Full Terms & Conditions of access and use can be found at
https://www.tandfonline.com/action/journalInformation?journalCode=ihip20
Hypertension in Pregnancy
ISSN: (Print) (Online) Journal homepage: https://www.tandfonline.com/loi/ihip20
Serum survivin is decreased in women with
preeclampsia
Onur Güralp, Koray Gök, Nevin Tüten, Kübra Hamzaoglu, Melike Makul,
Yahya Ozgun Oner, Huri Bulut, Meike Schild-Suhren, Eduard Malik &
Abdullah Tüten
To cite this article: Onur Güralp, Koray Gök, Nevin Tüten, Kübra Hamzaoglu, Melike Makul,
Yahya Ozgun Oner, Huri Bulut, Meike Schild-Suhren, Eduard Malik & Abdullah Tüten (2021): Serum survivin is decreased in women with preeclampsia, Hypertension in Pregnancy, DOI: 10.1080/10641955.2021.1917601
To link to this article: https://doi.org/10.1080/10641955.2021.1917601
View supplementary material
Published online: 20 May 2021.
Submit your article to this journal
Article views: 1
View related articles
ARTICLE
Serum survivin is decreased in women with preeclampsia
Onur Güralpa, Koray Gökb, Nevin Tütenc, Kübra Hamzaoglud, Melike Makuld, Yahya Ozgun Onerd, Huri Bulute,
Meike Schild-Suhrena, Eduard Malika, and Abdullah Tütend
aKlinikum Oldenburg Aör, Carl Von Ossietzky Oldenburg University, University Hospital for Gynecology and Obstetrics, Oldenburg, Germany; bObstetrics and Gynecology, Sakarya University, Education and Research Hospital, Sakarya, Turkey; cObstetrics and Gynecology, Kanuni Sultan Suleyman Education and Research Hospital, Istanbul, Turkey; dDepartment of Obstetrics and Gynecology, Istanbul Cerrahpasa University, Istanbul, Turkey; eFaculty of Medicine, Medical Biochemistry Department, Istinye University, Istanbul, Turkey
ABSTRACT
Objective: To evaluate the serum survivin level in preeclampsia.
Methods: Eighty-eight pregnancies complicated with preeclampsia and 88 gestational-age (GA)- matched uncomplicated pregnancies were evaluated.
Results: Mean serum survivin was detected to be significantly decreased in the early- (EOPE) and late-onset (LOPE) preeclampsia subgroups than the GA-matched control-groups; and were com-parable in EOPE- and LOPE-groups after correction for GA. Serum survivin had weak negative correlations with systolic and diastolic arterial blood pressure.
Conclusion: The serum survivin level was decreased in preeclamptic patients than the GA- matched controls. More comprehensive studies are needed to clarify the timing and extent of placental apoptosis in preeclampsia.
ARTICLE HISTORY
Received 21 March 2020 Accepted 11 April 2021
KEYWORDS
Survivin; apoptosis; early- onset preeclampsia; late- onset preeclampsia; hypertension
Introduction
Preeclampsia is known to be associated with various placental pathologic processes such as increased apop-tosis of the placental villous trophoblasts (1–3), increased syncytial knots (4), and increased debris of apoptotic syncytiotrophoblast in maternal circulation (5). Protein inhibitors of apoptosis are expressed in placental villous tissue. Only three of them, X-linked inhibitor of apoptosis protein (IAP), survivin, and neu-ronal apoptosis inhibitor protein (NAIP), are expressed in greater amounts in term villi compared to the villi in the first trimester (6).
Survivin belongs to the IAP superfamily. The pla-cental survivin was firstly isolated in 2001, and it was shown that its expression was increased in neo-plastic placental tissues, such as in hydatidiform mole, with the human telomerase reverse transcriptase (hTERT) pro-tein compared to non-neoplastic placental tissues. Non- neoplastic placental tissue examination was performed in normal pregnant women from each of the three trimesters and post-term pregnant women as well as preeclamptic pregnant women (7).
In adulthood, survivin is expressed in transformed cell lines and many cancer cell types (8,9). The studies about placental survivin expression in preeclampsia
showed inconsistent results. In three studies, its expres-sion was detected to decrease (10–12), which is in accordance with the theory that increased apoptosis in the placenta is associated with reduced placental survi-vin. However, few other study groups have found that the expression was increased or not significantly chan-ged (13,14). Our medical literature research did not reveal any study which evaluated the serum survivin levels in preeclampsia.
Early- and late-onset preeclampsia share common pathophysiological features, including shallow placental implantation (15). However, late-onset preeclampsia (LOPE) is seen in roughly 80% of all preeclampsia cases, whereas early-onset preeclampsia (EOPE) is asso-ciated with 80% of the complications related to pre-eclampsia (16). Therefore, the clinical outcome is known to be more severe in EOPE than in LOPE, which may be related to the differences in underlying pathophysiological mechanisms such as increased hypoxic injury or apoptosis in the placental tissue (15).
