Effects of Some Additives on Copper Losses to Matte Smelting
Slag
AYDIN RUSEN,1,4AHMET GEVECI,2YAVUZALI TOPKAYA,2 and BORA DERIN3
1.—Department of Materials Science and Engineering, Karamanoglu Mehmetbey University, 70100 Karaman, Turkey. 2.—Department of Metallurgical and Materials Engineering, Middle East Technical University, 06800 Ankara, Turkey. 3.—Department of Metallurgical and Materials Engineering, Istanbul Technical University, 34469 Istanbul, Turkey. 4.—e-mail: aydinrusen@hot-mail.com
Copper is lost to slag between 0.7 and 2.3 wt.% during the industrial copper matte smelting stage. In the present study, the aim was to minimize these losses in the slag phase by adding some fluxing agents like CaO, B2O3 and calcium borate (namely colemanite—2CaOÆ3B2O3Æ5H2O). Eti Copper Inc. (EBI) flash furnace smelter slag containing 0.88 wt.%Cu and matte with the addition each of CaO, B2O3and colemanite up to 10 wt.% of the total charge were melted in a silica crucible placed in a vertical tube furnace at 1250°C under nitrogen atmosphere for 2 h. The experimental results of matte–slag– flux mixtures showed that the addition of all additives up to 4 wt.% led to a gradual decrease of the copper content in the final slags. Beyond this value, the copper losses to slag increased markedly with the increasing CaO and B2O3 additions. On the other hand, the colemanite addition of more than 4 wt.% did not substantially affect the copper losses to slag.
INTRODUCTION
The copper losses in slags (0.7–2.3 wt.% Cu) are one of the major problems encountered in all new and conventional copper smelting techniques. There are several factors which affect copper losses to slag; such as matte grade, temperature, partial pressure of oxygen, slag composition, and slag properties.1–4 Since silica is widely used as a main fluxing agent to promote good separation of matte and slag phases by forming fayalite-type slag (2FeOÆSiO2), silica saturation level is the main parameter in slag composition. In addition, the slag properties such as its viscosity, density and melting point are affected by slag composition (amount of silica and other oxides).
According to investigations, copper found in the form of Cu2O, Cu2S or metallic Cu can be lost to smelting slag in two different forms, namely, mechanical and physicochemical losses. The former originates from mechanically entrainment of matte and/or metal components, and the latter is related to dissolved copper species in slag in both oxide and sulfide forms.5–7 The ratio of mechanically
entrained versus dissolved copper differs from plant to plant because factors (operating conditions) affecting copper losses to slag alter for each plant. However, the general opinion is that, at lower matte grades, most of the copper losses arise from mechanically entrained matte and metallic copper. As for the higher matte grades (>70 wt.%Cu), the majority of the losses result from physico-chemical losses.4,8,9Considering the matte grade at a reason-able level (between 45 wt.%Cu and 60 wt.%Cu) in most of the copper matte smelting processes, the copper losses to slag arise from entrained copper losses (mechanical) due to high slag viscosity. Smelting conditions which favor mechanically entrapped inclusions to settle easily to the matte phase, should be improved by decreasing slag viscosity, by increasing settling duration and by minimizing the slag layer. However, all these conditions cannot be applied simultaneously to a smelting furnace.
It is possible to reach the best matte–slag sepa-ration with silica satusepa-ration in the smelting stage. However, the usage of high amounts of silica results in an increase of the slag viscosity with the presence Ó2016 The Minerals, Metals & Materials Society
of magnetite. The higher slag viscosity leads to more copper losses due to the lowered settling rate of copper inclusions. Therefore, in some smelting processes, apart from silica, limestone (CaCO3) is added to the smelting charge to balance slag basicity, and to decrease the melting point and viscosity of the slag.7,10–13 In addition, some researchers10,11,14–16 have studied colemanite (2CaOÆ3B2O3Æ5H2O) as flux in the steel industry and proved that the colemanite addition leads to lower viscosity and the melting temperature of the steelmaking slags. Therefore, this study has aimed to investigate the effect of some fluxing agents (CaO, colemanite or B2O3) on copper losses to slag for the copper matte smelting process in order to reduce copper losses in the slag phase. By this way, comparative results could be obtained for all fluxing agents used in the experiments and the changes in liquidus temperature could be estimated by com-puter software (FactSage).17
EXPERIMENTAL PART Materials
Slag and Matte
Representative flash furnace slag and matte samples were supplied by Eti Copper Inc., the copper smelter plant in Samsun–Turkey, in powder form (100 lm) so that they were ready to be used in
the experiments. Flash furnace slag with
0.88 wt.%Cu, 36.1 wt.%SiO2, 43.9 wt.%FeO and 3.7 wt.%Fe2O3, and, matte with 50.2 wt.%Cu, 27.4 wt.%Fe and 19.8 wt.%S were labeled as the flash furnace slag (FFS) and flash furnace matte (FFM), respectively.
