Materials Reinforced with Different Nanoparticles
Muhammet Karci, DDS ,1Necla Demir, DDS, PhD,1& Sakir Yazman, PhD21Department of Prosthodontics, Selcuk University, Faculty of Dentistry, Konya, Turkey 2Ilgın Technical Collage, Selc¸uk University, Konya, Turkey
Keywords
Flexural strength; nanoparticle; polymethylmethacrylate; SEM.
Correspondence
Muhammet Karci, Faculty of Dentistry, Department of Prosthodontics, Selcuk University, Konya 42080. Turkey. E-mail: muhammetdt48@hotmail.com This study was supported by Selcuk University Scientific Research Projects (project number 16102013).
The authors deny any conflicts of interest related to this study.
Accepted July 12, 2018
doi: 10.1111/jopr.12974
Abstract
Purpose: To evaluate the effect of adding Al2O3, SiO2, and TiO2nanoparticles in
ratios of 1, 3, and 5 wt% to different acrylic resins on flexural strength.
Materials and Methods: A total of 210 specimens were prepared in 30 groups (n= 7/group) (Control, 1% Al2O3, 3% Al2O3, 5% Al2O3, 1% SiO2, 3% SiO2, 5% SiO2,
1% TiO2, 3% TiO2, 5% TiO2). The specimens were polished with 200-, 400-, and
600-grit abrasive paper to provide a standard surface before testing and then suspended in distilled water for 30 days. Flexural strength was measured via three-point bending tests. Subsequently, SEM analysis was performed for one specimen from each group. Homogeneity of data was assessed by Kolmogov–Smirnov test followed by two-way ANOVA and Tukey HSD tests (α = 0.05).
Results: There was a significant increase in the flexural strength of polymethyl-methacrylate (PMMA) after addition of 1% nanoparticles in both heat-polymerized and autopolymerized acrylic resins (p ˂ 0.05). The flexural strength values of the groups to which Al2O3and TiO2nanoparticles were added exceeded those of
the group with SiO2 addition (p˂ 0.05). The electron microscopy images revealed
that the nanoparticles were more homogeneously dispersed in PMMA with higher flexural strength.
Conclusions: The mechanical properties of PMMA can be improved by the addition of nanoparticles to PMMA; however, the flexural strength values of PMMA decrease with the addition of nanoparticles at higher percentages (3-5%). Hence, the ideal filler ratio corresponds to 1%.
Poly (methyl methacrylate), PMMA, is widely used in pros-thetic rehabilitation of totally and partially edentulous patients due to its satisfactory esthetics, low cost, ease of use, and stabil-ity in the mouth.1However, poor mechanical properties and low
fracture resistance constitute its major disadvantages.2
Mid-line fracture of maxillary complete prostheses is a common problem.3 Hargreaves et al4 reported that 68% of complete dentures were broken within the first 3 years. These fractures are caused by chewing mastication typically at the midline of the prosthesis or dropping a denture, leading to fracture of the acrylic base.5-7
Denture base resins used in dentistry are polymerized by heat, chemicals, visible light, and microwave energy.8
Heat-polymerized acrylic resins are frequently used in denture base materials. The use of microwave energy for PMMA polymer-ization is an increasingly popular method in which the poly-merization process occurs in a very short time when compared to that of the heat-polymerization technique.9 The particulate
structures of autopolymerized acrylic resins are irregular. They undergo plastic deformation in a short time. The polymerization
process is rapid, albeit not complete, and a residual monomer is detected at different ratios after polymerization.10However,
mechanical properties, such as flexural strength, elastic modu-lus, and surface hardness, are also important parameters.11The
minimum flexural strength value of standard dental base poly-mers is prescribed as 65 MPa by the ISO 1567 International Standard.12 The strength values of any filler and modifying agent to be added must not be lower than this limit.13
Different techniques including a rubber phase, metal wires, metal oxides, and fibers have been used to improve their me-chanical properties and clinical use.14Developments in the field
of nanotechnology, nanoparticles, nanotubes, and nanofibers were recently used to reinforce acrylic resins.15
Significant attention has focused on the incorporation of inorganic nanoparticles into PMMA to improve properties.16
Nanoparticles are characterized by their small size, large spe-cific surface area, and strong interfacial interaction with or-ganic polymers.17The surface of the material is in nanometer
dimension, and this leads to unique properties. Hence, when compared to bulk properties, different mechanical, chemical,
Karci et al Flexural Strength of Denture Base Materials
electrical, optical, magnetic, electro-optic, and magneto-optic properties may appear due to the significantly wide surface area relative to the volume of the material.18The properties of
polymer nanocomposites depend on the type of incorporating nanoparticles, their sizes, and shapes as well as the concentra-tion and interacconcentra-tion with the polymer matrix.16Commonly used nanoparticles include silver (Ag), aluminum oxide (Al2O3),
sil-icon dioxide (SiO2), zirconium dioxide (ZrO2), and titanium
dioxide (TiO2).
