Contents lists available atScienceDirect
Results in Physics
journal homepage:www.elsevier.com/locate/rinp
An investigation on shielding properties of BaO, MoO
3
and P
2
O
5
based
glasses using MCNPX code
O. Agar
a, M.I. Sayyed
b, H.O. Tekin
c,d, Kawa M. Kaky
e, S.O. Baki
f,⁎, I. Kityk
gaKaramanoğlu Mehmetbey University, Department of Physics, Karaman, Turkey bUniversity of Tabuk, Department of Physics, Tabuk, Saudi Arabia
cUskudar University, Vocational School of Health Services, Radiotherapy Department, Istanbul 34672, Turkey dUskudar University, Medical Radiation Research Center (USMERA), Istanbul 34672, Turkey
eCommunication and Information Technology, Council of Representatives of Iraq, Conferences Palace, Baghdad, Iraq fDepartment of Physics, Faculty of Science, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia
gInstitute of Optoelectronics and Measuring Systems, Faculty of Electrical Engineering, Czestochowa University of Technology, 17 Armii Krajowej Str, 42-200 Czestochowa,
Poland
A R T I C L E I N F O
Keywords: MoO3-based glass
Radiation shielding Attenuation coefficients WinXCom
MCNPX
A B S T R A C T
In the present work, some radiation shielding quantities (mass attenuation coefficients, effective atomic number, effective electron density, half value layer and mean free path) for various BaO–MoO3–P2O5ternary glass
sys-tems have been determined within the 0.015–15 MeV energy range, using WinXCom program. Additionally, the mass attenuation coefficients of all the investigated glasses have been calculated using MCNPX simulation code (version 2.6.0) and compared to those of WinXCom results. Among the studied glasses, BaMoP8 glass sample with MoO3content of 70% mol is found to have superior gamma-ray shielding characteristics. Moreover, the
glasses studied in this paper possess better radiation shielding properties by providing shorter half value layer (HVL) than RS-253 G18 commercial glass and some concrete samples namely ordinary, hematite-serpentine and ilmanite-limonite.
Introduction
High-energetic ionization radiation, especially X- and gamma ray, utilized in manyfields such as industrial, medical, agricultural, etc. is extremely dangerous for living beings, environment and electronic la-boratory instruments due to its harmful effects of neutral radiation. The most effective way to minimize its effects is to reduce the intensity of these radiation which is known as shielding[1,2]. It is mandatory to investigate the various parameters related to the passage of gamma ray radiation through any material [3]. Unlike lead (Pb) and standard concretes, glass materials draw particularly the attention for their transparency to visible and near-infrared light, and furthermore, its features can be modified with chemical composition and preparation methods. In addition, designing a new transparent shielding material is more suitable over opaque which utilizes in different applications for radiationfields such as isotope production center, research centers and nuclear reactors[4]. Toxic nature of Pb and Pb based compounds is the primary issue for the environment health, especially living being, al-though Pb-based glass is beneficial for radiation shielding purposes because of its good chemical and physical characteristics such as high
atomic number, high density, and high cross section [5]. The in-vestigations on radiation shielding features of various glass types have been applied for silicate[6], borate[7–9], tellurite[10–12], phosphate [13,14], barium[15,16]and zinc boro-tellurite[17,18]. On the other hand, an exceptional transition metal, molybdenum oxide (MoO3),
which can't purely form any glass can results in the formation of stable glasses when doped to different oxide glass formers[19,20]. The skills of ions of MoO3to be in multiple valence states namely +3, +4, +5 or
+6 encourage its network formation trend. Herewith, it can be com-posed different novel glass systems with interesting electrochromic, electrical and optical characteristics[21]. The present work has been aimed at the investigation of the selected glasses with different oxides (BaO, MoO3and P2O5) for gamma rays shielding material by utilizing
WinXCom software over a wide photon energy range from 0.015 to 15 MeV. Furthermore, in order to validate the obtained results the mass attenuation coefficient values of WinXCom for the selected glasses were compared to those results of MCNPX simulation code. Also, the results of half value layer have made the comparison with those of some or-dinary concretes and various glass systems.
https://doi.org/10.1016/j.rinp.2018.12.003
Received 9 November 2018; Received in revised form 30 November 2018; Accepted 1 December 2018
⁎Corresponding author.