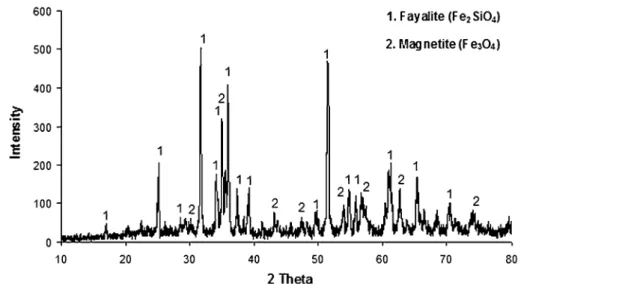
For mineralogical characterization of the slag sample, a Rigaku D/MAX2200/PC model x-ray pow-der diffraction (XRD) instrument available in the Metallurgical and Materials Engineering Depart-ment of the Middle East Technical University (METU) was used. The peaks of diffraction were recorded and plotted against a horizontal scale between 5° and 95° of 2h, which was the angle of the detector rotation using intervals of 0.02° with CuKa radiation. The x-ray pattern which belongs to the FFS sample is given in Fig.1.
As noted in the previous investigations,7,18–20 fayalite and magnetite were identified as the main phases of copper smelting slag. Although the pres-ence of other oxides such as CaO and Al2O3 were determined by the chemical analysis of FFS and also detected by other researchers21 with an elec-tron micro-probe analyzer, they could not be iden-tified in the XRD pattern. In order to get information about the sample surface’s topography and composition, scanning electron microscope (SEM) analyses were carried out on gold-coated FFS samples by using a FEI Nova Nano SEM 430 model equipped with energy dispersive x-ray spec-troscopy available in the Metallurgical and
Materials Engineering Department of METU. As seen in the SEM image of the representative FFS sample given in Fig.2, apart from iron silicate (fayalite) matrix and magnetite phase, matte/metal-lic copper particles with various sizes were present. Colemanite
Boron in elemental or compound form is widely used in several industries. However, it always occurs in nature as a mineral. There are many different types of boron minerals and one of them is colemanite with a chemical formula 2CaOÆ3B2O3Æ 5H2O. Ground colemanite is produced by Eti Mine Works Bigadic¸ Plant, Balıkesir, Turkey. In this study, ground colemanite with 75 lm particle size, supplied by the producer (Eti Mine Works General Management), was calcined at 400°C for 24 h in a muffle furnace with mixing every hour in order to decompose and eliminate any chemically bonded water that was present. The calcined colemanite (CC) with 51.7 wt.%B2O3, 27.7 wt.%CaO, 8.6 wt.%CaCO3, 7.9 wt.%SiO2and 4.1 wt.% other oxides (Al2O3, MgO and SrO) was kept in a desiccator to
prevent moisture pick up and used in the
experiments. Boric Oxide
It was obtained after the calcination of boric acid (H3BO3) provided by Merck at 900°C in a nickel crucible for 2 h. In this way, water in the boric acid was removed, and then, molten B2O3was poured on to a stainless steel plate. After cooling, it was powdered and stored in a desiccator to protect it from hydration.
Calcium Oxide
Reagent grade CaO supplied by Sigma Aldrich
(1305-78-8 CAS) was used in the related
experiments.