Several mechanisms have been described as occurring at the polymer surface. Chaudhary et al observed that fractured surfaces of the toughened polymers are very rough, and charac-teristic features are associated with microcracking, crack path deflection, particle yielding, crack pinning, particle bridging, and crack bowing mechanisms. Each of the aforementioned properties contributes toward the overall toughening.19
Heat-polymerized or autopolymerized acrylic resins were used in all previous studies; however, three acrylic resins, namely heat-polymerized, autopolymerized, and microwave acrylic resins, have not yet been compared within a single study. Therefore, the purpose of the present study is to evaluate the ef-fect of the addition of aluminum oxide (Al2O3), silicon dioxide
(SiO2), and titanium dioxide (TiO2) nanoparticles on the
flexu-ral strength of three different acrylic resins (heat-polymerized, autopolymerized, and microwave acrylic resins). The null hy-pothesis (H0) was that nanoparticle addition would not change
the flexural strength of denture base materials.
Materials and methods
A power analysis was performed (G*Power software v3.1.10) to calculate the sample size required for each group. The results indicated a power value of 95 for an effect size ofƒ = 0.8, α = 0.05, no centrality parameter of 18, and critical t value of 2.8. A requirement of 7 specimens in each group was determined.
In this study, three nanoparticles (Al2O3, SiO2, TiO2) were
added in ratios of weight 1, 3, and 5 wt% to a total of 210 acrylic resin specimens polymerized with three different meth-ods (heat, autopolymerization, and microwave) (n= 7). Each acrylic resin type was categorized into the following 10 groups: pure PMMA, PMMA with 1% Al2O3, PMMA with 3% Al2O3,
PMMA with 5% Al2O3, PMMA with 1% SiO2, PMMA with
3% SiO2, PMMA with 5% SiO2, PMMA with 1% TiO2, PMMA
with 3% TiO2, and PMMA with 5% TiO2. To examine the
distribution of nanoparticles in all the specimens, the fracture surfaces of all the specimens from the flexural tests were ex-amined by using scanning electron microscopy (SEM). The denture base materials, nanoparticles, and size and ratio of nanoparticles are listed in Table 1.
Standardized specimens (65× 10 × 3 mm3) were prepared,
as per the ISO 1567 standard, by mixing 10 g powder polymer and 4.3 ml liquid monomer for all acrylic resins. For prepa-ration of specimens from nanoparticle-added acrylic denture base resin, 10 g PMMA powder was supplemented with 1, 3, and 5 wt% Al2O3 nanoparticles (99.5% purity, 18 nm
parti-cle size, 140 m2/g specific surface area, 3.9 g/cm3 intensity,
white color), SiO2nanoparticles (99.5% purity, 15 nm particle
size, 150-550 m2/g specific surface area, 2.2 g/cm3 intensity, white color), and TiO2 nanoparticles (99.5% purity, 13 nm
Table 1 Materials used
Material Product Manufacturer
Heat-polymerized acrylic resin
Meliodent Heraeus Kulzer, Newbury Berkshire, UK Autopolymerized acrylic
resin
Meliodent Heraeus Kulzer, Newbury Berkshire, UK Microwave acrylic resin Acron MC GC Dental, Tokyo, Japan Aluminum oxide (Al2O3) Nanografi Nanografi, Ankara, Turkey (18 nm; 1%, 3%, 5%)
Silicon dioxide (SiO2) Nanografi Nanografi, Ankara, Turkey (15 nm; 1%, 3%, 5%)
Titanium dioxide (TiO2) Nanografi Nanografi, Ankara, Turkey (13 nm; 1%, 3%, 5%)
particle size, 60 m2/g specific surface area, 4.1 g/cm3
inten-sity, white color) (Fig 1). Nanoparticle powders were measured with precision scales to add the same to acrylic powder (Denver Instruments, Bohemia, NY).
To prepare heat-polymerized acrylic resin specimens, wax molds were flasked using Type II dental stone (Moldano; Her-aeus Kulzer, Hanau, Germany). After the wax boiled out, the acrylic powder and liquid were mixed in a ratio of 23.4 g/10 mL in accordance with manufacturer’s instructions. Afterward, the previously prepared mixture was left for 10 minutes and then packed in to the flask. The flasks were compressed by using a hydraulic press machine. All the flasks immersed in a water bath and were kept at 74± 1°C in a water-bath for 8 hours and subsequently boiled for 2 hours (Fig 1). With respect to autopolymerized acrylic resin specimens, acrylic powder and liquid were mixed in a ratio of 23.4 g/10 mL in accordance with manufacturer’s instructions and later kept in a flask for 10 minutes under 20 psi (Fig 1).