E-mail address:sharudinomar@upm.edu.my(S.O. Baki).
Available online 04 December 2018
2211-3797/ © 2018 The Authors. Published by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/BY-NC-ND/4.0/).
Materials and methods
Theoretical basis
The chemical compositions for the studied glasses containing BaO, MoO3and P2O5with different rates and their densities (g/cm3) have
been tabulated inTable 1 [22].
The mass attenuation coefficient (μ/ρ) is defined for a compound and mixture by the following relation[23,24]:
∑
= = μ μ ρ w μ ρ ( m glass) i( )i (1) where (μ/ρ)iand wirepresent the mass attenuation coefficient valueand the weight fraction of the ith element in the material, respectively. Theμ/ρ value of glass systems can be calculated for a specific energy range using WinXCom software based on the mixture rule[25].
Using the above basic parameter, it can be derived many quantities
namely total atomic and electronic cross-section, the effective atomic number and electron density, etc. The total atomic cross section (σa) can be determined as follow[17]: = ∑ σ μ N ( ) a m glass A i w A i i (2)
where NArepresents the Avogadro constant Aiis the atomic weight of
the ith element in the glass system, respectively. It is expressed in cm2/
atom.
The total electronic cross-section (σe) can be written as follows:
∑
= σ N μ ρ f A Z 1 ( ) e i i i i i (3)whereZiand fiare the atomic number and the fractional abundance of the ith element, respectively. It is expressed in cm2/electron.
The Zeff, dimensionless quantity, can be obtained fromσaandσe
using the next relation[26]: =
Z σ
σ
eff a
e (4)
The electron density (Neff) expresses the electron numbers per unit
mass of the interacting matter and given by Ref.[27]:
= ∑ N N Z f A e eff i i i (5) It is expressed in electron/g.
The half value layer (HVL) defines the thickness at which the transmitted intensity is one-half the incident intensity for the gamma radiation and depends on μ value[28]:
=
HVL ln
μ
2
(6) The mean free path (MFP) indicates the mean distance a photon can travel before interacting with the absorber. It has been estimated from
μas below[29]: = MFP μ 1 (7) MCNPX simulation code
In order to determine the µ/ρ values for the present glass systems, Monte Carlo N-Particle Transport Code System-extended (MCNPX) was carried out. The MCNPX 3-D view of photon attenuation scheme with several simulation equipments namely the glass sample as absorber, a point radioactive source, lead (Pb) collimator for primary radiation beam, Pb blocks to prevent from the scattered photons and F4 tally mesh detectionfield is exhibited inFig. 1. F4 tally detectionfield was located at the same line with a distance of 70 cm from point source to measure the gamma ray photons inter the detector per MeV.cm2.s−1.
This tally (F4) pattern provides information on the mean photonflux in the detectionfield. The selected glass samples were placed between the
Table 1
The densities and chemical compositions (mol%) of the selected samples.
Code Mole Fraction Wt. fraction of elements
BaO MoO3 P2O5 O P Mo Ba Density (g/cm3)
BaMoP1 0.50 0 0.50 0.32511 0.20980 – 0.46509 3.63 BaMoP2 0.45 0.10 0.45 0.32593 0.18929 0.06515 0.41963 3.69 BaMoP3 0.40 0.20 0.40 0.32675 0.16868 0.13062 0.37394 3.76 BaMoP4 0.35 0.30 0.35 0.32757 0.14797 0.19643 0.32803 3.83 BaMoP5 0.30 0.40 0.30 0.32840 0.12715 0.26257 0.28188 3.91 BaMoP6 0.25 0.50 0.25 0.32924 0.10623 0.32904 0.23549 3.94 BaMoP7 0.20 0.60 0.20 0.33007 0.08520 0.39585 0.18887 3.97 BaMoP8 0.15 0.70 0.15 0.33091 0.06406 0.46301 0.14202 3.99
Fig. 1. MCNPX 3-D simulation geometry.
source and the F4 tally mesh at a distance of 50 cm. For the detailed knowledge of the simulation used in this work we may refer to previous studies[30,31].