Methods and Models
In the experiments, a total of 60 g FFS + FFM (1:1 in ratio) and various additions of fluxes (CaO, B2O3or CC) up to 10 wt.% of the total charge were mixed and placed in a silica crucible (30 ± 1 mm inside diameter, 38 ± 1 mm outside diameter, 80 ± 2 mm height and 7 ± 1 mm bottom thickness), and then the crucible was put into a vertical MoSi2 electric resistance furnace. The furnace was heated slowly to 1250°C in N2 atmosphere with a rate of 4°C/min. The sample was held over 2 h in the nitrogen atmosphere at this temperature, and then slowly cooled. After separation from the crucible, the analyses of slag and matte samples used in the experiments were carried out by different tech-niques: wet chemical, inductively coupled plasma-mass spectrometer (ICP-MS), saturated magnetite analysis (SATMAGAN) and x-ray fluorescence
Rusen, Geveci, Topkaya, and Derin 2324
(XRF). By wet chemical analyses at EB_I Analysis Laboratory, the Cu, S and Fe contents of the matte, and the Cu, S, total Fe and SiO2contents of the slag were obtained. A Perkin Elmer DRC II model ICP-MS (at METU Central Laboratory) was especially used to analyze the boron (B) apart from the other elements (Cu, Fe, S, Si, Ca, Al, Zn, Pb) in the resultant slag samples after flux addition. The magnetite content of each sample was determined by SATMAGAN S135 with a maximum error of ± 0.2% of the measured values.
Thermochemical calculations were also carried out in the present study. The effects of additive oxides (i.e., CC, CaO, and B2O3) in the FeO-Fe2O3 -SiO2 system at different temperatures were calcu-lated and plotted as ternary phase diagrams by using the Phase Diagram module of the FactSage thermochemical software.17 FTOxide solution data-base was selected for the liquid and solid solutions
in the system, whereas all gas, liquid and other stoichiometric solid phases were selected from the FactPS database.
RESULTS AND DISCUSSIONS
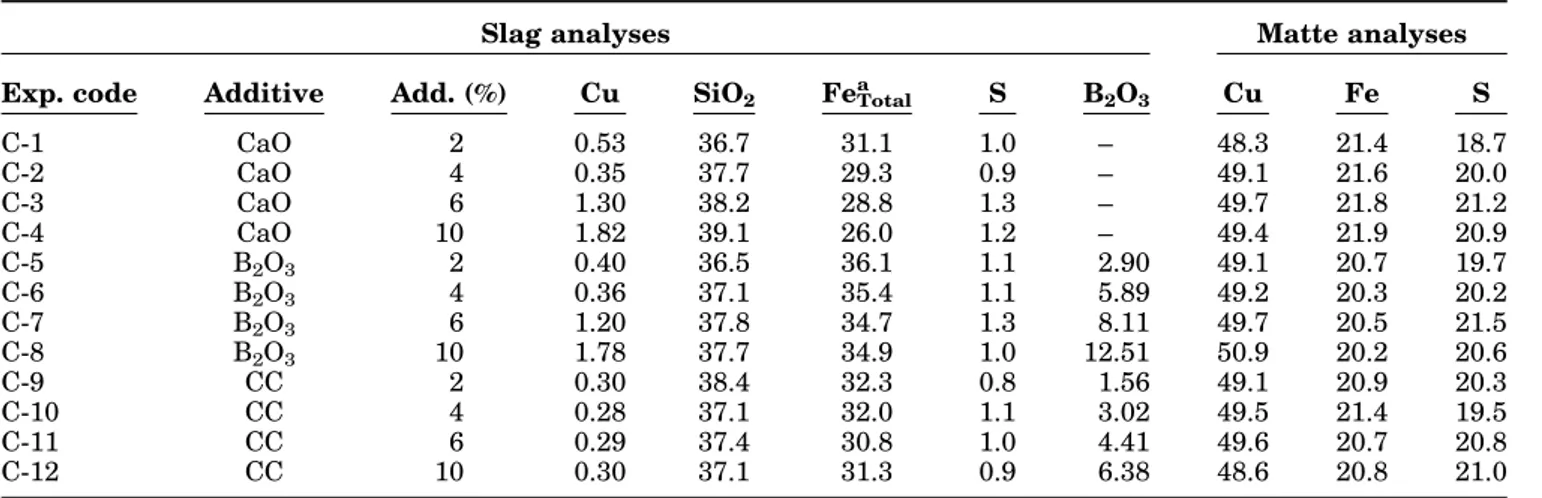
In this study, the effects of some additives such as CaO, B2O3and calcined colemanite (CC) as flux on the copper losses to slag were investigated by aiming to minimize the copper content in the slag. For this purpose, the flash furnace slag and matte (FFS-FFM) with the addition each of CC, CaO and B2O3up to 10 wt.% of the total charge were melted in a vertical tube furnace in silica crucibles at 1250°C under nitrogen atmosphere for 2 h. The experimental findings are given in Table I and Fig.3.
