Full Terms & Conditions of access and use can be found at
https://www.tandfonline.com/action/journalInformation?journalCode=ibih20
Biotechnic & Histochemistry
ISSN: 1052-0295 (Print) 1473-7760 (Online) Journal homepage: https://www.tandfonline.com/loi/ibih20
Effects of long term oral acrylamide
administration on alpha naphthyl acetate esterase
and acid phosphatase activities in the peripheral
blood lymphocytes of rats
Y. Yener, İ. Çelik, E. Sur, Y. Öznurlu & T. Özaydin
To cite this article: Y. Yener, İ. Çelik, E. Sur, Y. Öznurlu & T. Özaydin (2019) Effects of long term oral acrylamide administration on alpha naphthyl acetate esterase and acid phosphatase activities in the peripheral blood lymphocytes of rats, Biotechnic & Histochemistry, 94:5, 352-359, DOI: 10.1080/10520295.2019.1571227
To link to this article: https://doi.org/10.1080/10520295.2019.1571227
Published online: 13 Mar 2019.
Submit your article to this journal
Article views: 117
View related articles
Effects of long term oral acrylamide administration on alpha naphthyl acetate
esterase and acid phosphatase activities in the peripheral blood lymphocytes of
rats
Y. Yenera, İ. Çelikb, E. Surb, Y. Öznurlub, and T. Özaydinb
aEducation Faculty, Bolu Abantİzzet Baysal University, Bolu, Turkey;bVeterinary Faculty, Konya Selçuk University, Konya, Turkey ABSTRACT
Acrylamide is an important industrial chemical; it also is formed in starch-rich foodstuffs during baking, frying and roasting. Most acrylamide exposure occurs by ingestion of processed foods. We investigated possible immunotoxic effects of extended administration of low doses of acrylamide in rats. To do this, we measured alpha-naphthyl acetate esterase (ANAE) and acid phosphatase (ACP-ase) activities in peripheral blood lymphocytes. Male and female weanling Wistar rats were administered 2 or 5 mg acrylamide/kg/day in drinking water for 90 days. Peripheral blood was sampled at the end of the administration period. We found ANAE staining in eosinophils and T-lymphocytes, but not in monocytes, platelets, B-lymphocytes and neutrophils. ACP-ase was found in B-lymphocytes. We found a significant reduction of the ratio of ANAE:ACP-ase in lymphocytes of the experimental animals compared to controls. We found no statistically significant differences between the doses or sexes. We found that acrylamide ingested in processed foods might affect the immune system adversely by decreasing the population of mature T- and B-lymphocytes.
KEYWORDS Acid phosphatase; acrylamide; alpha-naphthyl acetate esterase; blood; enzyme histochemistry; lymphocytes; rats
Acrylamide is a colorless, odorless, water soluble monomer of vinyl that is formed by hydration of acrylonitrile. Acrylamide also is a byproduct formed during heating of starch-rich foods. Industrially, acrylamide is widely used to produce polymers in pulp and paper, and for water treatment and the textile industries. It also is used as a laboratory reagent. Because acrylamide has a reactive double bond and an amide group, it potentially is a human carcinogen (Adler et al. 1993; Kurenkov1997). The discovery of spontaneous formation of acrylamide during high temperature frying of carbohydrate-rich foods, such as potatoes, by two independent groups (Stadler et al. 2002; Tareke et al. 2002) indicated that people are exposed to this substance by ingestion of fried foodstuffs. The most important foodstuffs that contribute to acrylamide exposure include biscuits, coffee, crackers, crisp bread, fried potato products and soft bread (EFSA
2015). Acrylamide is absorbed rapidly after ingestion and is conjugated with glutathione for excretion in urine. In humans, ingested acrylamide is absorbed rapidly and metabolized; acrylamide has an average half-life of 3.1–3.5 h (Fennell et al.2006).
The discovery of acrylamide formation in fried foods has elicited a number of reports concerning its possible harmful effects. Harmful effects of acrylamide on the
nervous and reproductive systems of some animal species as well as carcinogenic effects have been documented by EFSA (2015), IARC (1994) and WHO (2005).
