Biochemistry
Research Article – 39269
Özlem Dilek
Spectroscopic characterization of synthetic
heteroatom-functionalized fluorescent probes
for bioimaging
sentetik heteroatom-fonksiyonelize floresans probların biyogörüntüleme uygulamaları için
spektroskopik karakterizasyonu
Abstract: Objective: Fluorescent probes are essential tools
for monitoring biological systems in cellular environment. Several boron dipyrromethene (BODIPY) derived fluores-cent probe derivatives have been synthesized by nucleop-hilic substitution reaction and their spectroscopic proper-ties have also been determined.
Methods: Initial BODIPY dye framework was prepared from the reaction of pyrrole-2-carbaldehyde and benzal-dehyde and then readily reacted with heteroatom-based nucleophiles at 3- and 5- position of BODIPY core. The spectroscopic properties of these new molecules have been reported.
Results: Amine based nucleophiles tend to produce broad absorption bands on the spectra. Our studies indicated that heteroatom-based nucleophiles on the BODIPY core resulted in a red shift on the absorption and emission spectra.
Conclusion: The synthesized fluorophores can therefore be potentially used as imaging agents for biological labeling.
Keywords: Boron dipyrromethene (BODIPY), fluorescent
probe, spectroscopic properties
Özet: Amaç: Floresan problar biyolojik sistemleri hücresel
ortamda takip edebilmek için gerekli araçlardır. Nükleofilik sübstitüsyon reaksiyonu kullanılarak floresan prob türevleri sentezlenmiştir ve spektroskopik özellikleri incelenmiştir.
Metod: İlk BODIPY boya anayapısı benzaldehit ve pirol-2-karbaldehitden hazırlanmış ve ardından hemen BODIPY kor yapısının 3- ve 5- pozisyonlarında heteroat-om-bazlı nükleofiller ile reaksiyona girmiştir. Bu yeni moleküllerin spektroskopik özellikleri belirtilmiştir. Bulgular: Amin bazlı nükleofiller spektrada geniş absorp-siyon bantları göstermiştir. Çalışmalarımıza göre heteroat-om-bazlı nükleofiller absorpsiyon ve emisyon spectra da kırmızı kaymaya yol açmıştır.
Sonuç: Bu yüzden sentezlenmiş floroforlar potansiyel görüntüleme ajanları olarak biyolojik işaretlemede kul-lanılabilir.
Anahtar Kelimeler: Boron dipiromethen (BODIPY),
flore-san prob, spektroskopik özellikler
DOI 10.1515/tjb-2015-0026
Received December 3, 2014; accepted June 10, 2015
Introduction
Most of the synthesized fluorescent probes in the past literature usually exhibit absorption and emission bands in the ultraviolet or visible bands, which are essential for variety of applications especially in biochemistry and cell biology [1]. Small molecule organic fluorophores, boron dipyrromethene dyes (BODIPY) are extensively received attention as fluorescent probes in recent years in diverse applications due to their high quantum yields, cost-effectiveness, tunable spectral characteristics and
Corresponding author: Özlem Dilek: Istanbul Kemerburgaz
University, Faculy of Medicine, Department of Medical Biochemistry, İstanbul, Turkey, e-mail: ozlem.dilek@kemerburgaz.edu.tr
photochemical stability [1–3]. Thus, BODIPY dyes have a widely range of applications in medicinal fields and one of the most favourable applications is the ability to visualize and monitor cellular systems in living cells [4,5]. Practical applications of these organic dyes mainly require specific modification on the fluorophore core and consequently different synthetic approaches have been developed for modifying the BODIPY nucleus. BODIPYs are usually amenable for functionalization at all posi-tions on the main core such as introducing ethynyl, halo-gens, methyl or aromatic groups onto the system [6–12]. The nucleophilic substitution reaction to functionalize the BODIPY core at 3- and 5- position is an easy synthe-tic approach to change the spectroscopic properties of the BODIPY compound [10,13–24]. According to recent literature, very few amine or heteroatom-based BODIPY compounds have been prepared and reported at 3-or 5-position on BODIPY core [10,13–15,24–27]. In addition to halogens, methyl or formyl or amine functional groups can be easily synthesized and activated to proteins or other biological systems [24,28–30]. Great efforts have been made to develop new fluorescent probes for fluore-scence imaging field in medicine. Due to its urgent need of fluorescent probes in biomedical field including with their interesting spectroscopic data, we therefore use this traditional straightforward method for the synthesis of some heteroatom-based BODIPYs at 3 and 5 positions and investigate their spectroscopic properties for further biological applications.