Results and discussions
Utilizing WinXCom package software [25]in the photon energy region between 0.015 and 15 MeV, the basic gamma radiation shielding quantities of BaO, MoO3and P2O5glasses studied in the present work
have been calculated. In the following subsections we discussed the mass attenuation coefficients (μ ρ/ ), the effective atomic number (Zeff),
the electron density (Neff), the half value layer (HVL) and the mean free
path (MFP) for the selected glasses.
Mass attenuation coefficients (μ/ρ)
The photon attenuation coefficients contain important information for gamma rays shielding characteristic. The variations of mass at-tenuation coefficient (μ/ρ) values as a function of the photon energy for glasses at different ratios (seeTable 1) of BaO, MoO3 and P2O5 are
presented graphically inFig. 2. Obviously, thisfigure indicates that the μ/ρ for all the investigated glasses has the largest values at the low energies (E < 500 keV) and thereafter, are gradually decreased with further increase in the photon energy however the μ/ρ values of BaMoP5, BaMoP6, BaMoP7 and BaMoP8 glass samples tend to increase slightly at 0.02 MeV (around the K absorption edges of Mo) and 0.04 MeV (around the K absorption edges of Ba). Furthermore, μ/ρ values reduce at a slower rate at the intermediate energies (between 0.5 and 5 MeV), while theμ/ρ values are smaller and incline to become
Table 2
Comparison of mass attenuation coefficient values of the investigated glasses using WinXCom and MCNPX.
Energy (MeV) BaMoP1 BaMoP2 BaMoP3 BaMoP4
XCOM MCNPX % Dev. XCOM MCNPX % Dev. XCOM MCNPX % Dev. XCOM MCNPX % Dev.
0.015 32.7100 32.7529 0.13 31.4300 31.9867 1.77 30.1500 30.8767 2.41 28.8600 28.9155 0.19 0.02 15.0700 15.1429 0.48 18.8100 19.0178 1.10 22.5600 22.7452 0.82 26.3400 26.5148 0.66 0.03 5.0850 5.1048 0.39 6.4310 6.6988 4.16 7.7840 7.8005 0.21 9.1430 9.1799 0.40 0.04 11.6800 11.9878 2.63 11.3900 11.6522 2.30 11.1000 11.2146 1.03 10.8100 11.0989 2.67 0.05 6.5850 6.6179 0.50 6.4070 6.5078 1.57 6.2280 6.2325 0.07 6.0480 6.0986 0.84 0.06 4.0940 4.1030 0.22 3.9780 4.0048 0.67 3.8620 3.8963 0.89 3.7450 3.8015 1.51 0.08 1.9470 1.9567 0.50 1.8900 1.9028 0.68 1.8320 1.8535 1.17 1.7750 1.7900 0.84 0.1 1.1110 1.1236 1.13 1.0790 1.1015 2.08 1.0460 1.1046 5.60 1.0140 1.0214 0.73 0.15 0.4383 0.4395 0.28 0.4273 0.4300 0.63 0.4163 0.4204 0.99 0.4052 0.4099 1.15 0.2 0.2546 0.2554 0.32 0.2495 0.2503 0.32 0.2444 0.2499 2.23 0.2393 0.2404 0.48 0.3 0.1448 0.1451 0.23 0.1432 0.1453 1.48 0.1415 0.1433 1.24 0.1398 0.1490 6.60 0.4 0.1096 0.1104 0.76 0.1088 0.1099 0.98 0.1080 0.1100 1.90 0.1073 0.1091 1.64 0.5 0.0924 0.0931 0.82 0.0920 0.0920 0.08 0.0916 0.0920 0.48 0.0911 0.0917 0.61 0.6 0.0818 0.0820 0.26 0.0816 0.0820 0.47 0.0813 0.0820 0.81 