The literature contains reports of acute immunotoxic effects of high doses of acrylamide (Zaidi et al.1994; Yener et al. 2013; Fang et al. 2014; Zamani et al. 2017a, b). Significant decreases in the weight of lymphoid organs, especially mesenteric lymph nodes, spleen and thymus have been reported. We reported earlier the acute effects of high acrylamide doses, 30, 45 and 50 mg/kg, given for 5 consecutive days or as single oral doses 125, 150 and 175 mg/kg, on the lymphoid organs of rats. These doses caused significant atrophy of the lymphoid folliles in rat ileal Peyer’s patches (IPPs). In those animals, lymphoid cell depletion in the follicles of IPPs was evident. The follicles were reduced in size as a result of the loss of lymphocytes. Also, germinal centers were regressed and reduced in size in acrylamide treated animals compared to controls. The numbers of alpha-naphthyl acetate esterase (ANAE) positive lymphocytes in both IPPs and peripheral blood were decreased significantly in all acrylamide treated animals in a dose-dependent manner (Yener et al.2013).
Identification of leukocyte enzymes may provide important information about the functional status and maturity of the immune system. Enzyme cytochemistry
CONTACTYeşim Yener yesimyener77@gmail.com Education Faculty, Bolu Abantİzzet Baysal University, Bolu, Turkey 2019, VOL. 94, NO. 5, 352–359
https://doi.org/10.1080/10520295.2019.1571227
provides useful information for diagnosing immune system disorders (Gul et al. 2007). The cells involved in the immune system include lymphocytes, and lymphocytes in the blood are classified as T- and B-lymphocytes. Although there are special techniques to differentiate these lymphocytes, these techniques are both very expensive and time-consuming. In recent years, ANAE has been used to identify these subtypes of lymphocytes in humans and some animals, because ANAE is found in T-lymphocytes and is not found in B-lymphocytes (Knowles et al. 1978; Osbaldiston and Sullivan 1978; Kajikawa 1983). Monocytes and macrophages exhibit diffuse granular staining for ANAE. It has been suggested that ANAE is responsible for the cytotoxic effects of T-lymphocytes and macrophages (Mueller et al. 1975; Catowsky1981).
Acid phosphatase (ACP-ase) is a lysosomal acid hydrolase that is found mainly in lymphocytes, megakaryocytes, mononuclear phagocytic system cells, myelocytes, plasma cells, polymorphic nuclear leukocytes and thrombocytes (Catowsky 1981). ACP-ase activity is particularly high in macrophages (Li et al. 1970). ACP-ase positive lymphocytes are observed mainly in B-lymphocyte regions of lymphoid organs of chickens, rodents and humans (Catowsky 1981).
Typical human exposure to acrylamide involves low doses over long periods. Consequently, our experimental design mimicked this pattern and the effects of acrylamide exposure were investigated by determining ANAE and ACP-ase positive peripheral blood lymphocytes.
Materials and methods
Chemicals
All chemicals used were obtained from Teknik Kimya Co., Ltd., Konya, Turkey. Pure acrylamide (Cas 79-06-1; analytical standard, ≥ 99.0%,) was purchased from Sigma-Aldrich (St. Louis, MO).
Animals
We used 25 male and 25 female 65–70 g 4-week-old Wistar rats obtained from 10 mothers obtained from Selcuk University Experimental Medical Research and Application Center. The animals were housed in polycarbonate cages, five animals/cage. Animals were maintained in the same location throughout the experiment at 21–23 °C and 50% relative humidity with a 12 h light:12 h dark cycle. Food and water were
available ad libitum. The Experimental Animal Ethics Committee of Selcuk University Experimental Medical Research and Application Center approved our experimental protocol (Protocool Number: 2010/98).
Experimental protocol
We used 2 and 5 mg/kg/day doses of acrylamide for our study to prevent characteristic signs of acrylamide-induced neurotoxicity such as hindlimb foot splaying (Burek et al.
1980). Acrylamide was administered in the drinking water for 90 days. Drinking water containing acrylamide was prepared daily and kept in a cool dark room.
The body weights and birth times of the animals used for our study were comparable. Prior to the experiment, the animals were divided randomly and equally into five cages, five of each sex in each cage. During the 1 week acclimatization period, the average daily water consumption of animals in each cage was recorded and the amount consumed by each animal was calculated. Because the ages of the animals were the same and the body weights were comparable to within 5 g, the slight differences among animals were ignored. We then calculated the amount of acrylamide to be given as mg/kg/day and dissolved this amount in the volume of water they normally comsumed.
We divided the animals into three groups. The untreated control group consisted of five animals of each gender. Experimental-I group animals consisted of ten animals of each gender that were given 2 mg acrylamide/kg/day in drinking water for 90 days ad libitum. The animals in the experimental-II group consisted of 10 animals of each gender that were given 5 mg acrylamide/kg/day for 90 days ad libitum. Peripheral blood samples
After acrylamide administration for 90 days, animals were weighed and blood samples were collected under anesthesia in heparinized tubes (10 IU heparin/ml) from the retro-orbital plexus. Six blood smears were prepared from each sample and air dried. Three smears were used for enzyme cytochemical staining for ANAE and three were used for ACP-ase.
