows to prevent an-timicrobial resistance. Sci. Rep. 8, 7237.
Yap, P.S., Lim, S.H., Hu, C.P., Yiap, B.C., 2013. Combination of essential oils and anti-biotics reduce antibiotic resistance in plasmid-conferred multidrug resistant bacteria. Phytomedicine 20, 710–713.
You, B.R., Kim, S.Z., Kim, S.H., Park, W.H., 2011. Gallic acid-induced lung cancer cell death is accompanied by ROS increase and glutathione depletion. Mol. Cell. Biochem. 357, 295–303.
Youle, R.J., Strasser, A., 2008. The BCL-2 protein family: opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 9, 47.