Material and Methods
Materials
Dry spectral grade of solvents were purchased from Acros Chimica or Aldrich. Deuterated solvents were obtained from Cambridge Isotope Laboratories. All other chemicals were commercially available.
Absorption and fluorescence analysis
Absorbance spectra were performed using HP 8453 diode array UV-vis spectrophotometer. Fluorescence studies were performed on a Spex FluoroMax-3 spectrofluorome-ter. The relative fluorescence quantum yields (φF) were determined in dilute solutions with an absorbance below 0.1 at the excitation wavelength. All solvents were dried before analysis. The slit width was adjusted to 2 nm for both excitation and emission. All spectra were recorded at 23°C. The relative quantum efficiencies of fluorescence were obtained with the following equation:
Equation 1: φFsample = φ
Fstandard × (Fsample- Fsolvent)/(Fstandard
-Fsolvent) × (η2 sample/η2 standard) × (Astandard/Asample)
where φ denotes for quantum fluorescence yield, F denotes the area under the fluorescence band, A denotes the absorbance at the excitation wavelength, and η2
denotes the refractive index of the solvent.
General Synthetic Procedures
Dry spectral grade of solvents were used for synthesis and spectral analysis. All moisture and air sensitive reactions were carried out under nitrogen or argon atmosphere using oven dried glassware. 1H, 13C, 11B NMR spectra were
recorded on instruments operating at a frequency of 360 MHz. 1H and 13C NMR spectra were referenced to CDCl
3 (7.26
ppm or 77.00 ppm). 11B NMR spectra were referenced to BF 3.
OEt2 (0 ppm). Chemical shift multiplicities are reported as s= singlet, t= triplet, q= quartet and m= multiplet. Flash column chromatography was performed using Baker alumina and silica gel 60–200 mesh or 200–400 mesh. All flash column experiments were performed using the same solvent conditions used for TLC.
Synthesis of BODIPY derivatives
Compound 1 (3,5-dichloro-3a, 4a-diaza-4,4-difluoro-8-phenyl boron dipyrromethene) as a starting material was synthesized as described before [6].
Compound 2 (3-Chloro-3a, 4a-diaza-4,4-difluoro-5-amino-8-phenyl boron dipyrromethene):
Compound 1 (25 mg, 0.07 mmol) was dissolved in dry methanol under nitrogen. Five equivalents of ammonia in methanol were added with stirring. The reaction mixture was refluxed overnight. The solvent was removed under reduced pressure. The crude compound was purified by silica gel flash column chromatography to afford the desired compound 2 as a dark solid (12 mg, 52% yield), (TLC conditions: 100% CH2Cl2); 1H NMR (CDCl 3): δ 5.91 (broad s, 2H), 6.11 (d, 2H, J = 5.12 Hz), 6.19 (d, 2H, J = 3.67 Hz), 6.36 (d, 2H, J =3.67 Hz), 6.86 (d, 2H, J = 5.12 Hz), 7.45 (m, 5H, aromatic). 13C NMR (CDCl 3): δ 112, 113, 121, 128, 128.2, 129, 129.3, 130.2, 132, 133, 133.2, 136, 161. 11B NMR (CDCl 3): δ 1.06 (t, J =30.5 Hz).
Compound 3 (3-Chloro-3a, 4a-diaza-4,4-difluoro-5-hy-droxylamino-8-phenyl boron dipyrromethene):
Compound 1 (25 mg, 0.07 mmol) was dissolved in dry acetonitrile under nitrogen. Two equivalents of hydro-xylamine (4.62 mg) and 3 equivalents of sodium hydride (5.04 mg) were added with stirring. The reaction mixture was refluxed overnight. The solvent was removed under reduced pressure. The crude compound was purified by silica gel flash column chromatography to afford the desired compound 3 as a dark solid (3 mg, 13% yield); (TLC conditions: 10:1 CH2Cl2 : MeOH); 1H NMR (CDCl 3): δ 5.82 (broad s, 2H), 6.10 (d, 2H, J = 5.13 Hz), 6.18 (d, 2H, J = 4.02 Hz), 6.36 (d, 2H, J = 4.02 Hz), 6.86 (d, 2H, J = 5.13 Hz), 7.45 (m, 5H, aromatic). 13C NMR (CDCl 3): δ 112.8, 113.2, 121, 127.9, 128.2, 128.4, 129.3, 130, 131, 133, 136, 136.3, 161. 11B NMR (CDCl3): δ 1.04 (t, J = 33.5 Hz).
