A Monostyryl-boradiazaindacene
(BODIPY) Derivative as Colorimetric and
Fluorescent Probe for Cyanide Ions
Zeynep Ekmekci,
†M. Deniz Yilmaz,
†,§and Engin U. Akkaya*
,‡ Department of Chemistry, Middle East Technical UniVersity, Ankara, Turkey, TR-06531, and Department of Chemistry, Bilkent UniVersity, Ankara, Turkey, TR-06800eua@fen.bilkent.edu.tr Received November 21, 2007
ABSTRACT
We developed a novel boradiazaindacene derivative to detect cyanide ions in solution at micromolar concentrations. This structurally simple chemosensor displays a large decrease in emission intensity and a reversible color change from red to blue on contact with cyanide ions. Highly fluorescent polymeric films can be obtained by doping with the chemosensor. Such polymeric materials can be used for the sensing of the cyanide ions in polymer matrices.
The development of selective optical signaling systems for anions is an emerging field of research in supramolecular photochemistry.1Cyanide is one of the most lethal poisons
known. A significant proportion of fatalities among fire victims is due to cyanide poisoning, as blood cyanide con-centrations reach a level of 23-26 µM.2 Furthermore,
cyanide concentrations in drinking water have to be lower
than 1.9µM.3With these considerations, a number of
prom-ising fluorescent probes and organic dyes have been reported
†Middle East Technical University. ‡Bilkent University.
§Present address: Laboratories of Supramolecular Chemistry and Technology and Molecular Nanofabrication, MESA+ Institute for Nano-technology, University of Twente, P. O. Box 217, 7500 AE, Enschede, The Netherlands.
(1) (a) Antonisse, M. M. G.; Reinhoudt, D. N. Chem. Commun. 1998, 443. (b) Snowden, T. S.; Anslyn, E. V. Curr. Opin. Chem. Biol. 1999, 3, 740. (c) Beer, P. D.; Gale, P. A. Angew. Chem., Int. Ed. 2001, 40, 486-516. (d) Amendola, V.; Esteban-Go´mez, D.; Fabbrizzi, L.; Licchelli, M.
Acc. Chem. Res. 2006, 39, 343-353. (e) Martı´nez-Ma´n˜ez, R.; Sanceno´n,
F. Coord. Chem. ReV. 2006, 250, 3081-3093.
(2) (a) Ishii, A.; Seno, H.; Watanabe-Suzuki, K.; Suzuki, O.; Kumazawa, T. Anal. Chem. 1998, 70, 4873-4876. (b) Moriya, F.; Hashimoto, Y. J.
Forensic Sci. 2001, 46, 1421-1425. (c) Kulig, K. W. Cyanide Toxicity;
U.S. Department of Health and Human Services: Atlanta, GA, 1991. (d) Baskin, S. I.; Brewer, T. G. Medical Aspects of Chemical and Biological
Warfare; Sidell, F. R., Takafuji, E. T., Franz, D. R, Eds.; TMM
Publica-tions: Washington, DC, 1997; pp 271-286.
ORGANIC
LETTERS
2008
Vol. 10, No. 3
461-464
10.1021/ol702823u CCC: $40.75 © 2008 American Chemical Society
as optical sensors for cyanide ions.4 Most of them display
color changes, but relatively few of them result in spectral shifts in both absorption and emission spectra.5
Boradiazaindacenes (BODIPY dyes) are well-known6
fluorophores with high quantum yields and large extinction coefficients, attracting considerable attention especially within the past few years in a variety of potential applications such as ion sensing and signaling,7energy transfer cassettes,8
light-harvesting systems,9 fluorescent labeling of
biomol-ecules,10and as potential sensitizers in photodynamic therapy.11
Here, we report the design and synthesis of new boradi-azaindacene (BODIPY)-based chemosensor for selective and sensitive reporting of cyanide ions. The structure and synthesis of compound 3 is depicted in Scheme 1. The target
chemosensor 3 was prepared by Knoevenagel-type conden-sation of the known BODIPY dye 2 (which was synthesized by reacting benzoylchloride and 2,4-dimethylpyrrole under
reflux in dichloromethane, followed by the addition of triethylamine and borontrifluoroetherate) and the correspond-ing aldehyde 1 in a Dean-Stark apparatus with azeotropic removal of water. The reactivity of the acidic 3- and/or 5-methyls on the boradiazaindacene core is now firmly established.7e,f,11,12 The reaction reached completion within
2 h with good isolated yields. The structure of 3 was confirmed by1H NMR, 13C NMR, and HRMS data
(Sup-porting Information).