ANAE cytochemistry
ANAE staining was performed using the method of Knowles et al. (1978) and Maiti et al. (1990). Briefly, the smears were fixed in glutaraldehyde-acetone, pH 6.8, for 10 min at−10 °C. We dissolved 10 mg substrate (ANAE; Merck, Darmstadt, Germany) in 0.4 ml acetone and
added this slowly to 80 ml 0.1 M buffered phosphate solution (1.42 g NaH2PO4 dissolved in water, pH
adjusted to 6.0 and distilled water added to bring the volume to 100 ml). Then, 2.4 ml 4% sodium nitrite solution (Merck) and 2.4 ml pararosaniline (Merck) (1 g pararosaniline, 20 ml distilled water, 5 ml concentrated HCl) were mixed and kept for 2 min to obtain 4.8 ml hexazotized pararosaniline. This mixture was added to the buffered phosphate solution containing the substrate. The pH of the prepared solution was adjusted to 5.8 with 2 N NaOH, filtered and used as the incubation medium. The smears were incubated in this solution for 2 h at 37 °C. When inspection by microscopy showed formation of reddish brown reaction granules within the cells, the incubation was terminated and the smears were washed three times in distilled water. Control smears were incubated in the buffered phosphate solution without ANAE. Nuclei were stained with 1% methyl green (Merck) in 0.1 M acetate buffer, pH 4.2, for 10 min. In these smears, the cells that exhibited lymphocyte morphology with 1−5 large reddish brown granules that are peculiar to lymphocytes were classified as ANAE positive lymphocytes (Mueller et al.1975; Knowles et al.
1978). In each ANAE stained specimen, 200 lymphocytes were counted and percentage of ANAE positive lymphocytes was recorded.
ACP-ase cytochemistry
ACP-ase was immunostained according to Catowsky et al. (1974). Blood smears were fixed in formal-calcium at 4 ºC for 10 min and rinsed three times in distilled water. Buffered Michael’s veronal acetate, pH 5.0 (Merck), was used as a buffer solution and 10 mg naphthol AS-BI phosphate, a substrate of ACP, (Merck) dissolved in 1 ml N,N-dimethylformamide was used as a substrate solution. One milliliter naphthol AS-BI phosphate (substrate) was mixed with 5 ml buffer solution and 13 ml distilled water was added, then 1.6 ml hexazotized pararosaniline solution (0.8 ml pararosaniline, 0.8 ml 4% sodium nitrite) was added to this mixture. The final pH of the mixture was adjusted to 5.0 with 1 N NaOH, then filtered. Blood smears were incubated at 37 ºC in the incubation solution for at least 2 h and monitored until
pink-red granules began to form. The smears were rinsed three times in distilled water and nuclei were stained with 1% methyl green (Merck) in 0.1 M acetate buffer, pH 4.2, for 10 min. Control smears were incubated in a solution without naphthol AS-BI phosphate. The lymphocytes with pinkish red reaction products were considered ACP-ase positive lymphocytes. The percentage of ACP-ACP-ase positive lymphocytes was determined by counting 200 lymphoid cells in each specimen and expressing stained cells as a percentage of the total.
Statistical analysis
The data were transformed using the arc-sin transforma tion. Significance of the differences between mean scores of the groups was calculated by Duncan’s test using SPSS, PC version 15.0, software (IBM, Chicago, IL). Values for p ≤ 0.05 were considered statistically significant.
Results
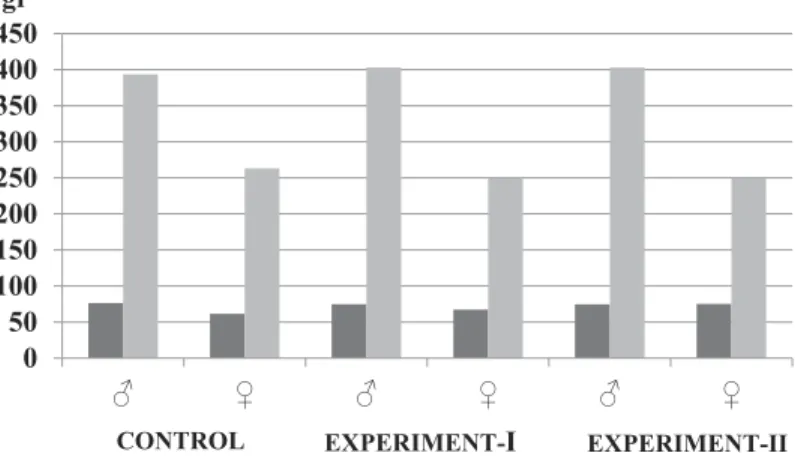
We found no morphological abnormalities in either the control or experimental animals. No indication of acrylamide toxicity including loss of sensation in the hindlegs, decreased activity or death were observed. The control animals gained body weight normally. Both male and female animals in the experimental groups weighed less than the controls, but the differences were not statistically significant (Table 1,Figure 1).