TableI shows the chemical analysis of resultant slags for Cu, S, Fe, SiO2and B2O3. Apart from these elements/oxides, the balance value for the resultant slags contains all the other oxides analyzed by XRF: ZnO (3.9–4.2 wt.%), Al2O3(2.2–5.0 wt.%), CaO (0.6– 4.3 wt.%), PbO (0.2 wt.%), BaO (0.6 wt.%), K2O (0.6 wt.%) and MgO (0.3 wt.%).
In order to correctly explain the results, some important information about the behavior of B2O3 should be given. Although boric oxide (B2O3) is not extensively employed in metallurgical applications like SRF lead production furnaces,22 it is widely used in the glass industry due to some important properties. For example, the addition of B2O3 to alkali-silicate compositions such as SiO2-Al2O3-CaO yields the very crucial group of alkali-borosilicate glasses with relatively easy melting and a good chemical durability especially to attack by acid.23As for viscosity, the effect of B2O3 is considerably different at high and low temperatures. Its struc-tural change, and the associated property change, depends on temperature variations. While the
building unit of boron structures at low
Fig. 1. X-ray diffraction pattern of FFS.
temperatures (<1000°C) as oxygen tetrahedron [BO4]5 leads to an increase in the viscosity of melt, at higher temperatures (>1000°C), a major part of the tetrahedrons turning into [BO3]
3
triangles causes the break up of the chains of the molten matrix and so decreases the viscosity.24 Moreover, B2O3 addition to the slag decreases the melting point. In other words, boric oxide tends to form a eutectic due to its very low melting point, which results in a more effective decrease in the slag viscosity.25,26Considering the copper smelting oper-ation temperature which is above 1200°C, the total effects of structure change and eutectic formation result in a decrease in the viscosity of molten slag. There is a clear consensus among research-ers10,11,14,15,27 studying B2O3 (or colemanite) sys-tems at high temperature that the addition of B2O3 (or colemanite) decreases the viscosity of the melt. After the addition of B2O3 to the flash smelting furnace, an increase of its temperature (as well as
the matte and slag temperatures) will be expected, while the supplied fuel is of the same amount due to the eutectics (combining of other oxides with B2O3) with low melting points. Therefore, the temperature can be decreased gradually to the operating value by lowering the fuel–oil/gas addition to the system. That is, a considerable amount of fuel saving can be obtained after B2O3 (or colemanite due to the contained B2O3) addition. However, it is well known that the liquidus temperature and the fluidity of slag play an important role in the furnace refractory life. Before applying industrially, it will be neces-sary to study experimentally at the laboratory scale the reactions between the refractory and slag having boron compounds.
As mentioned previously, a good/clear separation of matte and slag also depends on their density differences apart from other factors. Therefore, the addition of B2O3 leads to a lowering of slag density and also a better separation of matte and slag.