The treatment of compound 3 with Bu4NCN in acetonitrile
resulted in the formation of a blue nonfluorescent solution, and the emission was restored on the addition of trifluoro-acetic acid (Scheme 2). Further evidence of
chemosensor-cyanide interaction was obtained by studying the1H NMR
spectra which were recorded before and after the addition of tetrabutylammonium cyanide in acetonitrile-d6solution.
Most of the aromatic and olefinic protons exhibited an upfield shift (∼0.5 ppm) which is compatible with the proposed switching mechanism (Scheme 2 and Supporting Informa-tion).
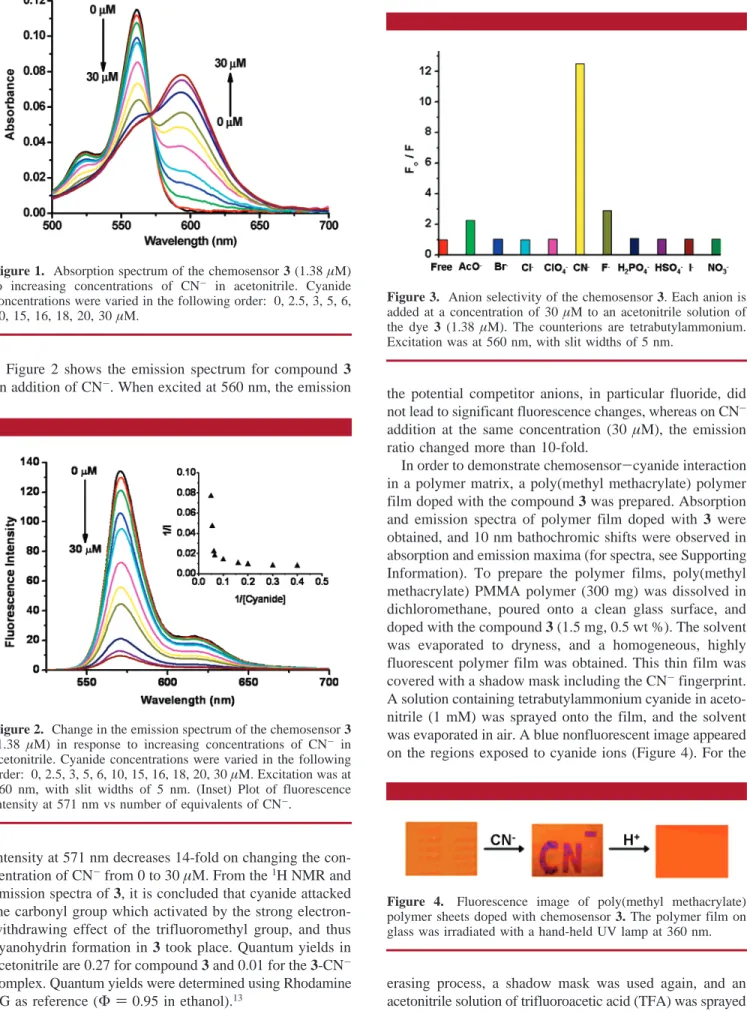
The absorption spectral changes of 3 on addition of cyanide in acetonitrile are shown in Figure 1. The absorption band at 561 nm decreased, while a new band at 594 nm appeared. A clean isosbestic point of 571 nm indicated an interconversion into single discrete chemical species during the titration process. When the titration was repeated with different anions, such as F-, Cl-, Br-, I-, AcO-, ClO4-,
H2PO4-, HSO4-, and NO3-, it was evident that only cyanide
ion produced a large spectral change in the absorption spectrum (33 nm bathochromic shift). The extinction coef-ficient of the chemosensor 3 at 561 nm is 83 000 M-1cm-1 and 56 400 M-1cm-1at 594 nm for the 3-CN-adduct. (3) (a) Guidelines for Drinking-Water Quality; World Health
Organiza-tion: Geneva, 1996. (b) Ullman’s Encyclopedia of Industrial Chemistry, 6th ed.; Wiley-VCH: New York, 1999.
(4) (a) Lakowicz, J. R. Principles of Fluorescence Spectroscopy, 2nd ed.; Kluwer/Academic Plenum Publishers: New York, 1997. (b) Lakowicz, J. R. Topics in Fluorescence Spectroscopy: Probe Design and Chemical
Sensing; Plenum Press: New York, 1994; Vol. 4. (c) Anzenbacher, P.;
Tyson, D. S.; Jursikova, K.; Castellano, F. N. J. Am. Chem. Soc. 2002,
124, 6232-6233. (d) Badugu, R.; Lakowicz, J. R.; Geddes, C. D. J. Am. Chem. Soc. 2005, 127, 3635-3641.