ANAE and ACP-ase cytochemistry
Most peripheral eosinophils, lymphocytes and monocytes exhibited granular ANAE staining in the control animals. Neutrophils, platelets and a few peripheral blood lymphocytes were ANAE negative. In the peripheral blood lymphocytes, we found staining of 1–5 reddish brown granules, whereas monocytes exhibited a strong, finely granular staining pattern (Figure 2). A small number of peripheral blood lymphocytes exhibited ACP-ase staining in the form of 1–3 pinkish red granules located mostly at the cell periphery (Figure 3).
At the beginning of the study, both male and female animals in the control group exhibited similar ANAE
Table 1.Mean body weights of the groups.
Groups
Body weight (g)
Begining of experiment End of the experiment
Male Female Male Female
Control (n = 10) 76.4 ± 10.0 61.6 ± 6.9 393.6 ± 67.9 263.0 ± 25.9 Experimental-I (n = 20) 74.8 ± 9.6 67.4 ± 10.8 403.0 ± 35.0 249.3 ± 28.0 Experimental-II (n = 20) 74.6 ± 6.6 75.2 ± 6.8 364.0 ± 73.9 247.7 ± 41.4 Data are means ± SD.
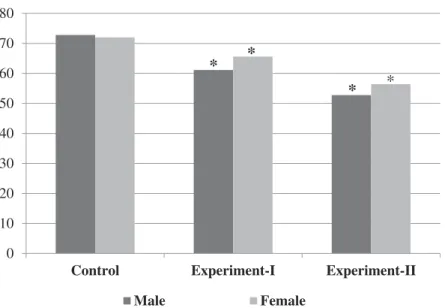
staining. In the experimental-I and experimental-II groups, the females exhibited significantly greater ANAE staining than the males (p < 0.05) (Table 2). The experimental groups exhibited significantly less ANAE staining than the controls (p < 0.05). In the experimental-II group, ANAE staining was weaker compared to ANAE staining in the control and experimental-I groups (p < 0.05,Table 2,
Figure 4).
We found that ACP-ase staining in experimental-I and experimental-II animals was decreased significantly compared to control animals (p < 0.05) (Table 2,Figure 5). We found no statistically significant difference in ACP-ase staining between male and female rats in experimental-I and experimental-experimental-Iexperimental-I groups (Table 2,Figure 5).
Discussion
Acrylamide is found in nearly all heated carbohydrate rich foods and more than a third of the average daily calorie intake comes from these foods (Petersen and Tran2005). The highest acrylamide levels are found in roasted potatoes and cereal products, such as bread and crackers (Lineback et al. 2012; EFSA CONTAM Panel 2015). Children and adolescents especially may consume large amounts of these foods and therefore are susceptible to harmful effects (Konings et al.2003; Dybing et al.2005).
Figure 1.Changes in the body weights of rats in all groups at the beginning and end of the study.
Figure 2.ANAE positive peripheral blood lymphocyte of an animal from the experiment-I group. Arrow: granular reaction products.
Figure 3.ACP-ase positive peripheral blood lymphocyte of an animal from the experiment-I group. Arrow: granular reaction product.
Table 2.ANAE and ACP-ase percentages.
Groups Gender ANAE (%) ACP-ase (%) Control (n = 10) Male 72.80 ± 2.4 25.20 ± 1.3 Female 72.00 ± 2.7 24.40 ± 2.1 Experimental-I (n = 20) Male 61.13 ± 2.1* 17.25 ± 2.5* Female 65.60 ± 1.8* 18.00 ± 1.1* Experimental-II (n = 20) Male 52.75 ± 2.0* 17.88 ± 1.8* Female 56.40 ± 2.3* 16.20 ± 1.6* Data are means ± SD. *Significantly different from control group,p < 0.05.