In this study, we aimed to investigate the serum survivin levels in preeclampsia, focus on its subgroups early- and late-onset preeclampsia for the first time in the literature, and contribute to a better understanding of the pathophysiology of the preeclampsia.
CONTACT Onur Güralp dronur@hotmail.com Klinikum Oldenburg Aör, University Hospital of Obstetrics and Gynecology, Carl Von Ossietzky Oldenburg University, Rahel-Strauß-Straße 10, Oldenburg 26133, Germany
Supplemental data for this article can be accessed here.
https://doi.org/10.1080/10641955.2021.1917601
Material and methods Design
This project was developed and carried out with the cooperation between Istanbul Cerrahpasa University, University Hospital for Obstetrics and Gynecology, Istanbul, Turkey, and Carl von Ossietzky University Oldenburg, University Hospital for Obstetrics and Gynecology in Klinikum Oldenburg Anstalt des öffentlichen Rechts (AöR), Oldenburg, Germany. We evaluated 88 pregnancies complicated with preeclamp-sia (the study group) and 88 uncomplicated pregnan-cies (the control group), examined or admitted between January 2018 and December 2019 at the Department of Obstetrics and Gynecology of Istanbul Cerrahpasa University Hospital, Istanbul, Turkey. All participants gave informed consent. Our protocol was authorized by the Ethics Committee of Istanbul Cerrahpasa University, School of Medicine (Date/number: 04.04.2014, Number: 8879 and 11/03/2019 – 39712 83045809–604.01.02).
The inclusion criteria for the study group were women between 18 and 40, singleton pregnancy com-plicated with preeclampsia. In order to study the EOPE and LOPE subgroups better, an equal number of con-secutive women with EOPE (n = 44) and LOPE (n = 44) were included.
The inclusion criteria for the control group included consecutive women between 18 and 40 years old, single-ton pregnancy, who had their regular pregnancy-care between the 24th gestational week (GW) and 40 GW in our outpatient-clinic, gestational-age (GA) matching (±1 week) according to the week of blood-sampling in the study group; and appropriate for GA (AGA).
Exclusion criteria for both the study and control groups were multifetal gestation, known fetal malfor-mations, known acute or chronic systemic diseases, any hypertensive disorders, any type of diabetes, preterm labor, and prelabor rupture of membranes, and other known inflammatory disorders as well as infections.
The diagnosis of preeclampsia was made with the new beginning of hypertension (measured twice with an interval of minimum 4 hours, blood pressure ≥140/ 90 mmHg) after the 20th gestational week (gestational week) and one or more of the followings: Proteinuria (300 mg/d or “≥0.3 protein to creatinine ratio” instead of “≥30 mg/ml protein to creatinine ratio“) AND/OR maternal organ dysfunction such as acute kidney injury; liver involvement ± pain in the right upper quadrant or epigastric region; neurological impairment; or hematological complications AND/OR placental dysfunction including intrauterine growth retardation
(IUGR), Doppler examination abnormalities of the umbilical artery, or fetal demise (17).
We further divided the preeclampsia into subgroups: (1) EOPE: delivery <34 + 0 gestational weeks (2) LOPE: delivery ≥34 + 0 gestational weeks GA was calculated using the Naegele’s formula based on the last menstrual period (LMP) or ultrasonographic measurement of the crown-rump-length (CRL) in the first trimester if LMP was not clearly known or there was a discrepancy of >7 days between GA according to the LMP and first-trimester CRL.
Ultrasonographic examinations were made by AT with Voluson 730 Pro ultrasonography (GEHealthcare, General Electric, Zipf, Austria). The estimated fetal weight (EFW) was calculated according to the Hadlock formula, and an EFW less than the 10th percentile was defined as small for GA (SGA).
Blood sampling and biochemical methods for measurements
The venous blood sampling was performed upon preeclampsia diagnosis prior to the onset of the labor in the preeclampsia group and the matching (±1 week) pregnancy week in the control group. The samples from all participants were collected in ethylenediami-netetraacetic acid (EDTA) for complete blood count and serum tubes for other measurements, including survivin level. The serum tubes were centrifugated for 15 minutes at 1000 g at 2–8°C in a maximum of 30 minutes of collection. The upper serum phase was collected and promptly put in the freezer at −80°C. The biochemical examinations were performed by HB in a blinded fashion.
Serum survivin was measured with an enzyme- linked immunosorbent assay (ELISA) method using the commercial kits (SL2201Hu Human Survivin (Surv) ELISA Kit, Sunlong Biotech, Hangzhou, China). The optical density (OD) was measured spec-trophotometrically at a wavelength of 450 nm. The detection range was 6 pg/mL to 400 pg/mL, and the sensitivity was 1.2 pg/mL.
Complete blood count (CBC), urea, total bilirubin, creatinine, uric acid, aspartate- (AST), and alanine- transaminase (ALT) levels were measured accordingly.