(5) (a) Tomasulo, M.; Raymo, F. M. Org. Lett. 2005, 7, 4633-4636. (b) Tomasulo, M.; Sortino, S.; White, A. J. P.; Raymo, F. M. J. Org. Chem.
2006, 71, 744-753. (c) Chung, Y. M.; Raman, B.; Kim, D. S.; Ahn, K. H. Chem. Commun. 2006, 186-188. (d) Ros-Lis, J. V.; Martı´nez-Ma´n˜ez, R.;
Soto, J. Chem. Commun. 2005, 5260-5262. (e) Miyaji, H.; Sessler, J. L.
Angew. Chem., Int. Ed. 2001, 40, 154-157. (f) Chen, C.-L.; Chen, Y.-H.;
Chen, C.-Y.; Sun, S.-S. Org. Lett. 2006, 8, 5053-5056. (g) Yang, Y.-K.; Tae, J. Org. Lett. 2006, 8, 5721-5723.
(6) (a) Treibs, A.; Kreuzer, F.-H. Justus Liebigs Ann. Chem. 1968, 718, 208-223. (b) Haugland, R. P. The Handbook. A Guide to Fluorescent
Probes and Labeling Technologies, 10th ed.; Molecular Probes, Inc.:
Eugene, OR, 2005.
(7) (a) Turfan, B.; Akkaya, E. U. Org. Lett. 2002, 4, 2857-2859. (b) Goze, C.; Ulrich, G.; Charbonnie`re, L.; Cesario, M.; Prange´, T.; Ziessel, R. Chem. Eur. J. 2003, 9, 3748-3755. (c) Gabe, Y.; Urano, Y.; Kikuchi, K.; Kojima, H.; Nagano, T. J. Am. Chem. Soc. 2004, 126, 3357-3367. (d) Rurack, K.; Kollmannsberger, M.; Resch-Genger, U.; Daub, J. J. Am. Chem.
Soc. 2000, 122, 968-969. (e) Coskun, A.; Akkaya, E. U. J. Am. Chem. Soc. 2005, 127, 10464-10465. (f) Coskun, A.; Yilmaz, M. D.; Akkaya, E.
U. Org. Lett. 2007, 9, 607-609.
(8) (a) Burghart, A.; Thoresen, L. H.; Chen, J.; Burgess, K.; Bergstro¨m, F.; Johansson, L. B.-Å. Chem. Commun. 2000, 2203-2204. (b) Goze, C.; Ulrich, G.; Ziessel, R. Org. Lett. 2006, 8, 4445-4448. (c) Wan, C.-W.; Burghart, A.; Chen, J.; Bergstro¨m, F.; Johansson, L. B.-Å.; Wolford, M. F.; Kim, T. G.; Topp, M. R.; Hochstrasser, R. M.; Burgess, K. Chem. Eur.
J. 2003, 9, 4430-4441.
(9) (a) Yilmaz, M. D.; Bozdemir, O. A.; Akkaya, E. U. Org. Lett. 2006,
8, 2871-2873. (b) Goeb, S.; Ziessel, R. Org. Lett. 2007, 9, 737-740.
(10) Yee, M.; Fas, S. C.; Stohlmeyer, M. M.; Wandless, T. J.; Cimprich, K. A. J. Biol. Chem. 2005, 280, 29053-29059.
(11) Atilgan, S.; Ekmekci, S.; Dogan, A. L.; Guc, D.; Akkaya, E. U.
Chem. Commun. 2006, 4398-4400.
(12) (a) Saki, N.; Dinc, N.; Akkaya, E. U. Tetrahedron 2006, 62, 2721-2725. (b) Dost, Z.; Atilgan, S.; Akkaya, E. U. Tetrahedron 2006, 62, 8484-8488. (c) Coskun, A.; Deniz, E.; Akkaya, E. U. Tetrahedron Lett. 2007,
48, 5359-5361.