The immune system is sensitive to a variety of chemical and physical stresses of internal and external origin. Consequently, this system is a sensitive tool for studying the subclinical effects of chemical exposure (Luster et al. 1988). Immunotoxic chemicals may affect the function of the immune system (Singh et al.
2016). Also many chemicals induce changes in the immune system that cause asthma, autoimmune disorder, chronic infections and even cancer (Jorsaraei et al.2014).
We investigated the possible harmful effects of acrylamide on the immune system using doses of 2 or 5 mg acrylamide/kg/day for 90 days and assessing ANAE and ACP-ase staining in peripheral blood lymphocytes. Earlier reports (Chapin et al. 1995; Fang et al.2014; Zamani et al.2017b) indicated that medium and high acrylamide doses decreased normal body weight gain and that this reduction was due to the possible systemic effects of acrylamide. Although we found that the mean body weight of the acrylamide
0 10 20 30 40 50 60 70 80
Control Experiment-I Experiment-II
Male Female % ratios
*
*
*
*
Figure 4.Changes of ANAE positive lymphocyte scores of the groups (%). Asterisks indicate significant difference from control group (p < 0.05). 0 5 10 15 20 25 30
Control Experiment-I Experiment-II
Male Female
% ratios
*
**
*Figure 5.Changes of ACP-ase positive lymphocyte scores of the groups (%). Asterisks indicate significant difference from control group (p < 0.05).
treated animals was less than for control animals, the differences between the groups were not statistically significant. It is possible that longer exposure periods might result in significant loss of body weight.
We found that both the ANAE and ACP-ase staining patterns for peripheral blood lymphocytes and monocytes were similar to those reported earlier (Catowsky 1981; Çelik et al. 1991; Özparlak 2011). ACP-ase staining has been used to diagnose lymphoproliferative disorders (Wehinger and Mobius1976). Strong ACP-ase staining has been reported in one third of B-cell pro-lymphocytic leukemias (Catowsky et al.1974).
ANAE staining of T-lymphocytes and monocytes differed. ANAE activity is reported to be T-lymphocyte-specific and localized as a few large granules (Catowsky
1981; Özparlak2011), whereas the activivity in monocytes is diffuse and finely granular (Mueller et al.1975; Knowles et al.1978; Çelik et al.1991). We found that the majority of the peripheral blood lymphocytes of the animals in all groups exhibited ANAE staining, whereas a minority of the lymphocytes exhibited staining for ACP-ase. Similarly, Poore et al. (1981) reported that ACP-ase was present in 45% of peripheral blood B-lymphocytes. Therefore, the percentage of ACP-ase positive lymphocytes was lower than the percentage of ANAE positive lymphocytes.
There was no significant difference between genders, which is consistent with the report by Morris and Komocsar (1997). Because it has been reported that ANAE positivity of T-lymphocytes increases during the late period of thymic maturation (Basso et al.1980), the enzyme has been reported to be specific for mature T-lymphocytes and absent in B-lymphocytes (Higgy et al.1977).
Carcinogenic, developmental, genotoxic, neurotoxic and reproductive effects of dermal, dietary and respiratory exposure to acrylamide have been documented (LoPachin
2005; Dybing et al. 2008; Yener and Dikmenli 2009; Hogervorst et al. 2010). Studies of the effects of acrylamide exposure on immunological status have suggested that acrylamide exposure might damage the immune system. Guo et al. (2017) reported an association between age, sex, smoke exposure and allergic reactions and exposure to acrylamide and its epoxide metabolite, glycidamide, in the general US population. Fang et al. (2014) administered 4, 12 and 36 mg/kg/day acrylamide for 30 days to BALB/c mice to evaluate toxicity of acrylamide. These investigators reported that acrylamide significantly decreased the mean body weight and the weight of the spleen and thymus, and reduced the number of T-lymphocytes in the animals. These investigators concluded that acrylamide exhibited toxic effects on the cellular and humoral immune response. Zamani et al. (2017a) applied 0, 5, 10 and 25 mM
acrylamide to mouse splenocyte cultures for 2 h at 37 °C. Acrylamide increased apoptosis by activating caspases 8 and 9, and also caused mitochondrial oxidative damage in splenocytes. Zamani et al. (2017a) suggested that mitochondrial dysfunction caused by acrylamide causes weakening of the immune system. Zamani et al. (2017b) reported the effects of oxidative stress and apoptosis in acrylamide induced immunotoxicity and the protective effect of L-carnitine. Significant decreases in peripheral blood lymphoid cells together with decreased thymus and spleen weights were observed in animals following 12.5, 25 and 50 mg acrylamide/kg administration for 30 days (Zamani et al. 2017b). These investigators suggested that the acrylamide toxicity might have been due to oxidative stress and apoptosis. Raju et al. (2015) investigated short term toxic effects of 5, 10 and 50 mg/kg/day acrylamide administered to male F344 rats for 10 weeks. Hematocrits and the number of lymphocytes in rats that received the highest acrylamide dose were decreased significantly. The weight of the spleen in these animals also decreased significantly. The reduced number of lymphocytes was explained by the reduced spleen weight. We reported earlier that 0, 30, 45 and 60 mg/kg/day acrylamide for five consecutive days and 0, 125, 150 and 175 mg/kg/day single doses of acrylamide caused ANAE stained cells in both peripheral blood and Peyer’s patches to decrease significantly in a dose-dependant manner (Yener et al.