With respect to autopolymerized acrylic resin specimens, acrylic powder and liquid were mixed in a ratio of 23.4 g/10 mL in accordance with manufacturer’s instructions and later kept in a flask for 10 minutes under 20 psi (Fig 1).
Fiber-reinforced plastic flasks (FRP Flask; GC Industrial Corp, Tokyo, Japan) were used while preparing acrylic resin specimens polymerized with microwave energy. After the flask was compressed with screws and closed, it was microwaved for a minute inside a microwave oven (Arcelik Intellowave MD554, Bolu, Turkey) with an amplitude of 2450 MHz microwave and a power of 500 W. Acron MC acrylic resin (Microwave Curing Denture Base Resin; GC Dental) was polymerized for 3 min-utes after mixing powder/liquid in a ratio of 100 g/43 mL ac-cording to the manufacturer’s instructions (Fig 1). After the flasks were left to cool at room temperature for 30 minutes, they were placed under running cold water for approximately 20 minutes to ensure complete cooling. The finishing processes of the obtained specimens were performed by using abrasive papers (200, 400, and 600 grit, waterproof silicon carbide pa-per; English Abrasives Ltd., London, UK) by a clinician for 5 minutes.
Nanoparticles were mixed for homogeneous distribution for 2 hours at room temperature (Pulverisette-5; Fritsch Inter-national, Idar-Oberstein, Germany) with a rotation speed of
Figure 1 Schematic appearance of specimen preparation.
400 cycles/minute in a dry condition. Steel balls (7 mm diam-eter) were used for the mixing process (Fig 1).
To determine the flexural strength of the specimens, three-point bending tests were performed with an Instron universal test machine (Universal Testing Machine; Instron Corp., Nor-wood, MA) at a speed of 5 mm/min. The specimens were placed on two supports, and the distance between the supports was 50 mm. A load was applied until the specimen fractured. Flexural strength was calculated as follows:
σ = 3Pl
2bd2 (1)
whereσ = flexural strength (N/mm2= MPa), F = the
max-imum load during fracture (N), l= the distance between the supports (50 mm), b = the specimen width (mm), and d = specimen thickness (mm). SEM analysis was performed via the SEM device FEI (Quanta FEG-250) at the Selcuk Univer-sity Advanced Technology Research and Application Center. Statistical analysis
The SPSS 22.0 statistical program (IBM, Armonk, NY) was used for the statistical analyses. To preserve the homogeneity of the study, data were evaluated by the Kolmogorov-Smirnov test. A two-way ANOVA test was used to compare averages between groups, and Tukey HSD tests were used for multiple comparisons (α = 0.05).
Results
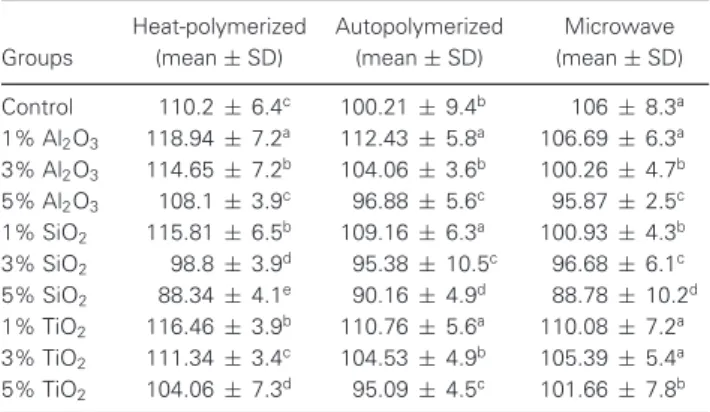
Generally, the flexural strength values of heat-polymerized acrylic resin specimens exceeded those of autopolymerized and microwave acrylic resin specimens (Table 2). With respect to heat-polymerized acrylic specimens, the flexural strength
val-Table 2 Flexural strength values of groups (MPa)
Groups Heat-polymerized (mean± SD) Autopolymerized (mean± SD) Microwave (mean± SD) Control 110.2± 6.4c 100.21 ± 9.4b 106± 8.3a 1% Al2O3 118.94 ± 7.2a 112.43 ± 5.8a 106.69 ± 6.3a 3% Al2O3 114.65 ± 7.2b 104.06 ± 3.6b 100.26 ± 4.7b 5% Al2O3 108.1 ± 3.9c 96.88± 5.6c 95.87± 2.5c 1% SiO2 115.81 ± 6.5b 109.16 ± 6.3a 100.93 ± 4.3b 3% SiO2 98.8± 3.9d 95.38± 10.5c 96.68± 6.1c 5% SiO2 88.34 ± 4.1e 90.16± 4.9d 88.78± 10.2d 1% TiO2 116.46 ± 3.9b 110.76 ± 5.6a 110.08 ± 7.2a 3% TiO2 111.34 ± 3.4c 104.53 ± 4.9b 105.39 ± 5.4a 5% TiO2 104.06 ± 7.3d 95.09± 4.5c 101.66 ± 7.8b SD: Standard Deviation; same letters in the column denote no statistically sig-nificant differences between groups (p> 0.05).