Based on all the positive effects mentioned above, it was expected that the copper losses to slag should decrease with the addition of B2O3. From the data given in TableIand also Fig.3, it can be seen that the addition of B2O3(up to 4 wt.%) to slag decreases the copper content in the slag to a relatively low value as expected. However, beyond this point, the copper content in the slag increased as the B2O3 addition increased. This might be attributed to the climbing of the slag including small amounts of matte particles on the side walls of the crucible, which occurred after the excessive addition of B2O3 in this study. On the other hand, as seen in Fig.4c, there are several holes on the slag surface observed only for 6 wt.% and 10 wt.%B2O3 additions, which may be attributed to the evaporation of boron. Figure4d shows an example of the slag climbing after an experiment with 10 wt.% B2O3 addition to the matte–slag system. Since B2O3 in the slag
Table I. Chemical analysis results of experiments with FFS and FFM with various additions of CC, CaO and B2O3as wt.% (under nitrogen atmosphere at 1250°C for 2 h)
Slag analyses Matte analyses
Exp. code Additive Add. (%) Cu SiO2 FeTotal
a S B2O3 Cu Fe S C-1 CaO 2 0.53 36.7 31.1 1.0 – 48.3 21.4 18.7 C-2 CaO 4 0.35 37.7 29.3 0.9 – 49.1 21.6 20.0 C-3 CaO 6 1.30 38.2 28.8 1.3 – 49.7 21.8 21.2 C-4 CaO 10 1.82 39.1 26.0 1.2 – 49.4 21.9 20.9 C-5 B2O3 2 0.40 36.5 36.1 1.1 2.90 49.1 20.7 19.7 C-6 B2O3 4 0.36 37.1 35.4 1.1 5.89 49.2 20.3 20.2 C-7 B2O3 6 1.20 37.8 34.7 1.3 8.11 49.7 20.5 21.5 C-8 B2O3 10 1.78 37.7 34.9 1.0 12.51 50.9 20.2 20.6 C-9 CC 2 0.30 38.4 32.3 0.8 1.56 49.1 20.9 20.3 C-10 CC 4 0.28 37.1 32.0 1.1 3.02 49.5 21.4 19.5 C-11 CC 6 0.29 37.4 30.8 1.0 4.41 49.6 20.7 20.8 C-12 CC 10 0.30 37.1 31.3 0.9 6.38 48.6 20.8 21.0 a
Resultant slags contain from 0.9 to 2.5 wt.% magnetite (determined by SATMGAN S135).
Fig. 3. Effect of some additives (CaO, B2O3, CC) on copper losses
to slag (under nitrogen atmosphere at 1250°C for 2 h).
Rusen, Geveci, Topkaya, and Derin 2326
lowers the surface tension between the slag and the crucible by occupying the surface,28 the addition of B2O3 above a certain value partially caused this climbing of the slag up the sides of the crucible.
Several researchers1,9,29,30 have reported that CaO addition decreased the copper solubility in the slag since it could replace any oxidic copper in the slag associated with silica. In addition, the presence of CaO in slag decreased its viscosity by breaking complex long silicate chains, which allowed easy settling and promoted a decrease in the copper losses to slag. Interfacial energy/tension is also an important parameter in the copper losses to slag. When the interfacial tension is high between matte inclusions and slag, a good separation encour-ages an increasing rate of settling. In contrast, as the interfacial tension between them decreases, the separation of these two phases becomes more diffi-cult. According to the literature, the effect of CaO on the interfacial tension between the matte and the slag exhibits a non-linear behavior with a peak at 4 wt.%CaO. After reaching the maximum value, the
interfacial tension between the copper matte and the slag decreases gradually with increasing CaO addition.31–33This effect was explained by Li et al.32 as follows; ‘‘This may be interpreted by a stronger interaction between S in matte and Ca2+ in slag. With increasing Ca2+ concentration in slag, the amounts of transition of S from the matte to the slag increases’’. As can be seen from Fig.3 and TableI, the effect of CaO on the copper losses to slag as well as the sulfur concentration in the slag indicated a similar behavior to this, i.e., when CaO was added to the matte–slag system up to 4 wt.%CaO, the copper and sulfur contents of slag decreased. After this point, any further increase in CaO addition caused a decrease in interfacial tension and also an increase in both copper and sulfur contents in the slag. Additionally, according to Ducret and Ran-kin,34CaO addition to the slag generally results in a slight decrease in liquidus temperature of the slag, but increasing oxygen partial pressure of the system at a further addition of CaO (>3 wt.%) leads to a moderate rising of the liquidus temperature. Fig. 4. The separated matte–slag phases after experiments (at 1250°C for 2 h under nitrogen atmosphere) with the addition of B2O3: (a) 2 wt.%,
(b) 4 wt.%, (c) 6 wt.% and (d) 10 wt.% (climbing of the slag including the matte on the sides of the silica crucible). (*m.p. matte or metallic copper particles).