Compound 4 (3, 5-Diphenol-3a, 4a-diaza-4,4-difluoro-8-phenyl boron dipyrromethene):
Compound 1 (50 mg, 0.15 mmol) was dissolved in 5 mL of absolute acetonitrile under nitrogen. 4 equiva-lents of phenol and 2 equivaequiva-lents of NaH was added with stirring. Stirring was continued at room temperature for overnight. The solvent was removed under reduced pres-sure and The crude compound was purified by alumina packed gel flash column chromatography to afford the desired compound 4 as a dark solid (10.2 mg, 15% yield); (TLC conditions: 7:3 EtOAc: Hexane), Rf = 0.23. IR (KBr):
υ 3582, 1580, 1445, 1231, 1023. 1H NMR (CDCl
3): δ 5.70 (d,
2H, J = 4.03 Hz), 6.70 (d, 2H, J = 4.03 Hz), 7.30 (m, 6H, aro-matic), 7.42 (m, 4H, aroaro-matic), 7.50 (m, 5H, aromatic). 13C
NMR (CDCl3): δ 103.1, 120.4, 120.7, 125.7, 126.5, 128.2, 129.8, 129.9, 130.1, 130.4, 146.2, 154.8, 158.8. 11B NMR (CDCl
3): δ
–0.16 (t, J =30.6 Hz).
Results and Discussion
Synthesis
Several mono- and di- substituted BODIPYs have been reported with different synthetic approaches in the lite-rature. By introducing various nucleophiles on BODIPY core, absorption and emission characteristics of BODIPY can be modulated [1]. Rohand, Boens and their co-workers reported the synthesis of initial reactant dichloro-BODIPY 1 and found the percentage yield for the compound 1 as 44% (Figure 1). In this paper, the reaction method
invol-Ab
sorb
anc
e
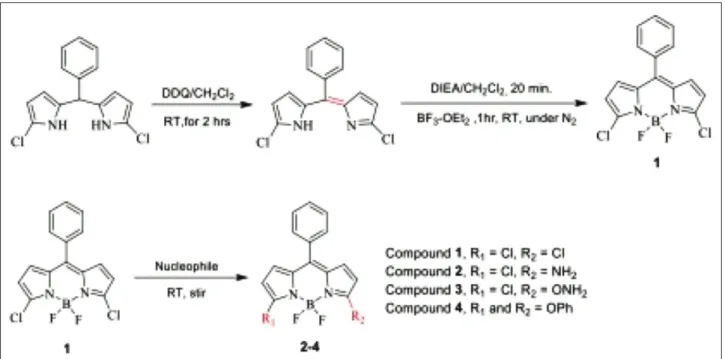
Figure 2: Absorption spectra of BDP compound 1 in ethanol.
Wavelength (nm) 0.6 0.5 0.4 0.3 0.2 0.1 0.0 600 500 400 300
ves the substitution of the chlorine groups on 3,5- posi-tion of compound 1 with amine, oxygen and sulfur based nucleophiles. Although many BODIPY compounds have been successfully investigated, their hydrazine derivati-ves displayed unusual absorption spectral features accor-ding to our previous papers [6]. In order to understand the origin of these phenomena, a few additional BODIPY com-pounds were synthesized and their spectral properties were examined. First, compound 1 is reacted slowly with ammonia in methanol under reflux overnight to obtain the compound 2. Relatively, hydroxylamine-compound 3 is also obtained upon treatment of dichloro-BODIPY 1 with hydroxylamine/sodium hydride in acetonitrile under reflux conditions. Next, the reaction of BODIPY-compound 1 with phenol/sodium hydride in acetonitrile at room temperature for overnight yields the monosubsti-tuted compound 4.