Scheme 1. Structure and the Synthesis of the Chemosensor 3
Scheme 2. Suggested Mechanism for ON-OFF Switching
within the Presence of CN-Ions
Figure 2 shows the emission spectrum for compound 3 on addition of CN-. When excited at 560 nm, the emission
intensity at 571 nm decreases 14-fold on changing the con-centration of CN-from 0 to 30µM. From the1H NMR and
emission spectra of 3, it is concluded that cyanide attacked the carbonyl group which activated by the strong electron-withdrawing effect of the trifluoromethyl group, and thus cyanohydrin formation in 3 took place. Quantum yields in acetonitrile are 0.27 for compound 3 and 0.01 for the 3-CN -complex. Quantum yields were determined using Rhodamine 6G as reference (Φ ) 0.95 in ethanol).13
The anion selectivity of the optical response of the chemosensor 3 was also studied (Figure 3). It is obvious that
the potential competitor anions, in particular fluoride, did not lead to significant fluorescence changes, whereas on CN -addition at the same concentration (30 µM), the emission ratio changed more than 10-fold.
In order to demonstrate chemosensor-cyanide interaction in a polymer matrix, a poly(methyl methacrylate) polymer film doped with the compound 3 was prepared. Absorption and emission spectra of polymer film doped with 3 were obtained, and 10 nm bathochromic shifts were observed in absorption and emission maxima (for spectra, see Supporting Information). To prepare the polymer films, poly(methyl methacrylate) PMMA polymer (300 mg) was dissolved in dichloromethane, poured onto a clean glass surface, and doped with the compound 3 (1.5 mg, 0.5 wt %). The solvent was evaporated to dryness, and a homogeneous, highly fluorescent polymer film was obtained. This thin film was covered with a shadow mask including the CN-fingerprint. A solution containing tetrabutylammonium cyanide in aceto-nitrile (1 mM) was sprayed onto the film, and the solvent was evaporated in air. A blue nonfluorescent image appeared on the regions exposed to cyanide ions (Figure 4). For the
erasing process, a shadow mask was used again, and an acetonitrile solution of trifluoroacetic acid (TFA) was sprayed Figure 1. Absorption spectrum of the chemosensor 3 (1.38µM)
to increasing concentrations of CN- in acetonitrile. Cyanide concentrations were varied in the following order: 0, 2.5, 3, 5, 6, 10, 15, 16, 18, 20, 30µM.
Figure 2. Change in the emission spectrum of the chemosensor 3
(1.38 µM) in response to increasing concentrations of CN- in acetonitrile. Cyanide concentrations were varied in the following order: 0, 2.5, 3, 5, 6, 10, 15, 16, 18, 20, 30µM. Excitation was at
560 nm, with slit widths of 5 nm. (Inset) Plot of fluorescence intensity at 571 nm vs number of equivalents of CN-.
Figure 3. Anion selectivity of the chemosensor 3. Each anion is
added at a concentration of 30µM to an acetonitrile solution of
the dye 3 (1.38 µM). The counterions are tetrabutylammonium.
Excitation was at 560 nm, with slit widths of 5 nm.
Figure 4. Fluorescence image of poly(methyl methacrylate) polymer sheets doped with chemosensor 3. The polymer film on glass was irradiated with a hand-held UV lamp at 360 nm.
onto film. The nonfluorescent cyanide image disappeared, and the fluorescence of the cyanide-exposed region of the film was restored.
In this work, we have successfully designed and synthe-sized a colorimetric and fluorimetric molecular probe for sensing and signaling cyanide ions. The signal transduction mechanism was investigated by UV/vis absorption, emission, and NMR spectroscopy. The interaction between the chemosensor 3 and cyanide ions causes large changes in both color and fluorescence emission intensity.
The results obtained using poly(methyl methacrylate) (PMMA) polymer matrix demonstrate that the use of the trifluoroacetanilide group with novel BODIPY dyes opens new avenues for the sensing of materials in solid state. The
signaling of cyanide ions takes place near the red end of the visible spectrum, which is a distinct advantage. Further work on this type of compoud is expected to yield novel cyanide-sensing platforms with practical utility. Work to that end is in progress in our laboratory.
Acknowledgment. This work was supported by Turkish
Scientific and Technical Research Council (TUBITAK) and Turkish Academy of Sciences (TUBA). M.D.Y. thanks TUBITAK for a scholarship.
Supporting Information Available: Syntheses,
experi-mental details, 1H and 13C NMR spectra and additional
spectroscopic data. This material is available free of charge via the Internet at http://pubs.acs.org.
OL702823U
(13) Du, H.; Fuh, R. A.; Li, J.; Corkan, A.; Lindsey, J. S. Photochem.
Photobiol. 1998, 68, 141-142.