SELCUK UNIVERSITY
THE GRADUATE SCHOOL OF NATURAL AND APPLIED SCIENCES
PREPARATION OF MODIFIED GLASSY CARBON ELECTRODES FOR VOLTAMMETRIC DETERMINATION OF
TRICLOSAN
Abdurrahman Taha GÜLDEREN THE DEGREE OF MASTER OF SCIENCE
IN CHEMISTRY
December-2018 KONYA All Rights Reserved
iv
ÖZET
YÜKSEK LİSANS TEZİ
TRİKLOSANIN VOLTAMETRİK TAYİNİ İÇİN MODİFİYE CAMSI KARBON ELEKTROTLARIN HAZIRLANMASI
Abdurrahman Taha GÜLDEREN Selçuk Üniversitesi Fen Bilimleri Enstitüsü
Kimya Anabilim Dalı
Danışman: Prof. Dr. Yasemin ÖZTEKİN 2. Danışman: Prof. Dr. Jiri BAREK
2018, 55 Sayfa Jüri
Prof. Dr. Yasemin ÖZTEKİN Doç. Dr. Mustafa ÖZMEN Dr. Öğretim Üyesi Mürsel EKREM
Triklosan, 5-kloro-2-(2,4-diklorofenoksi)fenol, temizlik ve kişisel bakım ürünlerinde yaygın olarak kullanılan, antibakteriyel ve antifungal etkilere sahip kimyasal bir bileşiktir. Birleşik Devletler Çevre Koruma Ajansı, bu molekülü pestisit kategorisinde değerlendirmektedir. Çevre ve insan sağlığı için zararlı olabilecek bu bileşiğin Avrupa ve Amerika’daki ürünlerdeki kullanımı %0.3 ile sınırlandırılmıştır.
Sudaki çözünürlüğü düşük olup bazik ortamda daha yüksek çözünürlüğe sahip olan triklosan, yaygın kullanımından ötürü yüzey sularında ve atık sularda yüksek oranda bulunabilmektedir. Bu nedenle su kalitesinin belirlenmesi için triklosanın doğru tayini önem arz etmektedir. Bunun için yüksek performanslı sıvı kromatografisi, gaz kromatografisi, kapiler elektroforez, spektrofotometri ve elektrokimya tabanlı pek çok teknik geliştirilmiştir.
Yapılan bu tez çalışmasında elektrokimyasal triklosan sensörü geliştirmek amacıyla camsı karbon elektrot yüzeyi, farklı oranlardaki çok duvarlı karbon nanotüp yokluğunda veya varlığında Au3+
iyonlarının elektrot yüzeyinde indirgenmesiyle modifiye edilmiştir. Modifiye edilen bu yüzeyler; elektrokimyasal, mikroskobik ve spektroskopik tekniklerle karakterize edilmiş ve elektrokimyasal triklosan tayini amaçlı kullanılmışlardır.
Anahtar Kelimeler: Altın nanoyapı, Camsı karbon elektrot, Çok duvarlı karbon nanotüp,
v
ABSTRACT MSc THESIS
PREPARATION OF MODIFIED GLASSY CARBON ELECTRODES FOR VOLTAMMETRIC DETERMINATION OF TRICLOSAN
Abdurrahman Taha GULDEREN
THE GRADUATE SCHOOL OF NATURAL AND APPLIED SCIENCES OF SELCUK UNIVERSITY
THE DEGREE OF MASTER OF SCIENCE IN CHEMISTRY
Advisor: Prof. Dr. Yasemin OZTEKIN 2nd Advisor: Prof. Dr. Jiri BAREK
2018, 55 Pages Jury
Prof. Dr. Yasemin OZTEKIN Assoc. Prof. Dr. Mustafa OZMEN
Assist. Prof. Dr. Mursel EKREM
Triclosan, 5-chloro-2-(2,4-dichlorophenoxy)phenol, is a chemical compound which is widely used as ingredient in cleaning and personal care products because of its antibacterial and antifungal effects. United States Environmental Protection Agency has classified triclosan as a pesticide. This compound, which can be harmful to the environment and human, is restricted to 0.3% in European and American products.
Triclosan, that is slightly soluble in water while its solubility could be higher in alkaline water, can be present in surface and waste waters due to its widespread usage. Therefore, accurate determination of triclosan is important for the determination of water quality. Thus, many techniques based on high performance liquid chromatography, gas chromatography, capillary electrophoresis, spectrophotometry and electrochemistry have been developed.
In this thesis, glassy carbon electrode was modified by reducing Au3+ ions in the absence or
presence of multi-walled carbon nanotubes for developing the electrochemical triclosan sensor. Subsequently, prepared surfaces were characterized with electrochemical, microscopic and spectroscopic techniques and applied for electrochemical determination of triclosan.
Keywords: Electrodeposition, Glassy carbon electrode, Gold nanostructure, Multi-walled carbon
vi
FOREWORD
This Thesis has been supervised by Prof. Dr. Yasemin ÖZTEKİN and presented as MSc Thesis to The Graduate School of Natural and Applied Sciences of Selcuk University. I would like to thank to my supervisor, Prof. Dr. Yasemin ÖZTEKİN, for her guidance, advice and support.
It is a great pleasure to thank to my co-supervisor, Prof. Dr. Jiří BAREK, for allowing me to study at UNESCO Laboratory of Environmental Electrochemistry, Charles University of Prague and also his supervision during my thesis. Also, I would like to express my thankfulness to Dr. Jan FISCHER for assisting me during my Erasmus term.
Many thanks to my colleague, Dr. Emre ASLAN and SEM specialist, Dr. Fatih ÖZCAN in Advanced Technology Research&Application Center of Selcuk University. Also, I would like to thank to each person who helped me during my MSc term like my family and my friends.
I would like to express my genuine gratefulness to ERASMUS+ Student Exchange Program for their financial support during my MSc term in Prague and also Scientific Research Projects Coordination Unit of Selcuk University (SELCUK-BAP) which supported this research under the number of 17201124.
Abdurrahman Taha GÜLDEREN KONYA-2018
vii INDEX ÖZET ... iv ABSTRACT ... v FOREWORD ... vi INDEX ... vii
SYMBOLS AND ABBREVIATIONS ... viii
1. INTRODUCTION ... 1
2. LITERATURE REVIEW ... 5
3. MATERIALS AND METHODS ... 12
3.1. Chemicals ... 12
3.2. Apparatus ... 13
3.3. Preparation of Solutions ... 14
3.3.1. Preparation of solutions for electrochemical characterization process ... 14
3.3.2. Preparation of solutions for electrodeposition process ... 14
3.3.3. Preparations of triclosan solution ... 15
3.3.4. Preparation of Britton-Robinson buffer solution ... 15
3.4. Cleaning Procedure of Working Electrode ... 15
3.5. Preparation of Surfaces ... 16
3.6. Characterization of Surfaces ... 17
3.6.1. Surface characterization with electrochemical techniques ... 17
3.6.2. Surface characterization with scanning electron microscope ... 18
3.6.3. Surface characterization with energy-dispersive X-ray spectroscopy ... 18
3.7. Electrochemical Determination of Triclosan ... 19
4. RESULTS AND DISCUSSION ... 20
4.1. Electrochemical Characterization of Surfaces ... 20
4.1.1. Electrochemical characterization with ferrocene ... 20
4.1.2. Real surface area calculations ... 23
4.2. Microscopic Characterization of Electrode Surfaces with Scanning Electron Microscope (SEM) ... 24
4.3. Spectroscopic Characterization of Surfaces with Energy-Dispersive X-ray (EDX) Spectroscopy ... 31
4.4. Electrochemical Triclosan Determination ... 34
5. CONCLUSIONS ... 39
REFERENCES ... 41
viii
SYMBOLS AND ABBREVIATIONS Abbreviations
A : Electroactive real surface area
Ag/Ag+ : Silver/silver nitrate reference electrode Ag/AgCl/KClsat : Silver/silver chloride reference electrode BR buffer solution : Britton Robinson buffer solution
C0 : Concentration of the oxidized molecules
CNT-X/AuNS-Y/GC : Carbon nanotube (X ppm)/gold nanostructure (Y modified glassy carbon electrode :
D : Diffusion coefficient
EDX : Energy-dispersive X-ray spectroscopy
EPA : United States Environmental Protection Agency FDA : United States Food and Drug Administration
GC : Glassy carbon
HAuCl4 : Hydrogen tetrachloroauric acid HCF (II)/(III) : Potassium ferro-ferricyanide
Ip : Peak current
MWCNT : Multi-walled carbon nanotube
n : Number of transferred electron
R2 : Coefficient of determination
SCE : Saturated calomel electrode
SEM : Scanning electron microscope
1. INTRODUCTION
Science, technology and innovation are quite important for the life quality of humanity. These interconnected fields have become the outcomes of human being. Curiosities have converted to discoveries and explores, thus, science has assisted a healthier life and technologies have increased quality of life (Van Montagu, 2018). These fields have been suited with knowledge and information, moreover have shaped the technology. Nevertheless, technology is not only a result of collaboration of knowledge and information but also another output of science. Primarily, humanity has tried to survive and right after saving their lives, they have tried to improve their life standards (Prutkin and Feinstein, 2002; Pennacchini et al., 2011). Day by day, their knowledge has grown accumulatively. However, before modern science this knowledge has not been found effective as much as today’s data. Integral forms of knowledge and technology have constituted the normative idea so that modern science has revealed and shaped as it is nowadays (Szymańska, 2018).