2013).
We found that chronic low level acrylamide exposure reduced peripheral blood ANAE and ACP-ase activity in peripheral blood lymphocytes in a dose-dependent manner. The decreased activity of both enzymes may reflect a decreased number of lymphocytes in the peripheral blood. Because all lymphocytes are derived mainly from the bone marrow in mature animals, detrimental effects of the acrylamide on bone marrow should also be investigated. Also, the nature of the lymphoid cells unstained for ANAE and ACP-ase in the peripheral blood requires clarification. Previous studies have shown that acrylamide exhibits a cytotoxic effect on bone marrow, which causes a decreased number of these cells (Adler et al.1993; Gassner and Adler1996; Sickles et al.
2007; Yener and Dikmenli2009,2011).
Chronic low dose acrylamide ingestion caused significant decreases in both ANAE and ACP-ase stained lymphocytes in a dose-dependent manner. The decreased percentage of peripheral blood lymphocytes may indicate immune system deficiency.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by Selcuk University Scientific Research Projects Coordination Units, Konya/TURKEY [grant number 11401013].
References
Adler ID, Zouh R, Schmid E.1993. Perturbation of cell division by acrylamide in vitro and in vivo. Mutat Res. 301:249–254. Basso G, Cocito MG, Semenzato G, Pezzutto A, Zanesco L.1980. Cytochemical study of thymocytes and T-lymphocytes. Br J Haematol. 44:577–582.
Burek JD, Albee RR, Beyer JE, Bell TJ, Carreon RM, Morden DC, Wade CE, Hermann EA, Gorzinski SJ.
1980. Subchronic toxicity of acrylamide administered to rats in the drinking water followed by up to 144 days of recovery. J Env. Pathol Toxicol. 4:157–182.
Çelik İ, Aştı RN, Ergene N. 1991. Determination of B, T-lymphocytes and null cells in human peripheral blood by means of esterase cytochemistry and immunoenzymatic staining of surface immunoglobulins. SÜ Tip Fak Derg. 7:497–503.
Catowsky D.1981. Leucocyte cytochemical and immunological techniques. In: Dacie JV, Lewis, SM, editors. Practical haematology. 7th ed. New York: Churchill Livingstone; p. 143–147.
Catowsky D, Galetto J, Okos A, Millard E, Galton DAG.
1974. Cytochemical profile of B and T leukaemic lymphocytes with special reference to acute lymphoblastic leukemia. J Clin Pathol. 27:767–771.
Chapin RE, Fail PA, George JD, Grizzle TB, Heindel JJ, Harry GJ, Collins BJ, Teague J. 1995. The reproductive and neuronal toxicities of acrylamide and three analogues in Swiss mice, evaluated using the continuous breeding protocol. Toxicol Sci. 27:9–24. doi:10.1093/toxsci/27.1.9
Dybing E, Farmer PB, Andersen M, Fennell TR, Lalljie SP, Müller DJ, Olin S, Petersen BJ, Schlatter J, Scholz G, Scimeca JA, Slimani N, Törnqvist M, Tuijtelaars S, Verger P. 2005. Human exposure and internal dose assessments of acrylamide in food. Food Chem Toxicol. 3:365–410. doi:10.1016/j.fct.2004.11.004
Dybing E, O’Brien J, Renwick AG, Sanner T. 2008. Risk assessment of dietary exposures to compounds that aregenotoxic and carcinogenic−an overview. Toxicol Lett. 180:110–117. doi:10.1016/j.toxlet.2008.05.007
EFSA CONTAM Panel. 2015. Scientific opinion on acrylamide in food. EFSA J. 13(4104):321. doi:10.2903/j. efsa.2015.4104
Fang J, Liang CL, Jia XD, Li N. 2014. Immunotoxicity of acrylamide in female BALB/c mice. Biomed Env. Sci. 27:401–409.