In Fig. 3, the curve belonging to CC addition shows better results with regards to the copper losses to the slag. Taking all the experimental results into account, this behavior could be explained by the double effect of CaO and B2O3. Since the CC included both CaO and B2O3, it was more effective than either of them separately. In any case, the addition of CC exceeding 4 wt.% had no beneficial effects, i.e., a decrease in copper content of the slag after 4 wt.%CC addition was insignificant. Considering the chemical analysis of CC (having 51.7 wt.B2O3, 27.7 wt.%CaO and 8.6 wt.%CaCO3), the addition of 4 wt.%CC of the total charge corresponded to nearly 2.1 wt.%B2O3 and 1.4 wt.%CaO addition of total charge. These values were within the positive effect limits for CaO and B2O3. Negative effects (high copper loss, slag climbing, low interfacial tension, etc.) of CC addi-tion are expected to start from 12 wt.%CC addiaddi-tion because only then does it include more than 4 wt.%CaO and 6 wt.%B2O3.
Experimental observations should also be given here to clearly understand the effect of CC, i.e., CaO and B2O3 additions to the system. Figures4,
5 and 6 show the separated matte–slag phases after experiments with the addition of B2O3, CaO and CC, respectively. As stated before, as more and more CC additions were carried out, the more easily the matte–slag separation took place. So the matte was easily separated from the slag by fingers without using any apparatus in all of the experiments performed with CC addition (even with 2%CC addition). However, as CC additions increased, the slag–crucible separation became more difficult due to the reaction with the crucible material. In B2O3 addition experiments, the sep-aration difficulties (matte–slag and slag–crucible) were very similar to those in CC addition exper-iments. However, the experiments with up to 4 wt.%CaO addition resulted in easy separation of matte and slag, as well as the slag from the crucible. Further addition of CaO led to a Fig. 5. The separated matte–slag phases after experiments (at 1250°C for 2 h under nitrogen atmosphere) with the addition of CaO: (a) 2 wt.%, (b) 4 wt.%, (c) 6 wt.% and (d) 10 wt.% (matte sticking to the slag).
Rusen, Geveci, Topkaya, and Derin 2328
stronger/hard slag, and matte–slag separation was quite difficult. This may be due to decrease in interfacial tension between matte–slag couples and the formation of a calcium silicate with a high melting point and a more rigid structure.
Calculation of Phase Diagrams by FactSage The change of the liquid slag region of fayalite-type flash furnace slags with 10 wt.% addition of each fluxing agent (CaO, B2O3 and CC) at different tem-peratures (1200, 1250 and 1300°C) was simulated and Fig. 6. The separated matte–slag phases after experiments (at 1250°C for 2 h under nitrogen atmosphere) with the addition of CC: (a) 2 wt.%, (b) 4 wt.%, (c) 6 wt.%, (d) 10 wt.%, and (e) without CC addition.
Fig. 7. Change in liquid slag region with 10 wt.% addition of B2O3
phase diagram of FeO-Fe2O3-SiO2 calculated by the ‘‘Phase
Dia-gram’’ module of FactSage 6.2.
Fig. 8. Change in the liquid slag region with 10 wt.% addition of CaO phase diagram of FeO-Fe2O3-SiO2calculated by the ‘‘Phase
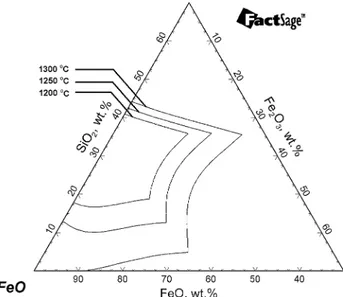
plotted by FactSage ‘‘Phase diagram’’ module. It should be noted that the oxides with small quantities ((ZnO < wt.%4.2, Al2O3< wt.%2.3, K2O < wt.%0.6, BaO < wt.%0.6, MgO < wt.%0.2 analyzed by XRF) present in FFS were neglected and only the FeO-Fe2O3-SiO2 phase diagram which is equilibrated under 1 atm total pressure was used when calculating each diagram. In addition, iron formation was not shown in the figures during the calculations. The calculated results for B2O3, CaO and CC can be seen in Figs.7,8and9, respectively.