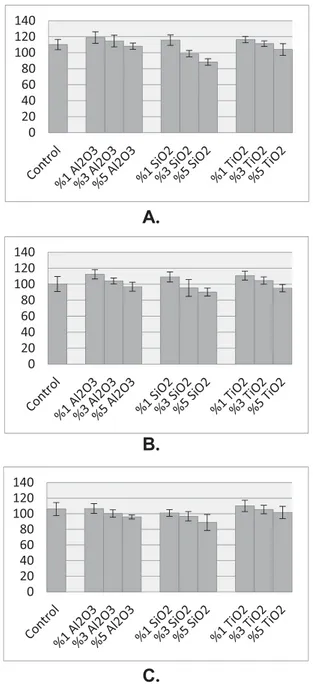
ues of groups with 1% ratio of nanoparticles were found to statistically significantly exceed those of the control group (p ˂ 0.05). There was no difference between the val-ues for Al2O3 and TiO2 nanoparticles (p ˃ 0.05);
how-ever, the values in the groups with SiO2 nanoparticles were
lower when compared with those of groups with other par-ticles (p ˂ 0.05) (Fig 2A). With respect to autopolymer-ized acrylic resin specimens, the flexural strength values of groups with a 1% ratio of nanoparticles were found to statistically significantly exceed those of the control group (p˂ 0.05) (Fig 2B). With respect to microwaved acrylic resin specimens, the highest flexural strength values were measured for groups with added TiO2nanoparticles; however, there were
no statistically significant differences (p˃ 0.05) (Fig 2C). Ad-ditionally, the lowest values in all groups were observed in the specimens added with 5% ratio of SiO2nanoparticles.
Karci et al Flexural Strength of Denture Base Materials 0 20 40 60 80 100 120 140 0 20 40 60 80 100 120 140 0 20 40 60 80 100 120 140
A.
B.
C.
Figure 2 Average values of flexural strength within 95% confidince
limits. (A) Heat-polymerized acrylic resin. (B) Autopolymerized acrylic resin. (C) Microwave acrylic resin.
Figure 3 shows the SEM images of heat-polymerized acrylic resin specimens. Figure 3A shows TiO2 nanoparticles in the
group with a 1% ratio of TiO2 nanoparticles added. Figures
3B and C show the groups with a 5% ratio of Al2O3and TiO2
nanoparticles and Al2O3agglomeration (red arrow:
agglomer-ation, white arrow: nanoparticle). This image indicates that the tendency for agglomeration increases with the ratio of nanopar-ticles. Figure 3D shows the crack pinning mechanism.
Figure 4 shows SEM images of autopolymerized acrylic resin specimens. The group added with 5% ratio of SiO2nanoparticle
exhibited more bubbles when compared with those observed in the heat-polymerized acrylic resin (Fig 4A). This reveals
that autopolymerized acrylic resins possess poor mechanical properties. The crack pinning mechanism of the same group is shown in Figure 4B. Figure 4C shows the Al2O3nanoparticles
and agglomerated structures in the group with the 1% ratio of Al2O3 nanoparticles. Figure 4D shows that more
agglomera-tions and pull-outs are present in the group with 3% ratio of TiO2nanoparticles (red arrow: pull-out, white arrow:
nanoparti-cle, black arrow: agglomeration). Increases in the percentage of nanoparticles make polymerization more difficult and weaken mechanical properties.
Figure 5 shows the SEM images of microwave acrylic resin specimens. In a manner similar to autopolymerized acrylic resin, extensive agglomerated particles and pull-outs are ob-served in the group with 1% Al2O3 nanoparticles (Fig 5A)
(black arrow: pull-out, red arrow: agglomeration). This result indicates that microwaved and autopolymerized acrylic resins exhibit similar mechanical properties. Figures 5B and 5C show SEM images of groups with 1% SiO2 nanoparticles.