Sample size calculation and statistical analysis
A pilot study was performed with ten women with preeclampsia and ten women with an uncomplicated
pregnancy. The serum survivin levels were 21.7 ± 12.4 pg/mL vs. and 35.2 ± 21.4 pg/mL in the preeclampsia and non-preeclampsia groups, respec-tively. The α-error was taken as 0.05, and the target power was accepted as 80%. The minimum required sample size was calculated as 40 women in each group. The patients included in the pilot study were excluded from the current analysis of the main study.
The Statistical Package for the Social Sciences (SPSS) software version 24.0 (SPSS Inc., Chicago, IL) was used for establishing the database and statistical calculations. The homogeneity of the distribution of continuous variables was evaluated using the Kolmogorov– Smirnov test. Age, BMI, systolic and diastolic arterial blood pressure (ABP), GA at sampling, WBC and pla-telet count, creatinine, total bilirubin, and survivin had homogenous distributions and were evaluated with parametric tests. Parity, uric acid, urea, AST, and ALT had non-homogeneous distributions and were evalu-ated with non-parametric tests. Univariate linear ana-lysis with correction for GA was used to compare the serum survivin levels in EOPE and LOPE groups. A partial correlation test was performed to evaluate the correlations between survivin and relevant para-meters with necessary corrections. The statistical sig-nificance threshold was <0.05.
Results
The patients’ characteristics in the study and control groups were shown in Table 1. There were no signifi-cant differences regarding the patient demographics as well as mean WBC, AST, ALT, and total bilirubin levels
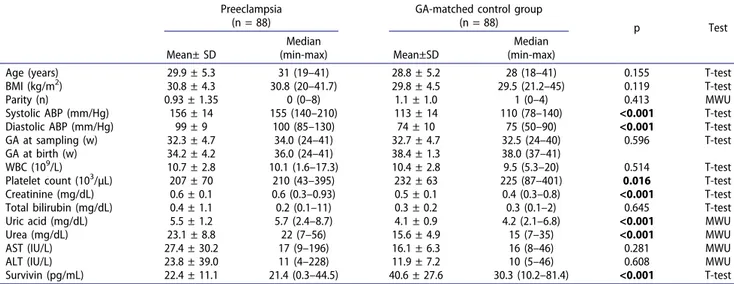
between the study and control groups. Mean creatinine, uric acid, urea levels were significantly higher, and the mean platelet count was significantly lower in pree-clamptic women than controls. Mean serum survivin was significantly lower in women with preeclampsia compared to the controls (22.4 ± 11.1 pg/mL vs. 40.6 ± 27.6 pg/mL, p < 0.001) (Figure 1).
The SGA rate was 39% (n = 35) in the preeclampsia group. Considering all women, survivin was signifi-cantly lower in the SGA-group (n = 35) compared to the AGA group (n = 131) (20.6 ± 12.1 pg/mL vs. 33.6 ± 23.6 pg/mL, p < 0.001). In the preeclampsia subgroup, however, the serum survivin level in the SGA-group (n = 35) and AGA-group (n = 53) were comparable (20.6 ± 12.1 pg/mL vs. 23.9 ± 10.4 pg/mL, respectively, p = 0.230). The SGA rate was 47.7% and 31.8% in EOPE and LOPE groups, respectively (p = 0.129).
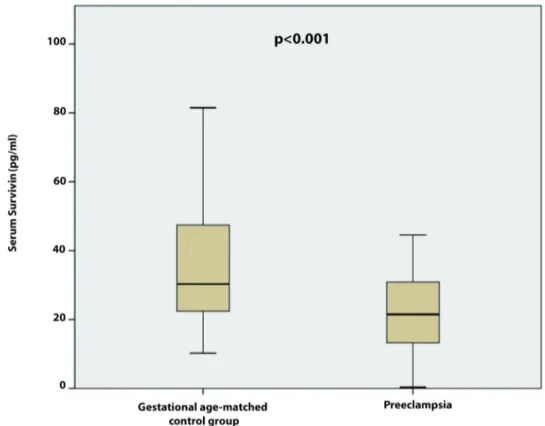
No significant difference regarding the patient demographics was seen between the EOPE group and the control group GA-matched for the EOPE group (Table 2). Mean systolic and diastolic blood pressure were significantly higher in the EOPE group com-pared to the GA-matched controls. The mean WBC, platelet count, AST, ALT, and total bilirubin values were similar in the two groups. Mean creatinine, uric acid, and urea levels were significantly elevated in pregnant women with EOPE than matched con-trols. Mean serum survivin was significantly decreased in pregnant women with EOPE compared to that of the GA-matched control group (20.1 ± 10.3 pg/mL vs. 36.9 ± 26.4 pg/mL, p < 0.001) (Figure 2(a,b)).