Science should be reckoned with all disciplines as an initiation of technology. By this way, today, to examine the nature from the observable universe scale to subatomic levels is the level that science reaches. Every science field has a different aspect; however science branches should work cooperatively to achieve a success such as cooperation between medicine and chemistry, pharmacology and chemistry or biology, engineering and medicine, agriculture and biology, chemistry or engineering (Looi, 2016; Copeland and Boriack-Sjodin, 2018; Gamble, 2018; Manoukian et al., 2018; Singh et al., 2018). To exemplify that, imaging and sensing systems can help to treat diseases more easily, cancer cells can be visible with microscopic techniques, environmental pollutants can be detected for sustainable life cycle or drug delivery systems can be formed by chemical advancements (Celotta, 1988; Pindelska et al., 2017; Bruno, 2018; Galvão et al., 2018). When these kinds of collaborations are considered as a new gate of science, it opens new waves of discoveries. Especially for few decades ago, researches at multidisciplinary level have gained more and more importance (Lin et al., 2014; Dahmash and Mohammed, 2016; Mitić et al., 2017).
During technological developments which increase life quality, environmental pollutions can be ignored intentionally or unintentionally not only by industrial producers but also beneficiaries. On the other hand, some of these pollutants can be used in the name of good intentions. For instance, using pesticides against to insects can
have a successful effect but in another situation it might be harmful for natural life cycle. From another point of view, after an industrial process some wastes can damage any kinds of water sources which threats millions of organisms. For these reasons, many governments and organisations have restrictions or rules for waste management. According to regulations such as United States Environmental Protection Agency (EPA) or United States Food and Drug Administration (FDA), wastes should be followed during or after processes and even if it is necessary a treatment for pollutants should be applied (Parolini et al., 2013; Bever et al., 2018). For this reason, several analytical methods have been developed as high performance liquid chromatography (Liu and Wu, 2012; Kim et al., 2013), gas chromatography (Jachero et al., 2013; Pintado-Herrera et al., 2014), capillary electrophoresis (Wang et al., 2013; Zhang et al., 2014), spectrophotometry (Atar et al., 2015; Carlson et al., 2015), electrochemistry (Beibei et al., 2014; Moyo et al., 2015; Yola et al., 2015), etc. Each method has its own advantages and disadvantages like lower cost, higher repeatability, higher reproducibility, easier pretreatments and so on. However, among them, electrochemical techniques are more advantageous because of their sensitivity, applications for in-situ analysis, portable systems in various fields, etc. (Moyo et al., 2015; Marques et al., 2017; Wu et al., 2017; Zheng et al., 2018).
Triclosan, (5-chloro-2-(2,4-dichlorophenoxy)phenol), is a chemical compound which is widely used as an ingredient in medical disinfectants, cleaning, personal care and some antibacterial products due to its antibacterial and antifungal effects (Lim et al., 2012; Peverly et al., 2014; Montaseri and Forbes, 2016). EPA has classified triclosan as pesticide due to its usage as antimicrobial pesticide. In addition, both American and European governments restrict to use triclosan in products up to 0.3% (Commission, 2013; Register, 2017) since it could be dangerous for sustainability of different kinds of lives (Fotouhi et al., 2010; Tohidi and Cai, 2017; Bever et al., 2018).
Triclosan is slightly soluble in water, but in alkaline water the solubility can be higher. It is possible to detect triclosan in any source of water because of its widespread usage (Ghanem et al., 2007; Liu et al., 2009; Huang et al., 2016; Wang et al., 2017). Accurate determination of triclosan is important for the determination of water quality due to its harmful effects such as testosterone and estrogen levels in placenta during pregnancy or neurodevelopmental disorders on newborns, allergic, weak androgenic, toxic for endocrine system and aquatic bacteria, etc. (Tsung Hsuan Tsai, 2011; Beibei et al., 2014; Moyo et al., 2015; Bo Kyung et al., 2016; Marques et al., 2017). Especially
due to widespread usage of triclosan, e.g. in toothpastes, liquid soaps, wound cleansers and body washes, it can directly enter the metabolism. With that it may change the hormone homeostasis and on some sensitive skins it may cause allergic reactions (Allmyr et al., 2006; Vidal et al., 2008). Other than, human body absorbs triclosan from mucosa or directly from the skin (Huang et al., 2016). Under ultraviolet light, triclosan can be degraded into dioxins or other phenolic compounds such as; 2,8-dichlorobenzo-p-dioxin, methyl triclosan, 2,4-dichlorophenol, 2,4,6-trichlorophenol (Montaseri and Forbes, 2016; Tohidi and Cai, 2017; Wu et al., 2017), which can enter underwater and mix with clean sources and affect the ecosystem. Thereby, the degradation products of triclosan have higher contaminative effects for environment than triclosan.
Several studies about triclosan have been performed for the determination of its concentration in any kinds of water sources. For this, carbon composites with several metal oxides such as nano-zinc oxide-functionalized multi-walled carbon nanotube on the glassy carbon electrode surface (Moyo et al., 2015), gold nanoparticle-functionalized polyoxometalates/reduced graphene oxide on the glassy carbon electrode surface (Yola et al., 2015), palladium nanoparticle, polypyrrole and iron oxide nanoparticle-modified carbon nanotube (Zheng et al., 2018), ultra thin films of poly(diallyldimethylammonium chloride) and carbon nanoparticles on the indium oxide substrate (Amiri et al., 2007), cellulosic nanocomposite poly(diallyldimethylammonium chloride) as thin films (Bonné et al., 2008), electropolymerized o-phenylenediamine on the glassy carbon electrode surface (Liu et al., 2009), layer by layer polymeric film structures with graphene oxide on an interdigitated array electrode (Marques et al., 2017), functionalized graphene nanostructures on the glassy carbon electrode surface (Beibei et al., 2014), multi-walled carbon nanotube films on the glassy carbon electrode surface (Yang et al., 2009), carbon nanodots on the glassy carbon electrode surface (Dai et al., 2012), carbon nanohorn structures with alkoxy silane hybrid films on the glassy carbon electrode surface (Dai et al., 2013), polyvinyl chloride-supported screen printed carbon electrode (Pemberton and Hart, 1999), tosyl-functionalized carbon nanoparticles on the glassy carbon or graphite electrodes (Vidal et al., 2008) have been applied for the electrochemical determination of triclosan. Especially, several studies have been reported for electrochemical determination of triclosan with the glassy carbon electrode (Pemberton and Hart, 1999; Liu et al., 2009; Dai et al., 2012; Yola et al., 2015; Wu et al., 2017). However, no application based on the electrochemical formation of gold nanostructures on the glassy carbon electrode surface for the determination of triclosan
has been reported yet. On the other hand, modification of surfaces by electrodeposition of gold have been applied for determination of other substances, such as gold structures for glucose sensing, pyramidal dendritic gold nanoparticles for azathioprine determination, dendritic gold nanostructures on beta-lactoglobulin-modified graphene oxide for glucose determination and hierarchical gold nanostructures for vorinostat determination (Shu et al., 2014; Du et al., 2015; Vais et al., 2015; Vais et al., 2018).
In this thesis, a facile electrodeposition method was applied for the formation of gold nanostructures on the glassy carbon electrode surface. The modification procedure included the reduction of Au3+ ions of HAuCl4 in the absence or presence of various concentrations of multi-walled carbon nanotube (MWCNT). By this way, various modified surfaces were prepared. All of these surfaces were characterized with electrochemical, microscopic and spectroscopic techniques. According to characterization results, some of these modified surfaces based on formation of the dendritic gold nanostructures were evaluated for the determination of triclosan for the first time.
2. LITERATURE REVIEW
Amiri et al. (2007) have applied a layer by layer deposition process at tin-doped indium oxide substrates for the formation of film composed of carbon nanoparticles and poly(diallyldimethylammonium chloride). Due to the positive binding sites of this film in aqueous phosphate buffer, pH 9.5, the surfaces have shown a binding ability for anionic systems. Because of this, triclosan, a negatively charged chlorinated poly-aromatic phenol, has been investigated at the carbon nanoparticle and poly(diallyldimethylammonium chloride)-modified electrode with that expected for simple electrostatic interaction of cationic binding sites. Oxidation of triclosan has been detected at 0.6 V vs. SCE as irreversible reaction. The regeneration of modified electrode could be possible with organic solvent. The linear range for triclosan oxidation has been reported between 0.5-50 µM concentration ranges.
Dai et al. (2013) have presented a simple and sensitive electrochemical sensor prepared with alkoxy silane and carbon nanohorns for the determination of malachite green, sudans, triclosan and bisphenol A. It has been reported that easily controlled composition of an organic–inorganic hybrid film was achieved because of the application of carbon nanohorns with its large surface area, good conductivity, admirable electrochemical activity and alkoxy silane with its excellent immobility. The kinetic parameters of modified electrode have been reported for triclosan and malachite green and respective protocols have been established. The proposed sensor has presented lower detection limit (1.2 nM), higher sensitivity and more broad linear range (1-1670 µM) for triclosan in comparison with previously published systems.