Fennell TR, Sumner SC, Snyder RW, Burgess J, Friedman MA. 2006. Kinetics of elimination of urinary metabolites of acrylamide in humans. Toxicol Sci. 93:256–267. doi:10.1093/toxsci/kfl069
Gassner P, Adler ID.1996. Induction of hypoploidy and cell cycle delay by acrylamide in somatic and germinal cells of male mice. Mutat Res. 367:195–202.
Gul ST, Ahmad M, Khan A, Hussain I. 2007. Haematobiochemical observations in apparently healthy equine species. Pak Vet J. 27:155–158.
Guo J, Yu D, Lv N, Bai R, Xu C, Chen G, Cao W. 2017. Relationships between acrylamide and glicydamide hemoglobin adduct levels and allergy-related outcomes in general US population, NHANES 2005–2006. Env. Pollut. 225:506–513. doi:10.1016/j.envpol.2017.03.016
Higgy EK, Bums FG, Hayhoe JGF.1977. Discrimination of B, T and null lymphocytes by esrerase cytochemistry. Scand J Haematol. 18:437–448.
Hogervorst JG, Baars BJ, Schouten LJ, Konings EJ, Goldbohm RA, van den Brandt PA. 2010. The carcinogenicity of dietary acrylamide intake: a comparative discussion of epidemiological and experimental animal research. Crit Rev Toxicol. 40:485–512. doi:10.3109/10408440903524254
IARC. (1994). Acrylamide. IARC Monographs on the evaluation of carcinogen risk to humans: Some industrial chemicals. Lyon, France: International Agency for Research on Cancer, vol. 60, p. 389–433.
Jorsaraei SGA, Maliji G, Azadmehr A, Moghadamnia AA, Faraji AA. 2014. Immunotoxicity effects of carbaryl in vivo and in vitro. Env. Toxicol Pharm. 38:838–844. doi:10.1016/j.etap.2014.09.004
Kajikawa O, Koyama H, Yashikawa T, Tsubaki S, Saito H.
1983. Use of alpha-naphthyl acetate esterase staining to identify T-lymphocytes in cattle. Am J Vet Res. 44:1549–1552.
Knowles DM, Hoffman T, Ferrarini M, Kunkel HG. 1978. The demonstration of acid alpha-acetate esterase activity in human lymphocytes usefulness as a T cell marker. Cell Immunol. 35:112–123.
Konings EJ, Baars AJ, van Klaveren JD, Spanjer MC, Rensen PM, Hiemstra M, van Kooij JA, Peters PW.2003. Acrylamide exposure from foods of the Dutch population and an assessment of the consequent risks. Food Chem Toxicol. 41:1569–1579.
Kurenkov VF (1997). Acrylamide polymers. In: Cheremisinoff NP, editor. Handbook of engineering polymeric materials tech and eng. NY: Marcel Dekker, Inc.; p. 61. ISBN 0-8247-9799-X
Li CY, Yam LT, Lam KW.1970. Acid phosphatase isoenzyme in human leukocytes in normal and pathologic conditions. J Histochem Cytochem. 18:473–481. doi:10.1177/18.7.473
Lineback DR, Coughlin JR, Stadler RH.2012. Acrylamide in foods: a review of the science and future considerations. Ann Rev Food Sci Technol. 3:15–35. doi: 10.1146/annurev-food-022811-101114
LoPachin RM. 2005. Acrylamide neurotoxicity: neurological, morphological and molecular endpoints in animal models. Adv Exp Med Biol. 561:21–37. doi:10.1007/0-387-24980-X_2
Luster MI, Munson AE, Thomas PT, Holsapple MP, Fenters JD, White KL Jr, Lauer LD, Germolec DR, Rosenthal GJ, Dean JH. 1988. Development of a testing battery to assess chemical-induced immunotoxicity: National Toxicology Program’s guidelines for immunotoxicity evaluation in mice. Fundam Appl Toxicol. 10:2–19. doi:10.1016/0272-0590(88)90247-3
Maiti NK, Saini SS, Sharma SN.1990. Histochemical studies on chicken peripheral blood lymphocytes. Vet Res Commun. 14:207–210.