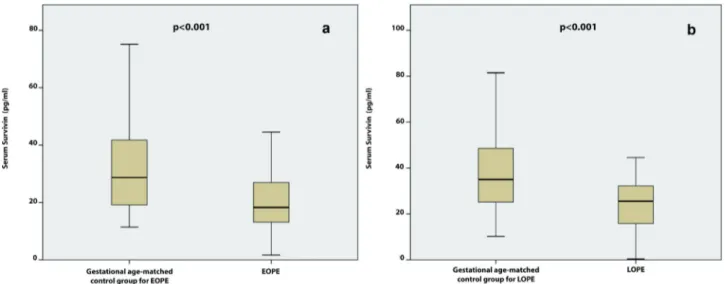
Table 1. Demographic, clinical and laboratory features of the women with preeclampsia and GA-matched control group. Preeclampsia
(n = 88)
GA-matched control group
(n = 88) p Test Mean± SD Median (min-max) Mean±SD Median (min-max)
Age (years) 29.9 ± 5.3 31 (19–41) 28.8 ± 5.2 28 (18–41) 0.155 T-test
BMI (kg/m2) 30.8 ± 4.3 30.8 (20–41.7) 29.8 ± 4.5 29.5 (21.2–45) 0.119 T-test
Parity (n) 0.93 ± 1.35 0 (0–8) 1.1 ± 1.0 1 (0–4) 0.413 MWU
Systolic ABP (mm/Hg) 156 ± 14 155 (140–210) 113 ± 14 110 (78–140) <0.001 T-test Diastolic ABP (mm/Hg) 99 ± 9 100 (85–130) 74 ± 10 75 (50–90) <0.001 T-test GA at sampling (w) 32.3 ± 4.7 34.0 (24–41) 32.7 ± 4.7 32.5 (24–40) 0.596 T-test GA at birth (w) 34.2 ± 4.2 36.0 (24–41) 38.4 ± 1.3 38.0 (37–41)
WBC (109/L) 10.7 ± 2.8 10.1 (1.6–17.3) 10.4 ± 2.8 9.5 (5.3–20) 0.514 T-test Platelet count (103/µL) 207 ± 70 210 (43–395) 232 ± 63 225 (87–401) 0.016 T-test Creatinine (mg/dL) 0.6 ± 0.1 0.6 (0.3–0.93) 0.5 ± 0.1 0.4 (0.3–0.8) <0.001 T-test Total bilirubin (mg/dL) 0.4 ± 1.1 0.2 (0.1–11) 0.3 ± 0.2 0.3 (0.1–2) 0.645 T-test Uric acid (mg/dL) 5.5 ± 1.2 5.7 (2.4–8.7) 4.1 ± 0.9 4.2 (2.1–6.8) <0.001 MWU
Urea (mg/dL) 23.1 ± 8.8 22 (7–56) 15.6 ± 4.9 15 (7–35) <0.001 MWU
AST (IU/L) 27.4 ± 30.2 17 (9–196) 16.1 ± 6.3 16 (8–46) 0.281 MWU
ALT (IU/L) 23.8 ± 39.0 11 (4–228) 11.9 ± 7.2 10 (5–46) 0.608 MWU
Survivin (pg/mL) 22.4 ± 11.1 21.4 (0.3–44.5) 40.6 ± 27.6 30.3 (10.2–81.4) <0.001 T-test ABP, arterial blood pressure; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body-mass-index; GA, gestational age; max, maximum; min,
minimum; SD, standard deviation; WBC, white blood cells P < 0.05 is significant (marked with bold)
There was no significant difference in mean age, BMI, parity, and GA at sampling between the LOPE and GA- matched control groups (Table 3). There were no signifi-cant differences in mean WBC, platelet count, total bilir-ubin, AST, and ALT values between the two groups. Mean creatinine, uric acid, and urea were significantly higher in
pregnant women with LOPE than controls. Mean serum survivin was significantly lower in pregnant women with LOPE than in the GA-matched control group (24.6 ± 11.6 pg/mL vs. 44.1 ± 28.5 pg/mL, p < 0.001).
Figure 1. The box-plot graphic presentation of the serum survivin levels’ comparisons: preeclampsia vs. gestational-age-matched controls.
Table 2. Demographic, clinical and laboratory features of the women with EOPE and women in the control group GA-matched for EOPE.