Du et al. (2015) have synthesized dendritic gold nanostructures by one step electrodeposition on reduced graphene oxide-functionalized by β-lactoglobulin which provided a superb platform for the growth of gold nanostructures because of its sulfhydryl groups. The modified structure has been characterized by scanning electron microscopy, Raman spectroscopy and X-ray spectroscopy. Electrocatalytic ability of nanocomposite has been investigated by cyclic voltammetry and chronoamperometry. The electrochemical determination of glucose has been presented as an application for modified glassy carbon electrode. The linear range of 0.05-6.00 mM, the detection limit of 22.9 µM and a rapid response time within 6 s has been reported. The prepared biosensor has been used for the determination of glucose in human serum.
Fotouhi et al. (2010) have developed an analytical method for the effect of surfactant on the voltammetric behavior, optimization and validation of parameters for the determination of trace amounts of triclosan. The determination process has been based on increasing the oxidation peak current of triclosan in the presence of sodium dodecyl sulphate with polished and rinsed glassy carbon rod electrode. Two different linear ranges have been reported as 10-600 µg/L and 1-8 mg/L. The detection limit has been calculated as 5.0 µg/L with a relative standard deviation lower than 4.5%. The real sample application has been performed for the analysis of waste water and personal care products.
Beibei et al. (2014) have functionalized β-cyclodextrin (β-CD) with graphene nano platelets (GNP) and constructed a new electrode by coating the glassy carbon (GC) electrode surface with β-CD/GNP for the determination of triclosan. The modified surface has been characterized by scanning electron microscopy and electrochemical impedance spectroscopy. It has been reported that the oxidation peak current of triclosan at the β-CD/GNP-modified GC electrode was three times higher than the GNP-modified GC electrode and seven times higher than the bare GC electrode. The detection limit has been calculated as 0.6 mM in the linear range of 2.0-100 mM for triclosan by applying differential pulse voltammetry. Higher sensitivity, wider dynamic response range and better stability towards the determination of triclosan have been also reported that would be promising for the environmental monitoring of triclosan in future.
Libansky et al. (2014) have reported the preparation of composite electrodes, combination of graphite, glassy carbon conductive microparticles and several types of polymers as non-conductive binders, for the determination of triclosan as the environmental pollutant. Among several polymers, polystyrene and polycarbonate have been selected for the combinations with graphitic carbon particles. 0.49 µmol/L and 0.25 µmol/L were calculated as detection limits for polystyrene and polycarbonate, respectively. Applications for the determination of triclosan in river water and toothpaste have been also reported.
Liu et al. (2009) have developed an electrochemical triclosan sensor based on electropolymerization of o-phenylenediamine (o-PD). Electrode preparation process has been performed at the glassy carbon electrode surface in the presence of triclosan. It has been reported that the template was renewable by NaOH(aq). After 15 minutes incubation in acetate buffer solution, pH 5.2, the detection limit (8.0×10−8 mol/L) and
linear range (from 2.0×10−7 to 3.0×10−6 mol/L) have been calculated. Furthermore, it has been also reported that elimination of other interferences, which had similar structure with triclosan, was not difficult.
Marques et al. (2017) have developed sensing systems consisting of interdigitated electrodes covered with different layer by layer films for the determination of picomolar concentration of triclosan in aqueous medium. Poly(ethyleneimine), graphene oxide, chitosan, poly[1-[4-(3-carboxy-4-hydroxyphenylazo) benzene sulfonamido]-1,2-ethanediyl, sodium salt] and poly (allylamine hydrochloride) have been used for the preparation of layer by layer films. The results have shown that the sensor could be applied for the determination of triclosan in aqueous solutions within 10−12-10−6 M concentration ranges. In addition to this, the results have been also promising that the methodology applied in this work could be used to detect triclosan in complex matrix solutions.
Moyo et al. (2015) have prepared a sensor for the electrochemical determination of triclosan by the modification of glassy carbon (GC) electrode surface with nanozinc oxide-multi-walled carbon nanotube (nZnO–MWCNT) composites. Cyclic voltammetry, differential pulse voltammetry, chronoamperometry and square wave voltammetry have been applied in the cell filled up with phosphate buffer solution, pH 7.0. It has been reported that modified GC electrode exhibited electrocatalytic behavior towards oxidation of triclosan which presented higher oxidation peak currents and lower oxidation peak potentials when compared to the bare GC electrode. An excellent response has been recorded for triclosan in the linear range from 1.5 µg/L to 2.0 mg/L with the detection limit as 1.3 µg/L and coefficient of determination (R2) as 0.9931. The diffusion coefficient of triclosan has been determined as 1.65×10−6 cm2/s. The electrochemical sensor has shown satisfactory stability, selectivity and reproducibility when it was stored at room temperature.
Pemberton and Hart (1999) have studied the electrochemical behaviour of triclosan at the screen-printed carbon electrode. A single anodic peak (+0.45 V vs. SCE), which was attributed to irreversible oxidation at the phenolic moiety, has been observed at pH values over 6.0. pKa value as 7.9 has been calculated from a graph of Ep vs. pH. The electrochemical behaviour of triclosan has been studied in diethanolamine buffer solution, pH 10.0, where the analyte existed as an anion which underwent electrosorption prior to electrochemical oxidation. The oxidation has had one-electron transfer at initial potential of 0 V. However, opposite to triclosan, triclosan
monophosphate has found to be electrochemically inactive when subjected to voltammetry under stated conditions. The electrochemical oxidation of triclosan at the screen-printed carbon electrode has been applied for its determination in commercial toothpaste and mouthrinse products using differential pulse voltammetry.
Peverly et al. (2014) have studied the direct reduction of methyl triclosan at the glassy carbon electrode in dimethylformamide containing 0.050 M tetramethylammonium tetrafluoroborate by cyclic voltammetry and controlled potential (bulk) electrolysis vs. the cadmium-saturated mercury amalgam reference electrode. In cyclic voltamograms three irreversible cathodic peaks at 1.72, 2.07 and 2.35 V attributed to scission of the ortho C–Cl bond, cleavage of two para C–Cl bonds and rupture of the C–O bond have been recorded, respectively. Controlled potential (bulk) electrolysis of methyl triclosan at carbon electrodes has resulted with the formation of mixtures of 4-chloro-1-(4-chlorophenoxy)-2-methoxybenzene, 4-chloro-2-methoxy-1-phenoxybenzene, 1-(4-chlorophenoxy)-2-methoxybenzene, 1-methoxy-2-phenoxybenzene, anisole and phenol.
Safavi et al. (2003) have developed a voltammetric method for the determination of triclosan. Tensammetric peak demonstrated adsorption and desorption potentials of triclosan between −1190 and −990 mV vs. Ag/AgCl/KClsat, depending on pH, scan rate, accumulation potential, accumulation time and presence of organic materials have been observed at differential pulse voltammograms. The increased peak current for tensammetric peak has been reported with the linear increase of the scan rate (80-500 mV/s) and also as the proportional to the concentration of triclosan over the range of 2.5–60 µg/L. pH 7.0 as the pH value, -450 mV as the accumulation potential and 90 s as the accumulation time have been determined as optimal conditions. Under these conditions, the detection limit has been calculated as 1.9 µg/L with relative standard deviation 3%. The real sample application has been performed for the determination of triclosan in toothpaste and wastewater.
Shu et al. (2014) have modified the glassy carbon electrode surface with dendrite-like gold nanostructures via potentiostatic method without any templates, surfactants or stabilizers. The effects of deposition time, potential and concentration of precursor solution on the formation of nanostructure and electrocatalytic activity of dendrite-like gold nanostructures have been investigated using electrochemical and microscopic techniques. The performance of modified surface fabricated as a non-enzymatic glucose sensor has been reported as good with its electrocatalytic
activity towards the electrooxidation of glucose in a neutral phosphate buffer solution. The system has shown a quick response (less than 2 s), a low detection limit (0.05 mM), a wide linear range (0.1–25 mM), good repeatability and stability.
Vais et al. (2015) have modified gold surfaces with gold hierarchical dendrites consisting of an array of parallel-arranged pyramidal nanoparticles in the presence of lysine. Potentiostatic electrodeposition method has been employed to synthesize the gold nanostructure and to prepare modified surfaces. The surfaces have been cleaned as in similar studies however differently from the most of them; surfaces have been also electrochemically treated with 500 mmol/L H2SO4 solution just before modification step. The surface has been modified in the cell containing 20 mmol/L HAuCl4, 0.5 mol/L H2SO4 and 150 mmol/L lysine at the potential of 0.0 mV for 600 s. The surfaces have been characterized by scanning electron microscopy and used for the determination of azathioprine in phosphate buffer solution, pH 7.40, by differential pulse voltammetry. The linear range as 0.095–900 µmol/L with a sensitivity of 0.14 A L/mol cm2 and detection limit as 0.090 µmol/L have been reported. The real sample application has been also performed with differential pulse voltammetry for the analysis of azathioprine tablets.