Exten-sive agglomerated, non-homogeneous, and porous areas are ob-served with an increase in the particle ratio (red arrow: bubble, black arrow: extensive pull-out). Homogeneous distribution of SiO2is difficult at high ratios, and this situation supports weaker
mechanical properties. Agglomeration, non-homogeneous dis-tribution, and pull-outs similar to those in autopolymerized acrylic resins are present when Al2O3nanoparticles are added
in high ratios (5%) (Fig 5D) (red arrow: agglomeration, white arrow: non-homogenous distribution, black arrow: nanoparti-cle, blue arrow: pull-out).
Discussion
The null hypothesis was rejected, since the flexural strength of denture base materials changed after nanoparticle addition. The aim of the present study involved changing the composition of PMMA with different fillers and/or different mixing meth-ods to improve its mechanical properties. Therefore, different fillers, such as fibers and nanoparticles or hybrid reinforcement methods, were used.20,21Fibers pose a few disadvantages such
as causing tissue irritation and weak binding to acrylic resins. Current reinforcement methods involve the addition of various nanofillers such as silver, aluminum oxide (alumina), titanium dioxide, and silicon dioxide (silica); However, clinicians have not agreed on an effective method.
In the process of improving the mechanical properties of polymer composites, shape and size of the filler particles, dis-tribution in the polymer matrix, and connection to the matrix play very important roles. The size of metal oxides should be sufficiently low for homogenous mixtures.22In this study,
approximately 15 nm metal oxide (Al2O3and TiO2) and
min-eral (SiO2) particles were mixed in approximately 121.2μm
acrylic powder. This prevents the formation of a heterogeneous mixture, and nanoparticles fill in the cracks between polymer particles, thereby preventing the movement of a polymer chain. Additionally, the percentage of filler should be kept low to ensure that the particles are embedded in the resin.23
Previous studies report that different percentages of nanopar-ticles can lead to positive and/or negative effects on the flex-ural strength of acrylic resins. Akkus et al24 added 1% and 3% of 40 to 50 nm Al2O3 and 15 nm SiO2 nanoparticles to
Figure 3 Scanning electron microscope images of heat-polymerized acrylic resin specimens. (A) 1% TiO2group (original magnification 10,000×). B, 5% TiO2 group (original magnification 10,000×). C, 5% Al2O3 group (original magnification 10,000×). D, Crack pinning mechanism (original magnification 250×).
heat-polymerized acrylic resin, and they observed the highest flexural strength values in the control group. Ahmed et al25
added 1% and 5% 40 nm TiO2 nanoparticles to traditional
heat-polymerized and high resistance heat-polymerized acrylic resin powder and reported a decrease in flexural strength val-ues. Nazirkar et al26examined the flexural strength values of
heat-polymerized acrylic resin specimens in which 0.5% and 1% 7 nm TiO2 nanoparticles were added and suggested that
flexural strength decreased with the addition of the nanoparti-cles. In contrast to the aforementioned studies, Harini et al27
added TiO2 nanoparticles (1%, 2%, and 5%) to PMMA and
revealed that the highest flexural strength values were observed in the group with 5% nanoparticles added. Also, Ihab and Moudhaffar28compared flexural strength values after addition of ZrO2 nanoparticles and reported a statistically significant
decrease of more than 5%. In our study, the highest flexural strength values were found in the group with the 1% ratio of nanoparticles, and the lowest flexural strength values were ob-served in the group added with the 5% ratio of nanoparticles. In concentrations exceeding 1%, a statistically significant de-crease was observed in flexural strength values.
Traditionally, complete removable dental prostheses are manufactured from PMMA resin, which requires heat-polymerization, autopolymerization or microwave polymeriza-tion and manual mixing of the resin powder and liquid.29,30
Computer-aided design and computer-aided manufacturing
(CAD/CAM) has been introduced for removable denture fabri-cation. Because CAD/CAM denture base resins are more highly condensed and have fewer porosities, they have superior me-chanical properties.31Srinivasan et al32compared the
mechan-ical properties of CAD/CAM and conventional PMMA resins and concluded that CAD/CAM resin presents improved me-chanical properties over traditional heat-polymerized PMMA resin; however, Steinmassl et al33 examined fracture
resis-tance of five CAD/CAM denture base resin specimens, a heat-polymerizing resin, and an autoheat-polymerizing resin. They indi-cated that CAD/CAM denture base resins do not generally have better mechanical properties than manually processed resins. The flexural strength values can be provided close to CAD/CAM resins, as in our study, which resulted in an in-crease of up to 12%.