BODIPY- hydrazine compounds showed broad peaks
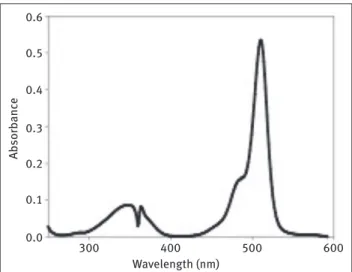
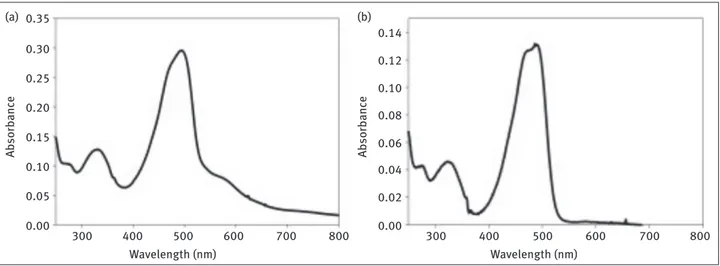
in this region of the absorption spectra [6]. The origin of this phenomenon is unclear; however, there are a few reports that indicate an amine substituent at this position may be responsible for this behavior [24–27]. For example, 3-amino-BODIPY compound displays a broad absorption spectrum in polar solvents [31]. Compounds 2 and 3 were therefore synthesized to study their spectroscopic proper-ties and compare their results with its hydrazine derivati-ves. The longest wavelength band on the absorption spec-trum of BODIPY compound 1 is typically sharp and narrow as shown in Figure 2. Compounds 2 and 3 displayed broad absorption spectra in methylene chloride (Figure 3). To explore the main reasons for having broad spectra for BODIPY-compounds 2 and 3 in detail, we synthesized 3,5-diphenoxy-BODIPY 4 as an example for disubstituted heteroatom compounds with having no amino groups at the fluorophore core (Figure 4). Although sulfur based heteroatom nucleophile (e.g. thiophenol) was also intro-duced on 3,5–position of BODIPY core, the disubstituted compound either decomposed or oxidized immediately after synthetic control experiments. Percentage yields of compound 3 and 4 were relatively lower than compound 2 due to rapid oxidation of the products during synthesis.
Ab sorb anc e Ab sorb anc e
Figure 3: Absorption spectra of BDP compounds 2 (Panel A) and 3 (Panel B) in dichloromethane.
Wavelength (nm) Wavelength (nm) (a) 0.35 (b) 0.14 800 800 700 700 600 600 500 500 400 400 300 300 0.30 0.12 0.25 0.10 0.20 0.08 0.15 0.06 0.10 0.04 0.05 0.02 0.00 0.00 Ab sorb anc e
Figure 4: Absorption spectrum of BDP compound 4 in dioxane.
Wavelength (nm) 0.12 0.10 0.08 0.06 0.04 0.02 0.00 -0.02 600 500 550 450 400
Table 1: spectral properties of BODIPY compounds 2–4.
Compound solvent λabsmax
(nm) λemmax(nm) (at 23 °C)ФF 2 Dioxane 487 522 0.096 2 Methanol 459 515 0.057 3 Dioxane 488 540 0.15 3 Methanol 469 534 0.085 4 Dioxane 518 533 0.17 4 Methanol 515 531 0.08
It should also be noted that all reactions must be perfor-med under argon or nitrogen atmosphere with oven dried glassware.
Photophysical properties
Rohand et al.,[6] indicated that the absorption maxima
of 3,5-di or mono-substituted-BODIPY’s are influenced by the electronic nature of substituents that are connec-ted to pyrrole ring. Compounds with nitrogen or oxygen directly bonded to the pyrrole ring tend to have lowest energy absorption maxima. These trends are also obser-ved for compounds 2,3 and 4. Compounds 2, 3 and 4 were characterized by absorption and fluorescence spectroscopy (Table 1). Amino-substituted compounds 2 and 3 exhibit absorption maxima at 459 and 469 nm in methanol and emission maxima at 515 and 534 nm, res-pectively. Compounds 2 and 3 in dioxane show stronger fluorescence than in methanol. The quantum yields of these compounds are listed in Table 1. Since the feature of any 3- and 5- substituent affects the spectral properties of the BODIPY, it was anticipated that amine or phenoxy based groups would change the spectral characteristics of the BODIPY. Compound 3 is the hydroxylamine analog of the compound 1. Its absorption feature is so similar to its amine derivative 2 that the spectrum is broad. Throug-hout this qualitative analysis of the absorption and emis-sion spectra, the origin of the broadness of the absorp-tion spectra of amines should be explained such as the presence of an amine nitrogen in the substituent induces broadening of the absorption spectrum, whether or not it is in direct conjugation with the BODIPY π-system. When the amine nitrogen is conjugated to the BODIPY chromo-phore, the quantum yield is therefore quite low. The long wavelength shoulder on the absorption spectrum of com-pound 2 may be due to the presence of a proton on amine nitrogen, which may engage in hydrogen bonding (inter-molecular or intra(inter-molecular with the fluorine on boron). Overlapping absorption peaks from both species also lead to the peak broadening (Figure 2). Absorption and emis-sion spectra of compound 4 are somewhat narrower than the amine substituted compounds 2 and 3.