EOPE
(n = 44) Control Group GA-matched for EOPE (n = 44) p Test Mean± SD
Median
(min-max) Mean±SD
Median (min-max)
Age (years) 30.5 ± 4.6 30 (19–39) 28.9 ± 4.1 29 (21–41) 0.081 T-test
BMI (kg/m2) 30.1 ± 3.9 30.8 (20–37) 28.8 ± 3.7 29.0 (21.2–45) 0.110 T-test
Parity (n) 0.9 ± 1.2 0 (0–5) 1.1 ± 1.0 1 (0–4) 0.510 MWU
Systolic ABP (mm/Hg) 161 ± 13 160 (140–210) 111 ± 15 110 (78–140) <0.001 T-test Diastolic ABP (mm/Hg) 103 ± 9 100 (90–130) 71 ± 9 70 (50–90) <0.001 T-test GA at sampling (w) 28.3 ± 3.0 28.0 (24–33) 28.7 ± 2.9 29.0 (24–33) 0.511 T-test GA at birth (w) 31.0 ± 3.6 31.0 (24–34) 38.2 ± 1.4 38.0 (37–41)
WBC (109/L) 11.2 ± 2.8 10.9 (5.7–17) 9.9 ± 2.8 9.3 (5.3–18.4) 0.068 T-test Platelet count (103/µL) 206 ± 67 217 (43–328) 232 ± 59 216 (143–401) 0.066 T-test Creatinine (mg/dL) 0.6 ± 0.1 0.6 (0.3–0.9) 0.4 ± 0.1 0.4 (0.3–0.7) <0.001 T-test Total bilirubin (mg/dL) 0.5 ± 1.5 0.2 (0.1–0.9) 0.3 ± 0.2 0.2 (0.1–1.0) 0.402 T-test Uric acid (mg/dL) 5.7 ± 1.3 5.9 (2.4–8.7) 4.0 ± 1.0 4.0 (2.1–6.8) <0.001 MWU
Urea (mg/dL) 26.2 ± 9.8 25 (7–56) 14.7 ± 4.6 14 (7–35) <0.001 MWU
AST (IU/L) 35.5 ± 39.8 19 (11–196) 15.1 ± 6.1 16 (5–46) 0.353 MWU
ALT (IU/L) 34.2 ± 51.8 13 (6–228) 10.9 ± 4.5 10 (5–46) 0.332 MWU
Survivin (pg/mL) 20.1 ± 10.3 18.5 (1.6–44.5) 36.9 ± 26.4 29.0 (11.4–81.4) <0.001 T-test ABP, arterial blood pressure; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body-mass-index; GA, gestational age; max, maximum; min,
minimum; SD, standard deviation; WBC, white blood cells P < 0.05 is significant (marked with bold)
MWU, Mann-Whitney-U Test 4 O. GÜRALP ET AL.
Mean serum survivin levels were comparable in EOPE and LOPE groups (20.1 ± 10.3 pg/mL vs. 24.6 ± 11.6 pg/ mL, p = 0.215) after correction for GA.
The correlation analysis of survivin and various parameters in all participants were presented in supple-mentary Table 1. Serum survivin had weak negative correlations with systolic and diastolic blood pressure. (Suppl Figure S1)
Discussion
Abnormal placentation has a central role in preeclamp-sia’s pathogenesis; however, the underlying cause is still unclear. Incomplete and belated trophoblastic invasion, as well as impaired remodeling of the spiral arteries, are primary placental pathologies associated with pree-clampsia (18,19). In physiological pregnancy, placental
Figure 2. A-b The box-plot graphic presentations of the serum survivin levels’ comparisons: EOPE vs. gestational-age-matched control group for EOPE (A), LOPE vs. gestational-age-matched control group for LOPE (B).
Table 3. Demographic, clinical and laboratory features of the women with LOPE and women in the control group GA-matched for LOPE.
LOPE
(n = 44) Control Group GA-matched for LOPE (n = 44) p Test Mean± SD
Median
(min-max) Mean±SD
Median (min-max)
Age (years) 29.3 ± 5.8 30 (19–41) 28.7 ± 6.1 28 (21–40) 0.641 T-test
BMI (kg/m2) 31.5 ± 4.6 31.1 (22–41.7) 30.7 ± 5.0 30.0 (21.2–35.2) 0.417 T-test
Parity (n) 0.8 ± 1.4 0 (0–8) 1.1 ± 1.0 1 (0–4) 0.410 MWU
Systolic ABP (mm/Hg) 152 ± 13 150 (140–200) 115 ± 12 110 (78–140) <0.001 T-test Diastolic ABP (mm/Hg) 96 ± 8 100 (85–120) 76 ± 11 75 (50–90) <0.001 T-test GA at sampling (w) 36.3 ± 1.7 36.0 (34.0–41.0) 36.9 ± 1.5 37.0 (34.0–41.0) 0.091 T-test GA at birth (w) 37.4 ± 1.5 37.0 (34.0–41.0) 38.6 ± 1.2 38.0 (37.0–41.0)
WBC (109/L) 10.1 ± 2.7 9.5 (1.6–17.3) 10.6 ± 2.8 10.2 (5.3–20.0) 0.403 T-test Platelet count (103/µL) 208 ± 74 191 (88–395) 231 ± 66 232 (143–375) 0.118 T-test Creatinine (mg/dL) 0.5 ± 0.1 0.6 (0.3–0.8) 0.5 ± 0.1 0.5 (0.3–0.8) 0.001 T-test Total bilirubin (mg/dL) 0.2 ± 0.1 0.2 (0.1–11.0) 0.3 ± 0.3 0.3 (0.1–2.0) 0.230 T-test Uric acid (mg/dL) 5.4 ± 1.1 5.4 (3.0–7.8) 4.2 ± 0.9 4.3 (2.1–6.6) <0.001 MWU
Urea (mg/dL) 20.0 ± 6.2 20 (7–42) 16.3 ± 5.1 15 (7–23) <0.001 MWU
AST (IU/L) 19.3 ± 11.4 16 (9–80) 16.7 ± 6.4 16 (4–25) 0.353 MWU
ALT (IU/L) 13.4 ± 13.0 10 (4–86) 12.7 ± 8.5 9 (4–26) 0.332 MWU
Survivin (pg/mL) 24.6 ± 11.6 25.3 (0.3–43.9) 44.1 ± 28.5 34.9 (11.41–75.14) <0.001 T-test ABP, arterial blood pressure; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body-mass-index; GA, gestational age; max, maximum; min,
minimum; SD, standard deviation; WBC, white blood cells P < 0.05 is significant (marked with bold)