On the other hand, because thermal and mechanical aging processes were not performed, and there was no saliva in the environment, it is not known whether these differences in values will be observed clinically. The lack of this factor may be a limitation in this study, but can be investigated in the future by in vivo studies.
The biggest issue in nanocomposite production is the for-mation of agglomerations. Wide surface areas of nanoparti-cles show a tendency for agglomeration.34Thus, both residual
monomers act as a plasticizer,35and the monomers negatively
Karci et al Flexural Strength of Denture Base Materials
Figure 4 Scanning electron microscope images of autopolymerized acrylic resin specimens. (A) 5% SiO2group (original magnification 51×). (B) 5% SiO2group crack pinning mechanism (original magnification 250×). (C) 1% Al2O3group (original magnification 1000×). (D) 3% TiO2group (original magnification 500×).
Figure 5 Scanning electron microscope images of microwaved acrylic resin specimens. (A) 1% Al2O3group (original magnification 1000×). (B) 1% SiO2group (original magnification 10,000×). (C) 5% SiO2group (original magnification 58×). (D) 5% Al2O3group (original magnification 1000×).
stress formation.34 A few extant studies recommend the use
of coupling agents, such as 3-methacrylo-propyl-trimethoxy-silane (MPTS), to prevent agglomeration of particles.36 Only
well-distributed particles form extensive contact areas and en-able the formation of new characteristics in the composite through the development of stress transfer. Thus, the improve-ment in mechanical properties are observed with respect to very low filling ratios.37In the present study, the increase in flexural
strength at low ratios (1%) when compared with the control group and the decrease at higher ratios (3% and 5%) were as-sociated with the increase in the agglomeration tendency of the nanoparticles. Additionally, SiO2exhibited the lowest density
among the nanoparticles used, and thus the particle amount per unit area in SiO2nanoparticles exceeded those observed in
other nanoparticles when equal amounts of nanoparticles were added. This explains the lower mechanical characteristics in SiO2nanoparticle-added groups, and it is also supported by the
appearance of agglomerated particles in the SEM images. The presence of agglomeration and non-homogeneous distri-bution has led to the discovery of new mixing methods for better distribution.37Mechanical or chemical methods such as melt
mixing, mixing in solution, mixing with shear force and high shear force during mechanical mixing, and mixing with ultra-sonic mixer are used.38Although some investigators have added
nanoparticles into the monomer (liquid),26,27,39-41 some
re-searchers have also mixed them into acrylic resin polymers.42-45
In addition, Mc Nally et al45 explained that SiO
2 cannot be
mixed homogeneously with more than 1% using manual mix-ing. As with Mc Nally et al45and Sodagar et al,39in our study, the nanoparticles were added to the acrylic resin polymer to prevent agglomeration of SiO2nanoparticles in liquid at high
concentrations.
Extant studies have not examined the mechanical prop-erties of heat-polymerized, autopolymerized, and microwave acrylic resin after the addition of nanoparticles. This study compared different acrylic resin types and suggested that heat-polymerized acrylic resin specimens exhibit improved mechan-ical properties when compared with the other acrylic resin types. This is attributed to the smoother fracture surfaces in heat-polymerized acrylic resins, pull-out of the nanoparticles on the surface, and less porosity. This is affected by the short poly-merization process of autopolymerized and microwaved acrylic resins. Additionally, this situation is also prevented by increas-ing the strength of the polymerization oven for microwaved acrylic resins.
Conversely, the materials used inside the mouth should be acceptable esthetically.38In recent studies, TiO2nanoparticles
were used to reinforce acrylic resins; however, it was simul-taneously reported to cause color changes.42,46,47Ghahremani
et al48 modified acrylic resins with color pigments after the
addition of TiO2 nanoparticles. In our study, visible opacity
was observed in specimens containing 5% TiO2nanoparticles.
Color pigments can be used in future studies to prevent color change.
Conclusions
1. SEM images show that particles were distributed more homogeneously in groups with high flexural strength
val-ues. A homogeneous distribution of particles exceeding 1% was not easy to achieve, and the agglomeration ten-dency increased with respect to the ratio of nanoparticles. 2. The most suitable ratio corresponds to 1% for nanopar-ticles that are added with mechanical methods without any surface application.
3. In vivo studies including aging are recommended to un-derstand the effect of clinical applications.
Acknowledgment
We would like to thank A. Nilgun Ozturk for contribution to the planning stage of this study.