Morris D, Komocsar WJ.1997. Immunophenotyping analysis of peripheral blood, splenic, and thymic lymphocytes in male and female rats. J Pharmacol Toxicol Methods. 37:37–46. Mueller J, Brun del RG, Buerki H, Keller HU, Hess MW,
Cottier H.1975. Nonspecific acid esterase activity: a criterion for differentiation of T- and B-lymphocytes in mouse lymph nodes. Eur J Immunol. 5:270–274. doi:10.1002/eji.1830050411
Özparlak H. 2011. Determination of the alpha-naphtyl acetate esterase (ANAE) and acid phosphates (ACP) activity in the peripheral blood lymphocytes in two different species of the ground squirrel. SÜ Fen Fak Fen Derg. 37:33–42.
Osbaldiston GW, Sullivan RJ.1978. Cytochemical demonstration of esterases in peripheral blood leukocytes. Am J Vet Res. 39:683–685.
Petersen BJ, Tran N.2005. Exposure to acrylamide: placing exposure in context. In: Friedman M, Mottram D, Eds. Chemistry and safety of acrylamide in food. New York: Springer Press; p. 63–76.
Poore E, Barrett SG, Kadın ME, Bainton DF. 1981. Ultrastructural localization of acid phosphatase in rosetted T- and B-lymphocytes of normal human blood. Am J Pathol. 102:72–83.
Raju J, Roberts J, Taylor M, Patry D, Chomyshyn E, Caldwell D, Cooke G, Mehta R. 2015. Toxicological effects of short-term dietary acrylamide exposure in male F344 rats. Env. Toxicol Pharmacol. 39:85–92. doi:10.1016/ j.etap.2014.11.009
Sickles DW, Sperry AO, Testino A, Friedman M. 2007. Acrylamide effects on kines in related proteins of the mitotic/meiotic spindle. Toxicol Appl Pharmacol. 222:111–121. doi:10.1016/j.taap.2007.04.006
Singh SK, Bano F, Mohanty B.2016. Vitamin E pretreatment prevents the immunotoxicity of dithiocarbamate pesticide mancozeb in vitro: a comparative age-related assessment in mice and chick. Pestic Biochem Physiol. 126:76–84. doi:10.1016/j.pestbp.2015.08.001
Stadler RH, Blank I, Varga N, Robert F, Hau J, Guy PA, Robert MC, Riediker S. 2002. Acrylamide from Maillard reaction products. Nature. 419:449–450. doi:10.1038/419449a
Tareke E, Rydberg P, Karlsson P. 2002. Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J Agric Food Chem. 50:4998–5006.
Wehinger H, Mobius W. 1976. Cytochemical studies on T and B-lymphocytes and lymphoblasts with special reference to acid phosphatase. Acta Haematol. 56:129–136. doi:10.1159/000207929
WHO (World Health Organization).2005. Summary report of the sixty-fourth meeting of the joint FAO/WHO expert committee on food additive (JECFA). Rome (Italy): The ILSI Press International Life Sciences Institute. Washington, DC; p. 1–47.
Yener Y, Dikmenli M.2009. Increased micronucleus frequency in rat bone marrow after acrylamide treatment. Food Chem Toxicol. 47:2120–2123. doi:10.1016/j.fct.2009.05.037
Yener Y, Dikmenli M.2011. The effects of acrylamide on the frequency of megakaryocytic emperipolesis and the mitotic activity on rat bone marrow cells. J Sci Food Agric. 91:1810–1813. doi:10.1002/jsfa.4388
Yener Y, Sur E, Telatar T, Oznurlu Y. 2013. The effect of acrylamide on alpha-naphthyl acetate esterase enzyme in blood circulating lymphocytes and gut associated lymphoid tissue in rats. Exp Toxicol Pathol. 65:143–146. doi:10.1016/j.etp.2011.07.002
Zaidi SI, Raisuddin S, Singh KP, Jafri A, Husain R, Husain MM, Mall SA, Seth PK, Ray PK. 1994. Acrylamide induced immunosuppression in rats and its modulation by 6-MFA, an interferon inducer. Immunopharmacol Immunotoxicol. 16:247–260. doi:10.3109/08923979409007093
Zamani E, Shaki F, Abedian-Kenari S, Shokrzadeh M. 2017a. Acrylamide induces immunotoxicity through reactive oxygen species production and caspase-dependent apoptosis in mice splenocytes via the mitochondria-dependent signaling pathways. Biomed Pharmacother. 94:523–530. doi:10.1016/j. biopha.2017.07.033
Zamani E, Shokrzadeh M, Ziar A, Abedian-Kenari S, Shaki F.
2017b. Acrylamide attenuated immune tissue function via induction of apoptosis and oxidative stress: protection by L-carnitine. Hum Exp Toxicol. 1–11. doi:10.1177/ 0960327117741753