Vais et al. (2018) have prepared new surfaces by the electrodeposition of leaf-like gold nanolayers and characterized with field emission scanning electron microscopy. Choline chloride has been used as shape directing agent. Modified electrode has been prepared by a potentiostatic electrodeposition method after pretreatment in 500 mmol/L H2SO4 solution by applying potential in range of cathodic and anodic edges of the electrolyte stability with 25 consecutive cycles. The main modifying process has been applied in the cell containing 20 mmol/L HAuCl4, 0.5 mol/L H2SO4 and 150 mmol/L choline chloride at the potential of 0.0 mV for 300 s. The electrooxidation behavior of vorinostat has been investigated at modified surface by voltammetric measurements in phosphate buffer solution, pH 7.40. An amperometric sensor designed by hierarchical leaf-like gold nanolayers for the determination of vorinostat has presented the linear range as 4.0–52 μmol/L with the calibration sensitivity of 7.7 mA L/mol. Besides, the detection limit has been calculated as 1.40 μmol/L. An amperometric method applied with hierarchical leaf-like gold nanolayers has been also evaluated for the analysis of vorinostat capsules.
Vidal et al. (2008) have studied on the electrochemical determination of benzophenone-3 (2-hydroxy-4-methoxybenzophenone) and triclosan
(5-chloro-2-(2,4-dichlorophenoxy)phenol) by an application of modified carbon nanoparticles. Analyte-free carbon nanoparticles have been immobilized at the graphite or glassy carbon electrode surfaces and directly immersed in analyte solution to bind benzophenone-3 and triclosan. Characteristic voltammetric responses as anodic for triclosan and cathodic for benzophenone-3 with a linear range of ca. 1–120 µM have been observed. The detection limit has been calculated as ca.5 µM (or 1.2 ppm) for benzophenone-3 and ca. 10 µM (or 2.3 ppm) for triclosan.
Wu et al. (2017) have synthesized poly (diallyldimethylammonium chloride)-functionalized graphene/palladium nanoparticles (PDDA-Gr/PdNPs) by a simple one pot method with the use of PDDA as stabilizing agents. The synthesized nanomaterial has been applied for the modification of the glassy carbon (GC) electrode surface. The electrochemical response of triclosan at the PDDA-Gr/PdNPs-modified GC electrode has been investigated with the comparison to the electrochemical responses of PDDA-Gr-modified or bare GC electrodes. The results have been discussed as an exhibition of nanocomposite system in the point of good electron transfer ability and catalytic activity. Under the optimal conditions, the oxidation peak current has increased linearly with the concentration of triclosan in the linear range of 9.0 nM-20.0 μM. The detection limit has been calculated as 3.5 nM (S/N=3). Additionally, electrochemical sensor has presented superior reproducibility, excellent anti-interference performance and long-term stability.
Yola et al. (2015) have developed a novel sensor for the determination of triclosan in wastewater with modified glassy carbon (GC) electrode by molecular imprinting method. For this aim, reduced graphene oxide (rGO) has been functionalized by polyoxometalate (POM) through electrostatic interaction between the POM and rGO nanosheets (POM/rGO). Gold nanoparticles (AuNPs) have been deposited on the POM/rGO surface without using any reducing agent and prepared nanomaterial (AuNPs/POM/rGO) has been applied for the modification of glassy carbon (GC) electrode surface (AuNPs/POM/rGO/GC) under infrared light. Modification of GC electrode surface has been approved with X-ray photoelectron spectroscopy, reflection-absorption infrared spectroscopy, scanning electron microscopy and transmission electron microscopy. Triclosan imprinted film has been generated on the AuNPs/POM/rGO/GC electrode surface via polymerization of phenol and triclosan. The linear range and detection limit for triclosan have been calculated as 0.5–50.0 nM and
0.15 nM, respectively. Waste and lake water treatments have been also presented with effective performance of proposed sensor.
3. MATERIALS AND METHODS
This thesis includes formation of gold nanostructures on carbon surface by one-step reduction of HAuCl4 as electrodeposition, characterization of nanostructures and application for the electrochemical determination of triclosan. The surfaces were prepared in the absence or presence of different concentrations of MWCNT towards to HAuCl4.
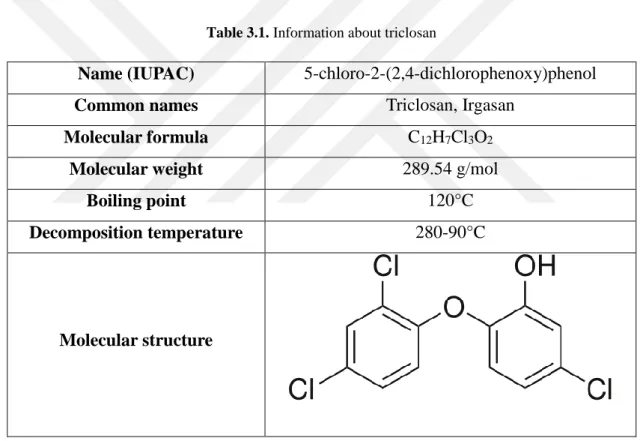
Triclosan, also known as Irgasan, is a chemical compound which has antibacterial and antifungal effects. It is also classified as a pesticide. Chemical information and specifications for triclosan are presented in Table 3.1. The hydroxyl part of molecule on an aromatic ring can be electrochemically oxidized.
Table 3.1. Information about triclosan
Name (IUPAC) 5-chloro-2-(2,4-dichlorophenoxy)phenol
Common names Triclosan, Irgasan
Molecular formula C12H7Cl3O2
Molecular weight 289.54 g/mol
Boiling point 120°C
Decomposition temperature 280-90°C
Molecular structure
3.1. Chemicals
In this study, all chemicals were used without further purification. All prepared solutions were stored in refrigerator at +4°C. Potassium chloride (KCl, Merck), sodium hydroxide pellets (NaOH, Merck), tetrabutylammonium tetrafluoroborate (C16H36BF4N, Sigma-Aldrich), ferrocene ((C5H5)2Fe, Aldrich), potassium ferrocyanide trihydrate (C6FeK4N6.3H2O, Sigma-Aldrich), potassium ferricyanide (C6FeK3N6, Sigma-Aldrich),
boric acid (H3BO3, Merck), multi-walled carbon nanotube (>8% carboxylic acid, Sigma-Aldrich), hydrogen tetrachloroaurate (III) trihydrate (HAuCl4.3H2O, Alfa Aesar), triclosan (C12H7Cl3O2, Sigma-Aldrich), hydrogen chloride (37.0% HCl(aq), Honeywell), ortho-phosphoric acid (85% H3PO4, Merck), acetic acid (96% CH3COOH, Merck), acetonitrile (≥99.9%, Sigma-Aldrich), ethyl alcohol (≥99.8%, Sigma-Aldrich) were the chemicals used during this research. Commercial buffer solutions having pH values as 4.0, 7.0 and 10.0 were also used for the calibration of pH meter. Alumina suspensions with 0.05 and 0.3 µm sizes were applied for electrode surfaces’ cleaning procedure.
3.2. Apparatus
All electrochemical measurements were carried out by using Gamry Reference 3000 Potentiostat/Galvanostat/ZRA with Gamry Instruments Framework Version 6.32. The cyclic voltammetry was performed with PHE 200 software and the data were analysed with Echem Analyst (Gamry Instruments, USA).
Glassy carbon and Pt wire electrodes were used as working and auxiliary electrodes, respectively, for all experiments. Silver/silver chloride reference electrode in 3.0 M KCl (Ag/AgCl/KClsat) and silver/silver nitrate reference electrode in acetonitrile (Ag/Ag+) were used as reference electrodes in aqueous and non-aqueous media, respectively.
VWR pHenomenal pH 1100L pH-meter was used for pH measurements. AND GR HR-250AZ Analytical Balance was used for weighing solids.
VWR circulating bath was used for keeping the temperature at 25°C during electrodeposition process.
Bandelin Sonorex sonicator was used for cleaning processes of working electrodes and also preparation of solutions.
Zeiss EVO-LS10 scanning electron microscope with LaB6 lamp under 5×10-7 atm pressure was used for taking surface images.
BRUKER X-ray spectroscopy with 1.23 eV EDX detector under 5×10-7 atm pressure was used for energy-dispersive calculations.
3.3. Preparation of Solutions
3.3.1. Preparation of solutions for electrochemical characterization process
3.3.1.1. Ferrocene solution
Ferrocene solution was used for electrochemical characterization of electrode surfaces. To prepare 1.0 mM ferrocene solution, 0.0093 g of ferrocene was weighed and dissolved in acetonitrile including 0.1 M tetrabutylammonium tetrafluoroborate in 50 mL flask. Prepared ferrocene solution was stored in refrigerator at +4°C and used at room temperature.
3.3.1.2. Potassium ferro-ferricyanide solution
Potassium ferro-ferricyanide (HCF (II)/(III)) solution was used for electrochemical characterization of electrode surfaces. To prepare 1.0 mM HCF (II)/(III) solution, 0.0211 g of potassium ferrocyanide trihydrate and 0.0165 g of potassium ferricyanide were weighed and dissolved in 0.1 M KCl in 50 mL flask. Prepared HCF (II)/(III) solution was stored in refrigerator at +4°C and used at room temperature.