proliferation and apoptosis should be kept in balance. The level of trophoblast apoptosis increases with advancing gestation and reaches the peak level at week 40 of pregnancy. Trophoblastic apoptosis begins in cytotrophoblasts and proceeds in syncytiotropho-blasts (20). Both proteins promoting and antagonizing apoptosis involve in the regulation of placental apopto-sis. The best known anti-apoptotic protein is survivin. Survivin is found in the cytoplasm, mitochondria, and nucleus (21,22). Survivin regulates mitosis in the nucleus as well as shows anti-apoptotic activity in the cytoplasm and the mitochondria (22,23). Survivin pre-vents apoptosis by passing from the mitochondria to the cytosol in response to apoptotic stimuli (22). When there is insufficient placental apoptosis, the maternal immune system is activated against the fetus. On the other hand, when there is excessive placental apoptosis, placentation is disturbed, maternal endothelium is damaged, and placental ischemia occurs. It was shown that placental apoptosis was higher in preeclamptic women (24).
In a study revealing the increase of syncytial nodes in preeclamptic pregnant women, the gene expression of anti-apoptotic proteins, including survivin, was examined. No decrease was observed in the gene expression of any anti-apoptotic proteins, including survivin, in the cytoplasm around syncytial nodes. This finding was explained by the fact that anti- apoptotic proteins in the cytoplasm should be sufficient to maintain the integrity of syncytiotrophoblast cells, unlike the syncytial nodes formed as a result of apop-totic degeneration of the nucleus. In other words, the level of survivin in the cytoplasm of trophoblasts may not provide accurate information about nucleus apop-tosis (4). In another study carried out by the same researchers in 2008, it was shown that apoptosis increased in the placentas of preeclamptic pregnant women. In the regulation of caspase activity leading to apoptosis, the gene expressions of the second mito-chondria-derived activator of caspases (SMAC), HtrA2/ Omi, XIAP, and survivin were investigated, and it was detected that only mitochondrial SMAC expression increased. They noted that the gene expression of sur-vivin did not decrease in their study, as in their pre-vious studies (14). In our study, it was found that serum survivin levels were reduced in the preeclamptic patient group compared to that of the GA-matched control group. In two different studies carried out by Heazell et al. (4,14), it was determined that the gene expression of placental survivin was not significantly changed. In the studies carried out by Heazell et al., if the gene expression of placental survivin was evaluated
in the nucleus rather than the cytoplasm, it would have given more accurate results.
In some other studies on the gene expression of placental survivin, it was found that there was a decrease in the preeclamptic pregnant women com-pared to the normal pregnant women (11,12,25). Li et al. found that the decline determined in the pree-clamptic pregnant women was correlated with the severity of disease (11). We have also found that the serum survivin levels decreased in the preeclamptic pregnant women, consistent with these studies.
In a study evaluating the maternal gene expression profile in the peripheral mononuclear cells of the pre-eclamptic and normal pregnant women, it was shown that the survivin expression increased in preeclamptic patients (10). The reason could be the attempt to com-pensate for the decrease in the expression of placental anti-apoptotic survivin in preeclampsia or or maybe to survive (or resist) maternal changes in preeclampsia such as endothelial dysfunction.
In a study on apoptosis-related proteins in the umbi-lical vein endothelial cells, apoptosis was increased in the preeclamptic pregnant women compared to the normal pregnant women. This increase was associated with the fact that anti-apoptotic Bcl-2 and survivin gene expression was suppressed while the pro- apoptotic BAX gene expression was activated, second-ary to P53 protein upregulation (26). It was shown that the survivin gene expression also decreased in the umbilical vein other than the placenta in the pree-clamptic pregnant women. The result was found to be consistent with the results of our study, showing that the survivin gene expression decreased in the pree-clamptic pregnant women.