Conclusion
In this work, new heteroatom-based BODIPY compounds 2, 3 and 4 have been synthesized by a traditional nuc-leophilic substitution reaction, which provides an easy
access to introduce multiple functional groups on BODIPY core. All compounds have been characterized using NMR spectroscopy and their spectral properties have also been studied by fluorescence spectroscopy. Amine based fluo-rophores tend to produce broader peaks than the oxygen or sulphur based nucleophiles. Finally, we believe that the synthesized molecules will extend the wide range of fluo-rescent probes library depending on the desired chemical and photochemical properties. Biological applications and great efforts to improve percentage yields of the syn-thesized probes are still in progress in our laboratory.
Acknowledgements: Thanks to Prof. Susan Bane and
Dr. Rebecca Kissling for their valuable scientific assis-tance and supportive discussions. I also thank to Dr. Jürgen Schulte for helping collecting 11B NMR spectra. We
gratefully acknowledge NIH grants (R01 CA69571 and R15 GM093941) for financial support.
Conflict of Interest: The authors have no conflict of interest.
References
[1] Loudet A, Burgess K. BODIPY dyes and their derivatives: syntheses and spectroscopic properties. Chem Rev 2007; 107(11):4891–932.
[2] Benniston AC, Copley G. Lighting the way ahead with boron dipyrromethene (Bodipy) dyes. Phys Chem Chem Phys 2009; 11(21):4124–31.
[3] Ulrich G, Ziessel R, Harriman A. The chemistry of fluorescent bodipy dyes: versatility unsurpassed. Angew Chem Int Ed Engl 2008; 47(7):1184–201.
[4] van swieten PF, Leeuwenburgh MA, Kessler BM, Overkleeft Hs. Bioorthogonal organic chemistry in living cells: novel strategies for labeling biomolecules. Org Biomol Chem 2005; 3(1):20–7. [5] Prescher JA, Bertozzi CR. Chemistry in living systems. Nat Chem
Biol 2005; 1(1):13–21.
[6] Rohand T, Baruah M, Qin W, Boens N, Dehaen W. Functio-nalisation of fluorescent BODIPY dyes by nucleophilic substitution. Chem Commun (Camb) 2006; (3):266–8. [7] Li L, Nguyen B, Burgess K. Functionalization of the
4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY) core. Bioorg Med Chem Lett 2008; 18(10):3112–6.
[8] Dilek O, Bane sL. synthesis of boron dipyrromethene
fluorescent probes for bioorthogonal labeling. Tetrahedron Lett 2008; 49:1413–6.
[9] Qin WW, Rohand T, Baruah M, stefan A, Van der Auweraer M, et al. solvent-dependent photophysical properties of borondipyr-romethene dyes in solution. Chem Phys Lett 2006; 420:562–8. [10] Rohand T, Lycoops J, smout s, Braeken E, sliwa M, et al.
Photophysics of 3,5-diphenoxy substituted BODIPY dyes in solution. Photochem Photobiol sci 2007; 6(10):1061–6. [11] Rohand T, Qin W, Boens N, Dehaen W. Palladium-Catalyzed
Coupling Reactions for the Functionalization of BODIPY Dyes with Fluorescence spanning the Visible spectrum. Eur J Org
Chem 2006; 20:4658–63.
[12] Brizet B, Bernhard C, Volkova Y, Rousselin Y, Harvey PD, et al. Boron functionalization of BODIPY by various alcohols and phenols. Org Biomol Chem 2013; 11(44):7729–37.
[13] Duran-sampedro G, Agarrabeitia AR, Garcia-Moreno I, Costela A, Bañuelos J, et al. Chlorinated BODIPYs: surprisingly Efficient and Highly Photostable Laser Dyes. Eur J Org Chem 2012; 32:6335–50. [14] Esnal I, Bañuelos J, López Arbeloa I, Costela A, Garcia-Moreno
I, et al. Nitro and amino BODIPYs: crucial substituents to modulate their photonic behavior. RsC Advances 2013; 3:1547–56.