3.3.2. Preparation of solutions for electrodeposition process
Solutions for electrodeposition process were prepared for the modification of the glassy carbon electrode surfaces. For this aim; different solutions in various concentrations of HAuCl4 (0, 0.04, 0.2, 0.4 or 0.8) and suspensions of MWCNT (0, 0.1, 0.5, 5.0, 20, 100 or 200 ppm) were prepared. Besides, all solutions included 0.1 M HCl and 0.1 M KCl to provide acidity and electrolytic conductivity.
Each solution was sonicated for five minutes and after then waited for thermal equilibration at 25°C in water bath.
3.3.3. Preparations of triclosan solution
10 mM stock solution of triclosan was prepared for electrochemical determination of triclosan. For that, 0.7238 g of triclosan was weighed and dissolved in ethanol in 250 mL flask. Stock solution (10 mM) was diluted between 1-100 µM concentration ranges with distilled water during electrochemical analysis. Stock solution was stored in refrigerator at +4°C and used at room temperature.
3.3.4. Preparation of Britton-Robinson buffer solution
Britton-Robinson (BR) buffer solution was used for electrochemical determination of triclosan. To prepare 0.2 M BR buffer solution, 2.472 g of H3BO3, 1.460 g of KCl were weighed; 2.29 mL of CH3COOH and 2.69 mL of 85% H3PO4 were mixed in 1.0 L flask. The pH of solution was arranged to 7.0 by addition of 0.2 M NaOH. Prepared buffer solution was stored in refrigerator at +4°C and used at room temperature.
3.4. Cleaning Procedure of Working Electrode
In an effort to acquire reliable results, all materials should be clean. Especially cleanliness of the working electrode is very important to reduce the impurity originated noise. For that, applied cleaning procedure of working electrode is expressed below:
Washing the glassy carbon (GC) electrode surface with distilled water.
Polishing the GC electrode surface with circular movements on micro cloth surface with alumina suspension.
Washing the alumina remains on electrode surface and sonicating for three minutes in distilled water.
Washing electrode surface with acetonitrile and sonicating for three minutes in acetonitrile.
3.5. Preparation of Surfaces
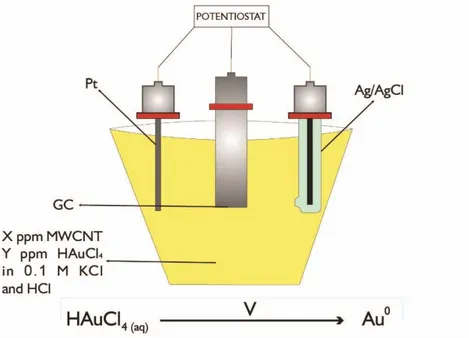
-0.3 V potential was applied to the polished glassy carbon electrode in the electrochemical cell including surface modification solutions for 90 minutes vs. Ag/AgCl/KClsat. Surface modification solutions contained X ppm (0, 0.1, 0.5, 5.0, 20, 100, or 200 ppm) MWCNT, Y ppm (0, 0.04, 0.2, 0.4 or 0.8 ppm) HAuCl4, 0.1 M HCl and 0.1 M KCl. The prepared surfaces were named as CNT-X/AuNS-Y/GC electrode surface in which MWCNT concentration (ppm) was coded as X and HAuCl4 concentration (ppm) was coded as Y. However, surfaces were not prepared with the deposition solution including neither 200 ppm MWCNT nor 0.8 ppm HAuCl4. Due to the effect of excessive concentration of MWCNT (200 ppm) in electrodeposition solution, deposition voltammogram was full of noise while due to over concentration of HAuCl4 (0.8 ppm) in electrodeposition solution, the electrochemical system overloaded. So that, both of these conditions for electrode modification process were not care about anymore.
The schematic diagram of electrochemical cell with three electrodes used for electrode surface modification process is presented in Figure 3.1.
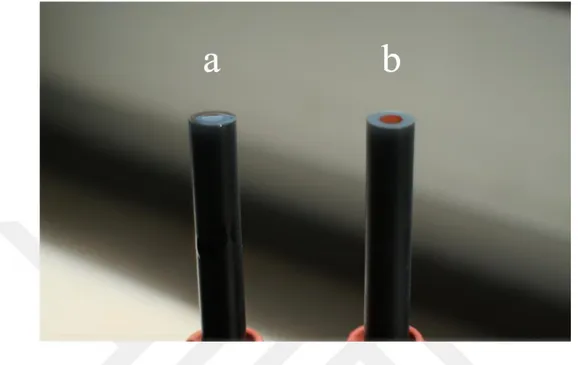
The images of the bare and modified GC electrode surfaces prepared with the presence of HAuCl4 and MWCNT in electrodeposition solution is presented in Figure 3.2.
Figure 3.2. The images of the bare (a) and modified (b) GC electrode surfaces
During the surface modification process, the temperature of modifying agent solution in water coated cell was fixed at 25°C by using circulating water bath.
3.6. Characterization of Surfaces
3.6.1. Surface characterization with electrochemical techniques
3.6.1.1. Surface characterization with cyclic voltammetry
For the electrochemical characterization of all surfaces, the redox behaviour of 1.0 mM ferrocene at the bare GC and CNT-X/AuNS-Y/GC electrodes was investigated by cyclic voltammetry between -0.2 and +0.4 V at the scan rate of 100 mV/s, vs. Ag/Ag+. Characterizations with cyclic voltammetry were carried out by Gamry Reference 3000 Potentiostat/Galvanostat/ZRA with Gamry Instruments Framework software of PHE 200 (Gamry Instruments, USA). The results were evaluated by comparing peak current changes of bare and modified electrodes.
3.6.1.2. Real surface area measurements
Real surface area studies were performed with the application of Randles-Sevcik equation due to reversibility of electrochemical process (Du et al., 2014; Moyo et al., 2015).
Ip=2.69 x 105AC0 n3/2D1/2v1/2 (3.1)
In equation (3.1); A reveals the electroactive real surface area, C0 is the
concentration of the oxidized molecules, n is the number of transferred electron, D signifies the diffusion coefficient, Ip means peak current and v stands for the scan rate.
By calculating A, electroactive surface areas of bare and modified electrodes were examined.
3.6.2. Surface characterization with scanning electron microscope
In order to have morphological characteristics of prepared surfaces, scanning electron microscope (SEM) was used (Zeiss, Germany). Images were obtained under 5×10-7 atm pressure with LaB6 lamp. Due to reflections of the bare GC electrode surface for SEM analysis, only the bare GC electrode surface was coated with gold. SEM results were evaluated by comparing gold and gold-MWCNT structures on electrode surfaces.
3.6.3. Surface characterization with energy-dispersive X-ray spectroscopy
In order to see elemental compositions, energy-dispersive X-ray spectroscopy (EDX) was used (Bruker, USA). EDX spectrograms were obtained with 1.23 eV EDX detector. Obtained spectrums were evaluated in the point of gold-carbon ratios for prepared electrodes.
3.7. Electrochemical Determination of Triclosan
All experiments based on electrochemical determination of triclosan were carried out at room temperature by cyclic voltammetry using Gamry Reference 3000 Potentiostat/Galvanostat/ZRA with Gamry Instruments Framework software of PHE 200 (Gamry Instruments, USA). The cyclic voltammograms were recorded between 0.6 and 1.1 V potentials at the scan rate of 100 mV/s vs. Ag/AgCl/KClsat in BR buffer solution, pH 7.0 (Safavi et al., 2003; Libansky et al., 2014; Wu et al., 2017). Each solution was deaerated with high-purity nitrogen gas before each voltammetric scan. 10 mM stock solution of triclosan was diluted with distilled water to obtain a solution which contains different concentrations of triclosan from 0 to 100 µM. Electrochemical results for triclosan determination were evaluated by comparing electrochemical oxidation capacity of triclosan at different electrodes.
4. RESULTS AND DISCUSSION
In this thesis, gold nanostructures were electrodeposited onto the GC electrode surface in the absence or presence of different concentrations of MWCNT towards HAuCl4. Bare and modified surfaces were characterized by electrochemical, microscopic and spectroscopic techniques. Subsequently, the results were evaluated for the electrochemical determination of triclosan. Optimal conditions applied for the determination of triclosan were followed as mentioned in Safavi, Libansky and Wu’s studies (Safavi et al., 2003; Libansky et al., 2014; Wu et al., 2017).