In another study performed in 2013, the placenta and blood samples from normotensive pregnancies complicated with severe intrauterine growth retarda-tion (IUGR) (regardless of being accompanied by pre-eclampsia) and from those with normal growth and development were compared. The gene expression of pro-apoptotic BAD and BIM and of anti-apoptotic Bcl- 2, BCL-XL, and survivin on these samples was mea-sured. In pregnant women with preeclampsia accom-panied by severe IUGR, the survivin gene expression measured in ribonucleic acid (RNA) extracts increased in both placenta and maternal blood. However, it was noted that in the patients with preeclampsia not accom-panied by IUGR, the survivin gene expression was not significantly changed in either placenta or maternal blood (13). The study carried out by Rajakumar et al. (10) did not address whether IUGR accompanied pre-eclampsia. According to the study carried out by
Whitehead et al. (13), severe IUGR seemed to be more effective in terms of increasing the gene expression of placental and maternal survivin compared to pree-clampsia. In the present study, we have shown that the serum survivin levels were decreased in the pree-clamptic pregnant women compared to the controls and women with SGA fetuses compared to the women with AGA fetuses. Since the control group included only AGA fetuses, the number of SGA fetuses was, needless to say, more frequent in our preeclampsia group, especially the EOPE subgroup compared to the LOPE-subgroup. Nevertheless, considering only the preeclamptic women, the serum survivin was compar-able in the SGA and AGA groups, which may be inter-preted so that the presence of preeclampsia has a greater effect on serum survivin levels than SGA. Our study was not originally designed to compare the survivin levels in SGA and AGA fetuses; for that rea-son, these results are rather exploratory, and we cannot make a convincing statistical comparison of serum survivin between the SGA and AGA groups of the EOPE and LOPE subgroups. Moreover, the number of patients in the studies carried out by Rajakumar et al. (10), and Whitehead et al. (13) may have been insuffi-cient to address the results compared to the number of women in the present study. Further, greater scale and properly designed studies are needed to address the possible associations between serum survivin between the SGA and AGA groups of the EOPE and LOPE subgroups.
The extent of placental apoptosis in EOPE and LOPE was evaluated in quite a few studies. Khodzhaeva et al. (27) have shown that the expression of apoptotic marker Apo-Cas was higher in early- compared to late-onset of PE. The expression of mar-kers of endoplasmic reticulum stress-induced apoptosis (GRP78, ATF4, CHOP, and caspase 12 proteins) was also significantly higher in women with early-onset severe PE compared to those with late-onset severe PE (28). In our study, mean serum survivin levels were comparable in EOPE and LOPE subgroups. Further studies are needed to evaluate the placental apoptosis in EOPE and LOPE.
The limitations of our study included the absence of gene expression of placental, maternal, and umbilical survivin. Mechanistic studies are needed to address the potential biomarker role of survivin in preeclampsia to understand the pathogenesis of preeclampsia and develop treatments. However, the number of patients in our study was higher than that of the studies reviewed.
Our results are valuable since the placental survivin in preeclamptic patients is consistent with the gene
expression decrease. It has been revealed that the decline in survivin, an anti-apoptotic protein, is closely related to the increased apoptosis in preeclamptic patients. As we have shown that the serum survivin was significantly lower in preeclamptic women, it may be beneficial to investigate the serum survivin levels in the first half of the pregnancy, namely during the first trimester or early second trimester, to see how early the increase in apoptosis begins in preeclampsia, which is important from pathophysiological point of view. Measuring the survivin levels in serum before the onset of preeclampsia may make it possible to predict preeclampsia alone or in combination with other serum markers. However, further studies with the appropriate design are needed to investigate this issue.
Consequently, it was shown for the first time that the serum survivin level in maternal blood decreased in preeclamptic patients, both in EOPE and LOPE sub-groups compared to the GA-matched controls. Mean serum survivin levels were comparable in EOPE and LOPE subgroups. Serum survivin had weak negative correlations with systolic and diastolic blood pressure. There is a need for more comprehensive studies with a greater population to clarify the timing and extent of increased placental apoptosis in preeclampsia.
Acknowledgments
We would like to thank our beloved nurse Medine Eltutan from our pregnancy outpatient clinic in Istanbul Cerrahpasa University Hospital for her kind support and dedication during the patient recruitment.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Funding
This study was financed by Istanbul Cerrahpasa University, Istanbul, Turkey, and Carl von Ossietzky University Oldenburg, University Hospital for Obstetrics and Gynecology in Klinikum Oldenburg AöR, Oldenburg, Germany.
References
[1] Allaire AD, et al. Placental apoptosis in preeclampsia. Obstet Gynecol. 2000;96(2):271–276.
[2] Leung DN, Smith SC, To KF, et al. Increased placental apoptosis in pregnancies complicated by preeclampsia. Am J Obstet Gynecol. 2001;184(6):1249–1250.
[3] Ishihara N, Matsuo H, Murakoshi H, et al. Increased apoptosis in the syncytiotrophoblast in human term placentas complicated by either preeclampsia or intrau-terine growth retardation. Am J Obstet Gynecol. 2002;186(1):158–166.