0.0811 0.0816 0.62 0.8 0.0689 0.0690 0.22 0.0687 0.0690 0.37 0.0686 0.0689 0.42 0.0685 0.0687 0.27 1 0.0607 0.0601 0.87 0.0606 0.0609 0.50 0.0606 0.0609 0.56 0.0605 0.0610 0.78 2 0.0426 0.0430 0.89 0.0426 0.0430 0.84 0.0426 0.0429 0.59 0.0426 0.0429 0.74 3 0.0364 0.0367 0.69 0.0364 0.0365 0.36 0.0364 0.0366 0.58 0.0364 0.0368 1.11 4 0.0335 0.0336 0.44 0.0335 0.0335 0.10 0.0335 0.0338 1.02 0.0335 0.0337 0.61 5 0.0319 0.0320 0.32 0.0320 0.0320 0.23 0.0320 0.0321 0.36 0.0320 0.0327 2.04 6 0.0311 0.0313 0.53 0.0312 0.0313 0.60 0.0312 0.0315 1.07 0.0312 0.0315 0.90 8 0.0306 0.0308 0.84 0.0306 0.0309 0.91 0.0307 0.0309 0.58 0.0307 0.0309 0.57 10 0.0307 0.0308 0.20 0.0308 0.0311 0.89 0.0309 0.0311 0.67 0.0309 0.0310 0.46 15 0.0320 0.0321 0.33 0.0321 0.0322 0.33 0.0322 0.0326 1.16 0.0322 0.0325 0.85
Energy (MeV) BaMoP5 BaMoP6 BaMoP7 BaMoP8
XCOM MCNPX % Dev. XCOM MCNPX % Dev. XCOM MCNPX % Dev. XCOM MCNPX % Dev.
0.015 27.5600 27.9245 1.32 26.2500 26.9856 2.80 24.9400 25.1048 0.66 23.6300 22.9877 2.72 0.02 30.1300 31.8796 5.81 33.9500 34.1245 0.51 37.7800 38.0146 0.62 41.6300 40.6986 2.24 0.03 10.5100 10.9867 4.54 11.8800 11.9855 0.89 13.2600 13.9515 5.21 14.6500 15.0779 2.92 0.04 10.5100 10.0976 3.92 10.2100 10.6887 4.69 9.9170 9.9531 0.36 9.6180 9.9856 3.82 0.05 5.8680 5.8959 0.48 5.6860 5.7105 0.43 5.5030 5.5169 0.25 5.3200 5.4165 1.81 0.06 3.6280 3.6572 0.80 3.5100 3.5355 0.73 3.3920 3.4109 0.56 3.2730 3.3128 1.22 0.08 1.7170 1.7270 0.58 1.6590 1.7101 3.08 1.6000 1.6204 1.28 1.5420 1.5786 2.37 0.1 0.9812 0.9909 0.99 0.9484 0.9501 0.18 0.9155 0.9201 0.51 0.8824 0.8934 1.25 0.15 0.3940 0.3975 0.90 0.3828 0.3867 1.02 0.3715 0.3753 1.03 0.3602 0.3688 2.39 0.2 0.2342 0.2388 1.95 0.2290 0.2305 0.65 0.2238 0.2314 3.40 0.2186 0.2237 2.31 0.3 0.1380 0.1400 1.48 0.1363 0.1399 2.61 0.1346 0.1352 0.41 0.1328 0.1366 2.85 0.4 0.1065 0.1099 3.17 0.1057 0.1089 3.00 0.1049 0.1071 2.14 0.1041 0.1099 5.55 0.5 0.0907 0.0910 0.35 0.0903 0.0909 0.69 0.0898 0.0902 0.43 0.0894 0.0903 1.02 0.6 0.0808 0.0810 0.28 0.0806 0.0809 0.45 0.0803 0.0806 0.40 0.0800 0.0820 2.48 0.8 0.0684 0.0687 0.41 0.0683 0.0687 0.51 0.0682 0.0686 0.53 0.0681 0.0690 1.31 1 0.0605 0.0607 0.40 0.0604 0.0608 0.65 0.0604 0.0605 0.24 0.0603 0.0608 0.79 2 0.0426 0.0430 0.87 0.0426 0.0429 0.69 0.0426 0.0428 0.47 0.0426 0.0430 0.93 3 0.0364 0.0367 0.69 0.0364 0.0366 0.47 0.0365 0.0366 0.46 0.0365 0.0370 1.44 4 0.0335 0.0338 0.76 0.0336 0.0339 1.03 0.0336 0.0325 3.07 0.0336 0.0343 2.03 5 0.0320 0.0322 0.65 0.0321 0.0326 1.57 0.0321 0.0324 0.84 0.0321 0.0326 1.60 6 0.0313 0.0313 0.13 0.0313 0.0320 2.25 0.0313 0.0315 0.57 0.0313 0.0315 0.57 8 0.0308 0.0309 0.53 0.0308 0.0310 0.77 0.0309 0.0310 0.44 0.0309 0.0310 0.34 10 0.0310 0.0310 0.28 0.0310 0.0315 1.65 0.0311 0.0312 0.58 0.0311 0.0316 1.47 15 0.0323 0.0326 0.86 0.0324 0.0324 0.10 0.0325 0.0326 0.51 0.0325 0.0327 0.47