4.1. Electrochemical Characterization of Surfaces
4.1.1. Electrochemical characterization with ferrocene
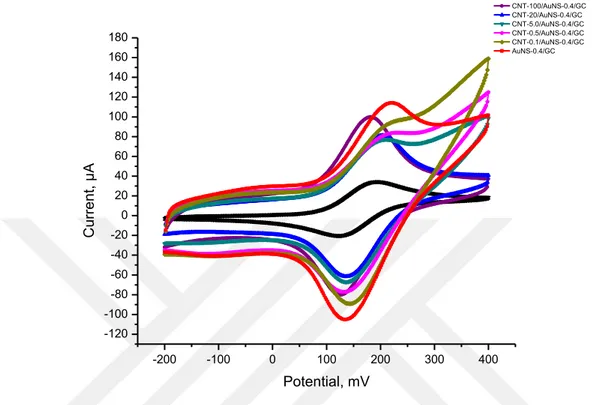
Electrochemical redox capacities of surfaces were investigated by cyclic voltammetry in electrochemical cell including 1.0 mM ferrocene solution through the recommendations of Gritzner and Kuta (Gritzner and Kůta, 1984; Wilburn et al., 2004). Figure 4.1 shows the comparisons of cyclic voltammograms of 1.0 mM ferrocene solution at the bare GC (Figure 4.1; black curve), AuNS-0.4/GC (Figure 4.1; red curve), CNT-0.1/AuNS-0.4/GC (Figure 4.1; dark yellow curve), CNT-0.5/AuNS-0.4/GC (Figure 4.1; magenta curve), CNT-5.0/AuNS-0.4/GC (Figure 4.1; dark cyan curve), CNT-20/AuNS-0.4/GC (Figure 4.1; blue curve) and CNT-100/AuNS-0.4/GC (Figure 4.1; purple curve) electrodes as the means of three measurements. Due to surface areas and conductivities of nanostructures, the electrochemical redox responses of ferrocene at modified electrodes are higher than at the bare GC (Dai et al., 2013). Among all these surfaces, AuNS-0.4/GC electrode has higher peak current (114 µA) than other surfaces with lower standard deviation, 7.10. Besides, CNT-20/AuNS-0.4/GC electrode is another one that has also reversible peak with low standard deviation, 6.61, of which has oxidation peak at 81.3 µA. On the other hand, CNT-100/AuNS-0.4/GC electrode has the second highest peak current (89.9 µA) with higher standard deviation, 12.83. In addition to 100/AuNS-0.4/GC electrode, CNT-5.0/AuNS-0.4/GC and CNT-0.5/AuNS-0.4/GC electrodes have irreversible peaks with high standard deviations. Furthermore, CNT-0.1/AuNS0.4/GC electrode has not a significant oxidation peak so that it can be accepted as irreversible peak. For this reason
four of them were not considered suitable for the determination of triclosan. As a result of reversible peaks with higher current but lower standard deviation, CNT-20/AuNS-0.4/GC and AuNS-CNT-20/AuNS-0.4/GC electrodes were chosen for further characterizations.
-200 -100 0 100 200 300 400 -120 -100 -80 -60 -40 -20 0 20 40 60 80 100 120 140 160 180 C u rre n t, µ A Potential, mV Bare GC CNT-100/AuNS-0.4/GC CNT-20/AuNS-0.4/GC CNT-5.0/AuNS-0.4/GC CNT-0.5/AuNS-0.4/GC CNT-0.1/AuNS-0.4/GC AuNS-0.4/GC
Figure 4.1. Cyclic voltammograms of 1.0 mM ferrocene at the bare GC and CNT-X/AuNS-Y/GC (X: 0,
0.1, 0.5, 5.0, 20 or 100; Y: 0.4) electrodes at 100 mV/s vs. Ag/Ag+
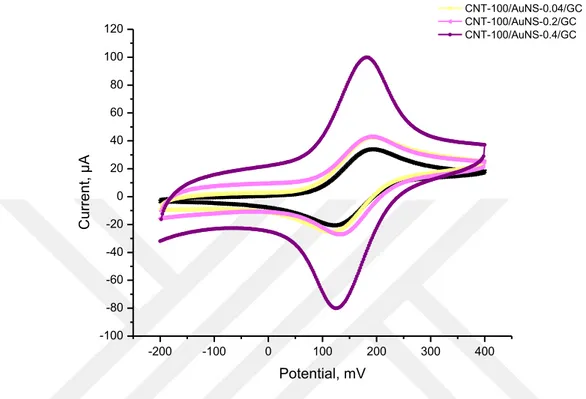
Figure 4.2 shows the comparisons of cyclic voltammograms of 1.0 mM ferrocene solution at the bare GC (Figure 4.2; black curve), CNT-100/AuNS-0.04/GC (Figure 4.2; light yellow curve), CNT-100/AuNS-0.2/GC (Figure 4.2; light magenta curve) and CNT-100/AuNS-0.4/GC (Figure 4.2; purple curve) electrodes as the means of three measurements. This comparison shows modified electrodes prepared in the presence of different concentrations of HAuCl4 (0.04, 0.2 or 0.4 ppm) and constant concentration of MWCNT (100 ppm). Each electrode has redox peaks of ferrocene. However, the peak currents of electrodes modified with less than 0.4 ppm of HAuCl4 was closer to the bare GC electrode. The reason of having higher peak current of the CNT-100/AuNS-0.4/GC electrode than other modified electrodes (CNT-100/AuNS-0.2/GC and CNT-100/AuNS-0.04/GC), is the gold concentration in electrodeposition solution. This situation has been also mentioned in the literatures that concentration of HAuCl4 has a significant role on the morphologies of formed gold nanostructures (Nguyen et al., 2015). Besides, CNT-100/AuNS-0.4/GC electrode has redox peaks at
184 mV and 126 mV, which indicates the difference between the peak potentials as 58 mV in harmony with one electron transfer in Nernst equation. This also shows a fast occurring electron transfer mechanism (Du et al., 2015; Moyo et al., 2015).
-200 -100 0 100 200 300 400 -100 -80 -60 -40 -20 0 20 40 60 80 100 120 C u rre n t, µ A Potential, mV Bare GC CNT-100/AuNS-0.04/GC CNT-100/AuNS-0.2/GC CNT-100/AuNS-0.4/GC
Figure 4.2. Cyclic voltammograms of 1.0 mM ferrocene at the bare GC and CNT-X/AuNS-Y/GC (X:
100; Y: 0.04, 0.2, 0.4) electrodes at 100 mV/s vs. Ag/Ag+
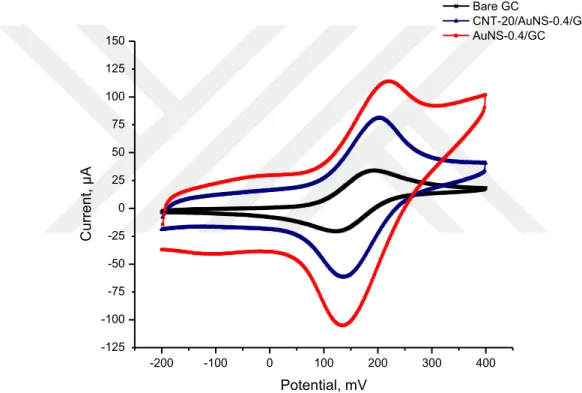
Figure 4.3 shows the cyclic voltammograms of 1.0 mM ferrocene at the CNT-20/AuNS-0.4/GC and AuNS-0.4/GC electrodes for comparison at the same conditions within Figures 4.1 and 4.2. Among these electrodes given in Figures 4.1 and 4.2 the CNT-20/AuNS-0.4/GC and AuNS-0.4/GC electrodes have not only higher peak currents but also lower standard deviations than the bare GC electrode. Due to these voltammetric characterization results, it is thought that CNT-20/AuNS-0.4/GC and AuNS-0.4/GC electrodes can be considered more homogenous as much as the bare GC electrode than other modified electrodes.
Anodic peak currents of the CNT-20/AuNS-0.4/GC and AuNS-0.4/GC electrodes are 81.3 µA and 114.0 µA, respectively. This shows oxidation capacity of AuNS-0.4/GC electrode is 1.4 times higher than CNT-20/AuNS-0.4/GC electrode. The difference between these electrodes is the presence of MWCNT in electrodeposition solution. Therefore, it can be thought that MWCNT existence in the structure is decreasing the oxidation capacity of the electrode (Wu et al., 2012).
The aim of this thesis is to choose the best electrode/electrodes for the electrochemical determination of triclosan. Triclosan has a hydroxyl group on its aromatic ring, so it is clear that triclosan can be determined by the oxidation of this hydroxyl group in its structure. From these electrochemical characterization results recorded by the oxidation of ferrocene at the bare and modified GC electrodes (Figures 4.1, 4.2 and 4.3), ferrocene is oxidized at the CNT-20/AuNS-0.4/GC and AuNS-0.4/GC electrodes better than others. Thus, in this situation as a prediction the CNT-20/AuNS-0.4/GC and AuNS-CNT-20/AuNS-0.4/GC electrodes are suitable for electrochemical determination of triclosan (Wu et al., 2017).
-200 -100 0 100 200 300 400 -125 -100 -75 -50 -25 0 25 50 75 100 125 150 C u rre n t, µ A Potential, mV Bare GC CNT-20/AuNS-0.4/GC AuNS-0.4/GC
Figure 4.3. Cyclic voltammograms of 1.0 mM ferrocene at the bare GC, CNT-20/AuNS-0.4/GC and
AuNS-0.4/GC electrodes at 100 mV/s vs. Ag/Ag+
4.1.2. Real surface area calculations
Real surface area calculations can be used to estimate electroactive areas on modified surfaces. Real surface area studies were carried out by cyclic voltammetry in 1.0 mM HCF (II)/(III). The calculations were computed according to Randles-Sevcik equation (Equation 4.1);
In Equation 4.1, Ip stands for the peak current of redox reaction which are 29.9,
116.0 and 102.8 µA for the bare GC, CNT-20/AuNS-0.4/GC and AuNS-0.4/GC electrodes, respectively. C0 is the concentration of HCF (II)/(III) solution which is the
probe solution, 1.0 mM. n shows the transferred electron number which is 1, D symbolises the diffusion coefficient of HCF (II)/(III) which is 6.70×10-6cm2/s and v indicates the scan rate which is 100 mV/s. Due to these presented data, calculated A, the real surface areas are presented in Table 4.1.