References
1. Acosta-Torres LS, Mendieta I, Nu˜nez-Anita RE, et al: Cytocompatible antifungal acrylic resin containing silver nanoparticles for dentures. Int J Nanomedicine 2012;7: 4777-4786
2. Faot F, da Silva WJ, da Rosa RS, et al: Strength of denture base resins repaired with auto- and visible light-polymerized materials. J Prosthodont 2009;18:496-502
3. Machado C, Sanchez E, Azer SS, et al: Comparative study of the transverse strength of three denture base materials. J Dent 2007;35:930-933
4. Hargreaves AS: The prevalence of fractured dentures. A survey. Br Dent J 2007;126:451-455
5. Beyli MS, Von Fraunhofer JA: An analysis of causes of fracture of acrylic resin dentures. J Prosthet Dent 1981;46:238-241 6. Darbar UR, Huggett R, Harrison A: Denture fracture-a survey.
Br Dent J 1994;176:342-345
7. Polyzois GL, Andreopoulos AG, Lagouvardos PE: Acrylic resin denture repair with adhesive resin and metal wires: effects on strength parameters. J Prosthet Dent 1996;75:381-387 8. Goldberg AJ, Burstone CJ: The use of continuous fiber
reinforcement in dentistry. Dent Mater 1992;8:197-202 9. Bartoloni JA, Murchison DF, Wofford D, et al: Degree of
conversion in denture base materials for varied polymerization techniques. J Oral Rehabil 2000;27:488-493
10. Bayındır F, Akyıl S¸, Kavrut R: Farklı Zaman Aralıklarında Suda Bekletmenin Protez Kaide Materyallerinin Transvers B¨uk¨ulme ve Transvers Dayanıklılık ¨Ozellikleri ¨Uzerindeki Etkisinin ˙Incelenmesi. Hacettepe Dis¸ Hek Fak Derg 2005;2:16-23 11. Aeran H, Kumar V, Uniyal S, et al: Nanodentistry: is just a
fiction or future? J Oral Biol Craniofac Res 2015;5:207-211 12. International Organization for Standardization. ISO 1567.
Dentistry denture base polymers 1999. Available at: http://www.iso.org/iso/store.html. Accessed 11/2/99 13. Andreotti AM, Goiato MC, Moreno A, et al: Influence of
Nanoparticles on color stability, Microhardness and flexural strength of acrylic resin specific for ocular prosthesis. Int J Nanomedicine 2014;9:5779-5787
14. Price CA: A history of dental polymers. J Prosthodont 1994;8:47–54
15. Memon MS, Yunus N, Razak AA: Some mechanical properties of a highly cross-linked, microwave-polymerized, injection-molded denture base polymer. Int J Prosthodont 2001;14:214-218 16. Jordan J, Jacop KL, Tannenbaum R, et al: Experimental trends in
polymer nanocomposites- a review. Mater Sci Eng 2005;393: 1-11
Karci et al Flexural Strength of Denture Base Materials
17. Han Y, Zhao Y, Xie C, et al: Color stability of pigmented maxillofacial silicone elastomers: effects of nano-oxides as opacifiers. J Dent 2010;38:e100-e105
18. Hajipour MJ, Fromm KM, Ashkarran AA, et al: Antibacterial properties of nanoparticles. Trends Biotechnol 2012;30:499-511 19. Chaudhary S, Parthasarathy S, Kumar D, et al: Simple
toughening of epoxy thermosets by preformed thermoplastics. SPE Plastic Research Online, 2014. https://doi.org/10.2417/ spepro.005409
20. Meng TR, Latta MA: Physical properties of four acrylic denture base resins. J Contemp Dent Pract 2005;6:93-100
21. Alla R, Raghavendra K, Vyas R, et al: Conventional and contemporary polymers for the fabrication of denture prosthesis: part I–overview, composition and properties. Int J Appl Dent Sci 2015;1:82-89
22. Unal H, Mimaroglu A: Influence of filler addition on the mechanical properties of nylon-6 polymer. J Rein Plast Com 2004;23:461-469
23. Korkmaz T, Dogan A, Usanmaz A: Dynamic mechanical analysis of provisional resin materials reinforced by metal oxides. Biomed Mater Eng 2005;15:179-188
24. Akkus B, Ozturk AN, Yazman S, et al: Effects of Al2O3and
SiO2nanoparticles on flexural strength of heat cured acrylic
resin. Int J Enhan Res Sci Tech 2015;4:158-163
25. Ahmed MA, El-Shennawy M, Althomali YM, et al: Effect of titanium dioxide nano particles incorporation on mechanical and physical properties on two different types of acrylic resin denture base. J Nano Sci Eng 2016;6:111-9
26. Nazirkar G, Bhanushali S, Singh S, et al: Effect of anatase titanium dioxide nanoparticles on the flexural strength of heat cured poly methyl methacrylate resins: An in-vitro study. J Indian Prosthodont Soc 2014;14:144-149