[15] Esnal I, Valois-Escamilla I, Gómez-Durán CF, Urías-Benavides A, Betancourt-Mendiola ML, et al. Blue-to-orange color-tunable laser emission from tailored boron-dipyrromethene dyes. Chemphyschem 2013; 14(18):4134–42.
[16] Ganapathi E, Madhu s, Chatterjee T, Gonnade R, Ravikanth M. synthesis, structure, spectral, electrochemical and sensing properties of 3-amino boron-dipyrromethene and its derivatives. Dyes and Pigments 2014; 102:218–27.
[17] Jiao L, Pang W, Zhou J, Wei Y, Mu X, et al. Regioselective stepwise bromination of boron dipyrromethene (BODIPY) dyes. J Org Chem 2011;76(24):9988–96.
[18] Leen V, Braeken E, Luckermans K, Jackers C, Van der Auweraer M, et al. A versatile, modular synthesis of monofunctionalized BODIPY dyes. Chem Commun (Camb) 2009; 30:4515–7. [19] Leen V, Gonzalvo VZ, Deborggraeve WM, Boens N, Dehaen
W. Direct functionalization of BODIPY dyes by oxidative nucleophilic hydrogen substitution at the 3- or 3,5-positions. Chem Commun (Camb) 2010; 46(27):4908–10.
[20] Leen V, Leemans T, Boens N, Dehaen W. 2- and 3-Monohalo-genated BODIPY Dyes and Their Functionalized Analogues: synthesis and spectroscopy. Eur J Org Chem 2011; 23:4386–96.
[21] Leen V, schevenels F, Cui J, Xu C, Yang W, et al. synthesis and substitution of 8-(4, 6-Dichloropyrimidin-5-yl)-BODIPY. Eur J Org Chem 2009; 34:5920–6.
[22] Leen V, Van der Auweraer M, Boens N, Dehaen W. Vicarious
nucleophilic substitution of α-hydrogen of BODIPY and its extension to direct ethenylation. Org Lett 2011; 13(6):1470–3. [23] Misra R, Dhokale B, Jadhav T, Mobin sM. Donor-acceptor
meso-alkynylated ferrocenyl BODIPYs: synthesis, structure, and properties. Dalton Trans 2013;42(37):13658–66. [24] Qin W, Leen V, Dehaen W, Cui J, Xu C, et al. synthesis,
spectroscopy, crystal structure, electrochemistry, and quantum chemical and molecular dynamics calculations of a 3-anilino difluoroboron dipyrromethene dye. J Phys Chem C 2009; 113:11731–40.
[25] Bañuelos J, López Arbeloa F, Arbeloa T, salleres s, Vilas JL, et al. Photophysical characterization of new 3-amino and 3-acetamido BODIPY dyes with solvent sensitive properties. J Fluoresc 2008;18(5):899–907.
[26] Osorio-Martínez CA, Urías-Benavides A, Gómez-Durán CF, Bañuelos J, Esnal I, et al. 8-AminoBODIPYs: cyanines or hemicyanines? The effect of the coplanarity of the amino group on their optical properties. J Org Chem 2012;77(12):5434–8. [27] Qin W, Leen V, Rohand T, Dehaen W, Dedecker P, et al.
synthesis, spectroscopy, crystal structure, electrochemistry, and quantum chemical and molecular dynamics calculations of a 3-anilino difluoroboron dipyrromethene dye. J Phys Chem A 2009; 113(2):439–47.
[28] Roacho RI, Metta-Magaña A, Portillo MM, Peña-Cabrera E, Pannell KH. 8-Amino-BODIPYs: structural variation, solvent-dependent emission, and VT NMR spectroscopic properties of 8-R2N-BODIPY. J Org Chem 2013; 78(9):4245–50.
[29] Volkova YA, Brizet B, Harvey PD, Averin AD, Goze C, et al. BODIPY Dyes Functionalized with Pendant Cyclic and Acyclic Polyamines. Eur J Org Chem 2013; 20:4270–9.
[30] Zatsikha YV, Yakubovskyi VP, shandura MP, Dubey IY, Kovtun YP. An efficient method of chemical modification of BODIPY core. Tetrahedron 2013; 69:2233–8.
[31] Liras M, Prieto JB, Pintado-sierra M, Arbeloa FL, García-Moreno I, et al. synthesis, photophysical properties, and laser behavior of 3-amino and 3-acetamido BODIPY dyes. Org Lett 2007; 9(21):4183–6.