As it is seen from Table 4.1 that electroactive surface areas of CNT-20/AuNS-0.4/GC and AuNS-CNT-20/AuNS-0.4/GC electrodes are 3.8 and 3.4 times higher than of the bare GC electrode, respectively. This shows us that with modification of the GC electrode surface, the electroactive surface area increases due to the application of nanostructures to the bare GC surface. On the other hand, the electroactive surface area of the CNT-20/AuNS-0.4/GC is 1.1 times higher than the AuNS-0.4/GC electrode surface which can be accepted as similar. The results of real surface area calculations are parallel with scanning electron microscope results which are stated in Section 4.2.
Table 4.1. Real surface area calculations of the bare GC, CNT-20/AuNS-0.4/GC and AuNS-0.4/GC
electrodes in 1.0 mM HCF (II)/(III)
Electrode Real Surface Area (cm2)
Bare GC 0.0043
CNT-20/AuNS-0.4/GC 0.0167
AuNS-0.4/GC 0.0148
4.2. Microscopic Characterization of Electrode Surfaces with Scanning Electron Microscope (SEM)
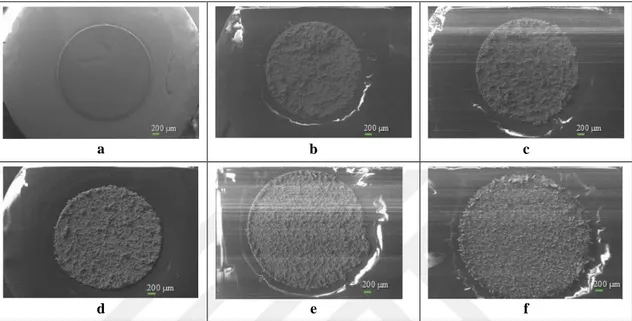
Scanning electron microscope (SEM) is a technique which allows to reach high magnifications to examine surface morphologies of materials by reflection of electrons. From the point of this view, the surface morphologies of the bare and modified electrodes were investigated by using SEM. The bare GC electrode surface was coated with gold for its comparison with modified electrode surfaces. The other surfaces were analysed without coating due to their modification with gold. Figure 4.4 shows SEM images of the bare GC (Figure 4.4a) and modified electrode surfaces,
CNT-100/AuNS-0.4/GC, CNT-20/AuNS-CNT-100/AuNS-0.4/GC, CNT-0.5/AuNS-CNT-100/AuNS-0.4/GC, CNT-0.1/AuNS-0.4/GC and AuNS-0.4/GC (Figure 4.4b-f) surfaces. While MWCNT:HAuCl4 ratio (from 250 to 0) is being decreased in electrodeposition solutions (b-f), homogeneousness of structural shapes are enhancing (Nguyen et al., 2015). Elaborated SEM images of images presented in Figure 4.4 are given in Figures 4.5-4.10.
a b c
d e f
Figure 4.4. SEM images of the bare GC (a), CNT-100/AuNS-0.4/GC (b), CNT-20/AuNS-0.4/GC (c),
CNT-0.5/AuNS-0.4/GC (d), CNT-0.1/AuNS-0.4/GC (e) and AuNS-0.4/GC (f) electrode surfaceson 50 X magnification under 5×10-7 atm pressure
Figure 4.5 shows the SEM images taken on different magnifications of the bare GC electrode surface. As it can be seen in Figure 4.5, the bare GC electrode has a homogenous and smooth surface on all magnifications. This feature of glassy carbon electrode allows to modify and compare with other surfaces easily (Pifferi et al., 2013).
a b
c d
Figure 4.5. SEM images of the bare GC electrode surface on 1K X (a), 5K X (b), 10K X (c) and 30K X
(d) magnificationsunder 5×10-7 atm pressure
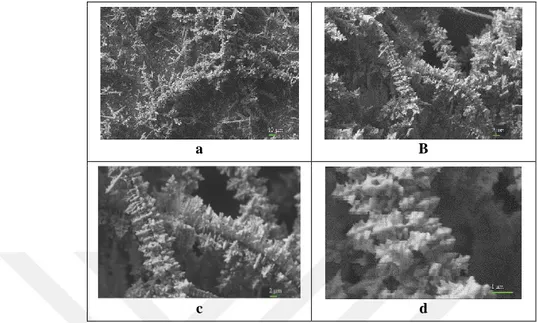
Figure 4.6 shows the SEM images taken on different magnifications of the CNT-100/AuNS-0.4/GC electrode surface. It is easy to see from Figure 4.6 that growing mechanism of gold nanostructures on the CNT-100/AuNS-0.4/GC electrode surface follows diffusion-limited aggregation due to random distributed branches with different sizes (Shu et al., 2014; Braga et al., 2015). In the presence of 100 ppm MWCNT in electrodeposition solution it is thought that inhomogeneous clusters as MWCNT-gold dendritic structures formed on the electrode surface. This thought can be easily seen from SEM images presented in Figure 4.6.
a b
c d
Figure 4.6. SEM images of the CNT-100/AuNS-0.4/GC electrode surface on 1K X (a), 5K X (b), 10K X
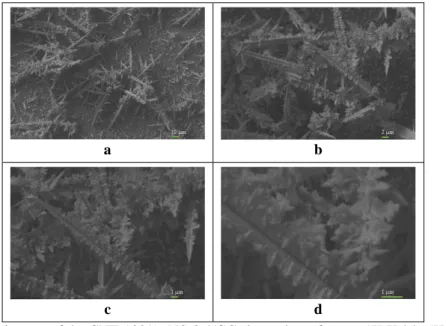
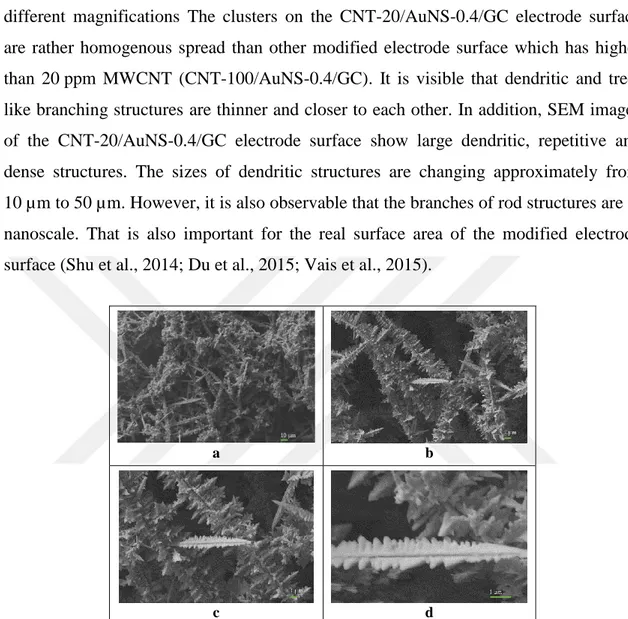
Figure 4.7 shows the SEM images of modified electrode surface prepared in the presence of 20 ppm MWCNT and 0.4 ppm HAuCl4. The SEM images were taken on different magnifications The clusters on the CNT-20/AuNS-0.4/GC electrode surface are rather homogenous spread than other modified electrode surface which has higher than 20 ppm MWCNT (CNT-100/AuNS-0.4/GC). It is visible that dendritic and tree-like branching structures are thinner and closer to each other. In addition, SEM images of the CNT-20/AuNS-0.4/GC electrode surface show large dendritic, repetitive and dense structures. The sizes of dendritic structures are changing approximately from 10 µm to 50 µm. However, it is also observable that the branches of rod structures are at nanoscale. That is also important for the real surface area of the modified electrode surface (Shu et al., 2014; Du et al., 2015; Vais et al., 2015).
a b
c d
Figure 4.7. SEM images of the CNT-20/AuNS-0.4/GC electrode surface on 1K X (a), 5K X (b), 10K X
(c) and 30K X (d) magnifications under 5×10-7 atm pressure
Figure 4.8 shows the SEM images taken on different magnifications of the CNT-0.5/AuNS-0.4/GC electrode surface. As it is shown in Figure 4.8, dendrite-like gold nanostructures on the CNT-0.5/AuNS-0.4/GC electrode surface have more deformation and smaller units than the surface prepared in the presence of 20 or 100 ppm MWCNT. SEM images of CNT-0.5/AuNS-0.4/GC shows that the distributions of gold structures have inhomogeneous patterns on the surface. It is visible that there are main wide structures together with smaller and narrow rod-like structures, so it can be told that the
growing mechanism of gold nanostructures is based on the formation of random lattice structures (Witten and Sander, 1981).