[4] Heazell AE, Moll SJ, Jones CJP, et al. Formation of syncytial knots is increased by hyperoxia, hypoxia and reactive oxygen species. Placenta. 2007;28(Suppl A): S33–40.
[5] Johansen M, Redman CW, Wilkins T, et al. Trophoblast deportation in human pregnancy–its rele-vance for pre-eclampsia. Placenta. 1999;20(7):531–539. [6] Ka H, Hunt JS. Temporal and spatial patterns of
expression of inhibitors of apoptosis in human placentas. Am J Pathol. 2003;163(2):413–422.
[7] Lehner R, Bobak J, Kim NW, et al. Localization of telomerase hTERT protein and survivin in placenta: relation to placental development and hydatidiform mole. Obstet Gynecol. 2001;97(6):965–970.
[8] Wheatley SP, McNeish IA. Survivin: a protein with dual roles in mitosis and apoptosis. Int Rev Cytol. 2005;247:35–88.
[9] Holcik M, Gibson H, Korneluk RG. XIAP: apoptotic brake and promising therapeutic target. Apoptosis. 2001;6(4):253–261.
[10] Rajakumar A, Chu T, Handley DE, et al. Maternal gene expression profiling during pregnancy and preeclamp-sia in human peripheral blood mononuclear cells. Placenta. 2011;32(1):70–78.
[11] Li CF, Gou WL, Li XL, et al. Reduced expression of survivin, the inhibitor of apoptosis protein correlates with severity of preeclampsia. Placenta. 2012;33(1):47–51. [12] Zhang Z, Yang X, Zhang L, et al. Decreased expression
and activation of Stat3 in severe preeclampsia. J Mol Histol. 2015;46(2):205–219.
[13] Whitehead CL, Walker SP, Lappas M, et al. Circulating RNA coding genes regulating apoptosis in maternal blood in severe early onset fetal growth restriction and pre-eclampsia. J Perinatol. 2013;33 (8):600–604.
[14] Heazell AE, Buttle HR, Baker PN, et al. Altered expres-sion of regulators of caspase activity within trophoblast of normal pregnancies and pregnancies complicated by preeclampsia. Reprod Sci. 2008;15(10):1034–1043. [15] Stanek J. Histological features of shallow placental
implantation unify early-onset and late-onset preeclampsia. Pediatr Dev Pathol. 2019;22 (2):112–122.
[16] Guralp O, Tuten N, Oncul M, et al. Neutrophil gelatinase-associated lipocalin levels in early and late onset preeclampsia. Gynecology Obstet Reprod Med. 2020;26(3):166–172.
[17] Poon LC, Shennan A, Hyett JA, et al. The international Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: a pragmatic guide for first-trimester screening and prevention. Int J Gynaecol Obstet. 2019;145(Suppl 1):1–33. [18] Özçimen EE. New approaches for preeclampsia: review
of the literature. Gynecology Obstet Reprod Med. 2016;21(3):174–176.
[19] Kaufmann P, Black S, Huppertz B. Endovascular tro-phoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod. 2003;69(1):1–7.
[20] Athapathu H, Jayawardana MA, Senanayaka L. A study of the incidence of apoptosis in the human placental cells in the last weeks of pregnancy. J Obstet Gynaecol. 2003;23(5):515–517.
[21] Fortugno P, Wall NR, Giodini A, et al. Survivin exists in immunochemically distinct subcellular pools and is involved in spindle microtubule function. J Cell Sci. 2002;115(Pt 3):575–585.
[22] Dohi T, Beltrami E, Wall NR, et al. Mitochondrial survivin inhibits apoptosis and promotes tumorigenesis. J Clin Invest. 2004;114(8):1117–1127. [23] Colnaghi R, Connell CM, Barrett RMA, et al.
Separating the anti-apoptotic and mitotic roles of survivin. J Biol Chem. 2006;281(44):33450–33456. [24] Levy R. The role of apoptosis in preeclampsia. Isr Med
Assoc J. 2005;7(3):178–181.
[25] Muschol-Steinmetz C, Friemel A, Kreis -N-N, et al. Function of survivin in trophoblastic cells of the placenta. PLoS One. 2013;8(9):e73337.
[26] Gao Q, Zhu X, Chen J, et al. Upregulation of P53 promoted G1 arrest and apoptosis in human umbilical cord vein endothelial cells from preeclampsia. J Hypertens. 2016;34(7):1380–1388.
[27] Khodzhaeva Z, Kogan E, Kholin A, et al. PP016. Relation of apoptosis, proliferation and angiogenesis in early and late onset of preeclampsia. Pregnancy Hypertens. 2013;3(2):73.
[28] Fu J, Zhao L, Wang L, et al. Expression of markers of endoplasmic reticulum stress-induced apoptosis in the placenta of women with early and late onset severe pre-eclampsia. Taiwanese J Obstetrics Gynecol. 2015;54 (1):19–23.