As previously described, boric oxide addition to the slag system tended to form a eutectic due to its very low melting point, which resulted in the liquid slag region increasing and it expanding with increasing temper-ature. However, 10 wt.%CaO addition to fayalite-type
slag showed limited increase in its liquid slag region with increasing temperature. On the other hand, when 10 wt.%CC is added to the system, the liquid slag region increases considerably by increasing the tem-perature from 1200°C to 1300°C, as seen in Fig.9.
In order to see the effects of additives on the FeO-Fe2O3-SiO2phase diagram, the change in the liquid slag region of fayalite-type slag with 10 wt.% addi-tion of CaO, B2O3 and CC each was calculated by FactSage ‘‘Phase diagram’’ module at 1250°C. It should also be stated again that other oxides (Al2O3, ZnO, K2O, BaO, etc.) present in FFS were omitted for this calculation. Figure10 gives all the fluxing agents addition on the FeO-Fe2O3-SiO2 phase diagram.
The calculated results at 1250°C indicated that when 10 wt.%CaO was added to the fayalite slag, the liquid slag region became narrower and shifted up (toward SiO2) probably due to need for more SiO2 to reach the equilibrium. On the other hand, 10 wt.% addition of B2O3with its very low melting point led to the extension of the liquid region towards the FeO corner. It was very clear that 10 wt.%CC addition greatly increased the liquid slag region in every direction (toward SiO2, FeO and Fe2O3corners) by maintaining its initial shape.
CONCLUSION
The objective of this study was to observe the effects of CaO and B2O3 additions on the copper losses to slag and to compare their results with those of calcined colemanite (CC) addition. As a conclusion, the experimental results of the matte– slag–flux mixture conducted at 1250°C for 2 h under nitrogen atmosphere showed that the addition of each additive such as CC, CaO and B2O3 up to 4 wt.% led to a gradual decrease in the copper content of the final slags. After this point, the copper losses to the slag increased markedly with the increasing CaO and B2O3 additions. On the other hand, the CC addition of more than 4 wt.% had a negligible effect on the copper losses to the slag and showed a plateau at about 0.3 wt.%Cu.
By using FactSage software, the phase diagrams of the system FeO-Fe2O3-SiO2 after additions of CaO, B2O3 and CC were calculated to observe the behavior of the liquid slag region. The phase diagrams calculated by FactSage indicated that the CC addition greatly increased the liquid slag region in every direction (toward SiO2, FeO and Fe2O3 corners) by maintaining its initial shape unlike the other additives.
ACKNOWLEDGMENTS
This study was supported by the National Boron Research Institute with project no. BOREN-2007-C0141 and METU. The support given by EB_I by supplying FFS-FFM samples and performing the wet chemical analysis is gratefully acknowledged. Fig. 9. Change in the liquid slag region with the 10 wt.% addition of
CC phase diagram of FeO-Fe2O3-SiO2calculated by the ‘‘Phase
Diagram’’ module of FactSage 6.2.
Fig. 10. Change in the liquid slag region with the 10 wt.% addition of CaO, B2O3and CC on phase diagram of FeO-Fe2O3-SiO2calculated
by the ‘‘Phase Diagram’’ module of FactSage 6.2.
Rusen, Geveci, Topkaya, and Derin 2330
REFERENCES
1. J.C. Yannopoulas, Can. Metall. Q. 10, 291 (1971).
2. J.M. Toguri, N.J. Themelis, and P.H. Jennigs, Can. Metall. Q. 3, 199 (1964).
3. M.E. Schlesinger, M.J. King, A.W. Davenport, and K.C. Sole, Extractive Metallurgy of Copper, 5th edn. (Oxford: Elsevier, 2011), pp.191–203.