a B
c d
Figure 4.8. SEM images of the CNT-0.5/AuNS-0.4/GC electrode surface on 1K X (a), 5K X (b), 10K X
(c) and 30K X (d) magnifications under 5×10-7 atm pressure
Figure 4.9 shows the SEM images taken on different magnifications of the CNT-0.1/AuNS-0.4/GC electrode surface. When the morphology of the surface is examined, foam-like structures can be seen. Unlike the other electrodes, the CNT-0.1/AuNS-0.4/GC electrode surface has an isotropic growth form, due to the foam-like morphological structures (Ben-Jacob et al., 1985; Wang and Xin, 2000). By this way, it is corrected that dendritic structures are getting smaller and having more deformed shapes due to the decreasing MWCNT amount in electrodeposition solution. As a consequence, CNT-0.1/AuNS-0.4/GC has no dendritic formations.
a b
c d
Figure 4.9. SEM images of the CNT-0.1/AuNS-0.4/GC electrode surface on 1K X (a), 5K X (b),
10K X (c) and 30K X (d) magnifications under 5×10-7 atm pressure
Figure 4.10 shows the SEM images taken on different magnifications of the AuNS-0.4/GC electrode surface. In Figure 4.10, it is significantly clear that leaf-like structures on the AuNS-0.4/GC electrode surface have growth mechanism like the other dendritic structures due to diffusion-limited aggregation (Hu et al., 2010; Braga et al., 2015). SEM images of the AuNS-0.4/GC electrode surface shows that leaf-like structures are arrayed homogenously and close to each other between the sizes of 25 µm and 50 µm (Shu et al., 2014; Worrall et al., 2016).
As a summary, it can be said that from the SEM images of modified electrode surfaces given between Figures 4.5-4.10, the shapes of gold nanostructures are different due to the ratio of MWCNT in electrodeposition solution. So, it can be easily claimed that MWCNT concentration has a huge effect on the shapes and the homogeneous distribution of gold nanostructures through the surface.
a b
c d
Figure 4.10. SEM images of the AuNS-0.4/GC electrode surface on 1K X (a), 5K X (b), 10K X (c) and
30K X (d) magnifications under 5×10-7 atm pressure
Figure 4.11 shows the SEM images taken on different magnifications of the CNT-20/GC electrode surface. The CNT-20/GC electrode surface was prepared in order to examine the effect of MWCNT on the bare GC electrode surface in the absence of Au3+ ions. As it seen from Figure 4.11 that in the absence of HAuCl4 in electrodeposition solution MWCNT could not hold on the surface easily. Only few clusters of MWCNT accumulated to the surface. Thus, this surface was not used on electrochemical experiments. On the other hand, it has been reported that thin films of carbon nanotubes were not suitable for electrodeposition (Abe et al., 2005). For electrodeposition of carbon nanotubes higher potentials, co-deposition materials or surfactants have been recommended (Abe et al., 2005; Yuan et al., 2011; Ramalingam et al., 2016). Thus, SEM images of the CNT-20/GC electrode surface shows only insufficient quantities of MWCNT clusters.
a b
c d
Figure 4.11. SEM images of the CNT-20/GC electrode surface on 1K X (a), 5K X (b), 10K X (c) and
30K X (d) magnifications under 5×10-7 atm pressure
4.3. Spectroscopic Characterization of Surfaces with Energy-Dispersive X-ray (EDX) Spectroscopy
Energy-dispersive X-ray spectroscopy (EDX) is a surface technique that allows to get information about elemental composition with electron beams by exciting inner shell of an atom. Figure 4.12 shows EDX spectrums of bare and modified electrode surfaces (CNT-100/AuNS-0.4/GC, CNT-20/AuNS-0.4/GC, CNT-0.5/AuNS-0.4/GC, CNT-0.1/AuNS-0.4/GC and AuNS-0.4/GC). Typical elemental composition of carbon peaks in EDX analysis were detected at 0.265 keV (Jyothirmayee Aravind et al., 2011). As well characteristic elemental composition of gold peaks were detected approximately about 2.1 keV. Additionally, gold also had other L-shell peaks near to 10 and 12 keV (Hu et al., 2006; Anuradha et al., 2015; Newbury and Ritchie, 2015).
2 4 6 8 10 12 14 16 18 20 keV 0 20 40 60 80 100 cps/eV 1 C a 2 4 6 8 10 12 14 16 18 20 keV 0 5 10 15 20 25 30 35 cps/eV 1 2 3 4 5 6 Au Au Au Au C b 2 4 6 8 keV10 12 14 16 18 20 0 5 10 15 20 25 30 35 cps/eV 1 2 3 4 5 6 Au Au Au Au C c 2 4 6 8 10 12 14 16 18 20 keV 0 5 10 15 20 25 30 35 cps/eV 1 2 3 4 5 6 Au Au Au Au C d 2 4 6 8 10 12 14 16 18 20 keV 0 2 4 6 8 10 12 14 cps/eV 1 2 3 4 5 6 Au Au Au Au C e 2 4 6 8 10 12 14 16 18 20 keV 0 2 4 6 8 10 12 14 16 18 20 22 cps/eV 1 2 3 4 5 6 Au Au Au Au f
Figure 4.12. EDX spectrum of the bare GC (a), CNT-100/AuNS-0.4/GC (b), CNT-20/AuNS-0.4/GC (c),
CNT-0.5/AuNS-0.4/GC (d), CNT-0.1/AuNS-0.4/GC (e) and AuNS-0.4/GC (f) electrode surfaces
Table 4.2 shows the EDX spectra data of the bare GC and modified electrode surfaces. As given in Table 4.2 that carbon percentage either weight% or atom% is independent from the concentration of MWCNT in electrodeposition solution. This indicates that structures are formed mostly based on gold nanostructures. From the point of this view the MWCNT are not the main composition of modified surfaces but due to ratios in the electrodeposition solution they are changing the electrical conductivities and correspondingly effecting the shapes of gold nanostructures (Yuan et al., 2011).
Table 4.2. EDX spectra data of the bare GC, 100/AuNS-0.4/GC, 20/AuNS-0.4/GC,
CNT-0.5/AuNS-0.4/GC, CNT-0.1/AuNS-0.4/GC and AuNS-0.4/GC electrode surfaces
Electrode Surface Element Weight % Atom %
Bare GC Carbon 100 100 Gold 0 0 CNT-100/AuNS-0.4/GC Carbon 2.48 29.46 Gold 97.52 70.54 CNT-20/AuNS-0.4/GC Carbon 2.18 26.80 Gold 97.82 73.20 CNT-0.5/AuNS-0.4/GC Carbon 1.53 20.35 Gold 98.47 79.65 CNT-0.1/AuNS-0.4/GC Carbon 1.72 22.29 Gold 98.28 77.71 AuNS-0.4/GC Carbon 0 0 Gold 100 100
Energy-dispersive X-ray spectroscopy mapping technique is an elemental imagination technique that allows to visualise atomic compositions (Honold et al., 2016). Due to the results of electrochemical and microscopic characterizations, the CNT-20/AuNS-0.4/GC and AuNS-0.4/GC electrode surfaces were thought more homogeneous, from the point of this view elemental mappings were generated by using SEM-EDX technique for these modified electrode surfaces. The results indicate that carbon (remarked as blue color in Figure 4.13a; inset) and gold (remarked as red color in Figure 4.13a, b; insets) elements were distributed throughout the CNT-20/AuNS-0.4/GC electrode surface as shown in Figure 4.13a (inset). On the other hand, only gold can be seen on the AuNS-0.4/GC electrode surface as it can be easily seen Figure 4.13b (inset) (Shahid et al., 2018).
a b
Figure 4.13. SEM images of the CNT-20/AuNS/GC (a) and AuNS/GC (b) electrode surfaces. Inset:
Elemental mappings (Carbon is given blue and gold is given red)
4.4. Electrochemical Triclosan Determination
The electrochemical oxidation mechanism of triclosan is shown in Figure 4.14 (Amiri et al., 2007; Vidal et al., 2008; Moyo et al., 2015). Triclosan has an easily oxidizable hydroxyl group on an aromatic ring. This oxidation reaction follows one electron pathway. As a result of this reaction triclosan gets polymerized on oligomeric levels with radical intermediates (Bonné et al., 2008; Montaseri and Forbes, 2016). Moreover, oxidation of phenolic compounds leads to formation of phenoxy radicals, subsequently, this is resulted with passive film formations (Wang and Farrell, 2004). Due to this polymerization reaction, this oxidation mechanism can not be reversible and oligomers are strongly adsorbed on electrode surface (Liu et al., 2009).
Figure 4.14. Electrochemical oxidation mechanism of triclosan.
Electrochemical properties of the bare GC, CNT-20/0.4/GC and AuNS-0.4/GC electrodes were investigated in BR buffer solution, pH 7.0 in the absence or presence of triclosan (10 µM). These electrochemical measurements were performed by cyclic voltammetry in the potential range of 0.6-1.1 mV at the scan rate of 100 mV/s vs. Ag/AgCl/KClsat. The cyclic voltammograms recorded by this way are presented in