27. Harini P, Mohamed K, Padmanabhan TV: Effect of titanium dioxide nanoparticles on the flexural strength of
polymethylmethacrylate: an in vitro study. Indian J Dent Res 2014;25:459-463
28. Ihab N, Moudhaffar M: Evaluation the effect of modified nano-fillers addition on some properties of heat cured acrylic denture base material. J Bagh Colleg Dent 2011;23:23-29 29. Nogueira SS, Ogle RE, Davis EL: Comparison of accuracy
between compression and injection-molded complete dentures. J Prosthet Dent 1999;82:291-300
30. Steinmassl PA, Klaunzer F, Steinmassl O, et al: Evaluation of currently available CAD/CAM denture systemsi Int J Prosthodont 2017;30:116-122
31. Infante L, Yılmaz B, McGlumphy E, et al: Fabricating complete dentures with CAD/CAM technology. J Prosthet Dent
2014;111:351-355
32. Srinivasan M, Gjengedal H, Cattani-Lorente M, et al:
CAD/CAM milled complete removable dental prostheses: an in vitro evaluation of biocompatibility, mechanical properties, and surface roughness. Dent Mater J 2018;37:526-533
33. Steinmassl O, Offermanns V, St¨ockl W, et al: In vitro analysis of fracture resistance of CAD/CAM denture base resins. Materials (Basel) 2018;11:E401
34. Han Y, Kiat-amnuay S, Powers JM, et al: Effect of nano-oxide concentration on the mechanical properties of a maxillofacial silicone elastomer. J Prosthet Dent 2008;100:465-473 35. Shibata T, Hamada N, Kimoto K, et al: Antifungal effect of
acrylic resin containing apatite-coated TiO2photocatalyst. Dent
Mater J 2007;26:437-444
36. Kanie T, Arikawa H, Fujii K, et al: Physical and mechanical properties of PMMA resins containing
γ -methacryloxypropyltrimethoxysilane. J Oral Rehabil
2004;31:166-171
37. Wetzel B, Rosso P, Haupert F, et al: Epoxy
nanocomposites—fracture and toughening mechanisms. Eng Frac Mech 2006;73:2375-2398
38. Xia H, Wang Q: Preparation of conductive polyaniline/nanosilica particle composites through ultrasonic irradiation. J Appl Polym Sci 2003;87:1811-1817
39. Ghaffari T, Hamedirad F, Ezzati B: In vitro comparison of compressive and tensile strengths of acrylic resins reinforced by silver nanoparticles at 2% and 0.2% concentrations. J Dent Res Dent Clin Dent Prospects 2014;8:204-209
40. Sodagar A, Kassaee MZ, Akhavan A, et al: Effect of silver nanoparticles on flexural strength of acrylc resins. J Prosthodont Res 2012;56:120-124
41. Salman TA, Khalaf HA: The influence of adding modified ZrO2-TiO2nanoparticles on certain physical and mechanical
properties of heat polymerized acrylic resin. J Bagh Colleg Dent 2015;27:33-39
42. Safarabadi M, Khansari N, Rezaei A: An experimental investigation of HA/Al2O3nanoparticles on mechanical
properties of restoration materials. Engin Sol Mechan 2014;2:173-182
43. Shirkavand S, Moslehifard E: Effect of TiO2nanoparticles on
tensile strength of dental acrylic resins. J Dent Res Dent Clin Dent Prospects 2014;8:197-203
44. da Silva LH, Feitosa SA, Valera MC, et al: Effect of the addition of silanated silica on the mechanical properties of microwave heat-cured acrylic resin. Gerodontology 2012;29:e1019-1023
45. Ismail IJ, Jasim BS: The effect of silanized alumina nano-fillers addition on some physical and mechanical properties of heat cured polymethyl methacrylate denture base material. J Bagh Colleg Dent 2014;26:18-23
46. Mc Nally L, O’Sullivan DJ, Jagger DC: An in vitro investigation of the effect of the addition of untreated and surface treated silica on the transverse and impact strength of poly(methyl methacrylate) acrylic resin. Biomed Mater Eng 2006;16: 93-100
47. Acosta-Torres L, Lopez-Marin L, Nunez-Anita RE, et al: Biocompatible metal-oxides nanoparticles: nanotechnology improvement of conventional prosthetic acrylic resins. J Nanomater 2011; 2011:941561
48. Ghahremani L, Shirkavand S, Akbari F, et al: Tensile strength and impact strength of color modified acrylic resin reinforced with titanium dioxide nanoparticles. J Clin Exp Dent 2017;9: e661-e665