4. H. Jalkanen, J. Vehvilainen, and J. Poijarvi, Scand. J. Me-tall. 32, 65 (2003).
5. R. Sridhar, J.M. Toguri, and S. Simeonov, Metall. Mater. Trans. B 28, 191 (1997).
6. F. Sehnalek, I. Imris, in Adv. Extr. Metall. Refin., IMM, London, 1972, pp. 39–62.
7. A. Geveci, in 5.th Sci. Congr., Turkish Scientific And Re-search Council, Ankara, pp. 289–305, (1975).
8. A. Yazawa, Can. Metall. Q. 13, 443 (1974).
9. W.J. Elliot, B.J., See, J.B., Rankin, Trans. Inst. Min. Met. C, 87, 204 (1978).
10. M. Timucin, N. Sevinc, Y. A. Topkaya, H. Eric, Use of Cole-manite in the Production of Iron and Steel, Ankara, (1986). 11. E. Ozmen, L., Inger, in Sohn Int. Symp., TMS, San Diego,
pp. 299–306, (2006).
12. Y. Lu, G. Zhang, and M. Jiang, Adv. Mater. Res. 233, 805 (2011). 13. K.H. Obst, J. Stradtman, J. S. Afr. Inst. Min. Metall., 158
(1972).
14. M. Sek, A. Aso, and Y. Okubo, Kawasaki Steel Tech. Rep. 15, 16 (1986).
15. Y. Pontikes, L. Kriskova, X. Wang, S. Arnout, E. Nagels, O¨ . Cizer, T. Van, in 2nd Int. Slag Valoris. Symp. (Ed.: O.C. P.T. Jones, Y. Ponitkes, J. Elssen), Katholieke Universtiteit Leuven, Leuven, pp. 313–326 (2011).
16. A. Rus¸en, A. Geveci, Y.A. Topkaya, and B. Derin, Can. Me-tall. Q. 51, 157 (2012).
17. FactSage, 2011.
18. B. Gorai and R.K. Jana, Resour. Conserv. Recycl. 39, 299 (2003).
19. Y.A. Topkaya, ATB Met. 30, 23 (1990).
20. I. Mihailova and D. Mehandjiev, J. Univ. Chem. Technol. Metall. 45, 317 (2010).
21. R. Ru¨ ffler and J. Davalos, Hyperfine Interact. 111, 299 (1998).
22. P.C. Hayes, M.E. Schlesinger, H.U. Steil, A. Siegmunt, in Proc. Lead-Zinc 2010, Canadian Institute of Mining, Metallurgy and Petroleum), Westmount, pp. 345–413 (2010).
23. K.J. Rao, Structural Chemistry of Glasses (Oxford: Elsevier, 2002).
24. V. Werner, Glass Chemistry (Berlin: Springer, 1994). 25. S. Ren, J. Zhang, L. Wu, W. Liu, Y. Bai, X. Xing, B. Su, and
D. Kong, ISIJ Int. 52, 984 (2012).
26. H. Aykut, Influence of B2O3Additions on the Microstructure
of Mica Based Glass—Ceramics, METU, M.Sc. Thesis (2005).
27. H. Wang, G. Li, Q. Dai, Y. Lei, Y. Zhao, B. Li, G. Shi, and Z. Ren, ISIJ Int. 46, 637 (2006).
28. K. Mills, The Estimation of Slag Properties, Johannesburg (2011).
29. R. Altman, Trans. Inst. Min. Met. C 87 (1978).
30. J.B. See, L.L. Oden, P.E. Sanker, and E.A. Johnson, Copper Losses and the Distribution of Impurity Elements between Matte and Silica-saturated Iron Silicate Slags at 1250°C (Pittsburgh, PA: U.S. Dept. of the Interior, Bureau of Mines, 1982).
31. F.J. Elliott and M. Mounier, Can. Metall. Q. 21, 415 (1982). 32. J. Li, K. Huang, and X. Chen, Acta Metal. Sin. B 2, 386
(1989).
33. R.H. Eric, J. S. Afr. Inst. Min. Metall. 499 (2004).
34. A.C. Ducret and W.J. Rankin, Scand. J. Metall. 31, 59 (2002).