almost stationary in the higher energy region up to 15 MeV. On other hand, the occurred trend in Fig. 2can be evaluated in terms of the radiation physical concepts. Depending on both the atomic number (Z) of the glass sample and the energy of the gamma radiation in different dominant interaction processes on different energy ranges, gamma photons interact with the absorber. It possess three energy regions re-lative to the partial processes namely effects of photoelectric absorption (PE), Compton scattering (CS) and pair production (PP) at low, inter-mediate and high energies, respectively. Therefore, theμ/ρ of all glass samples has high values in the energy region where the PE is dominant. This is due to the fact that the effective cross section of PE process is related to the photon energy and the atomic number as 1/E3.5and Z4,
respectively. Therefore, the highest differences in μ/ρ values between different glasses were observed in this energy region and also this clarified the higher μ/ρ values of BaMoP8 glass sample that consisted of large amount of high Z element. In other words, it indicates that
Fig. 3. The effective atomic number (Zeff) of the studied glass systems.
Fig. 4. The electron density (Neff) of the selected glass systems.
Table 3
Photon energies (in keV) of absorption edges of the elements under investiga-tion[32].
Element Z M3 M2 M1 L3 L2 L1 K
P 15 – – – – – – 2.1455
Mo 42 – – – 2.5202 2.6251 2.8655 19.9995
Ba 56 1.0622 1.1367 1.2928 5.2470 5.6236 5.9888 37.4406
Fig. 5. Comparison of HVL thicknesses of (a) the studied glass systems with (b) commercial glasses[36]and (c) concrete samples[37].
probabilities of interaction of photons with glass sample are more likely at low energies. After that, the cross section of the CS mechanism varies with the photon energy and atomic number as 1/E and Z, respectively and so, the μ/ρ minimizes exponentially with increment of gamma photon energy and became nearly constant. In high energy region, the PP is the dominant and the cross section of PP is proportional to Z2,
therefore we observed a slight increase in the mass attenuation coeffi-cient values in the high energy region[32,33].
Furthermore, the μ/ρ values of the selected glass systems were calculated utilizing MCNPX simulation code to validate the results with standard WinXcom data. The comparison of results obtained through both WinXCom software and MNCPX code are reported inTable 2. This table represents that, in general, the WinXCom data is in good agree-ment with those of MCNPX Monte Carlo. The difference between the WinXCom and MCNPX results has been calculated by Eq.(8):
⎜ ⎟ = ⎛ ⎝ − ⎞ ⎠ ∗ Diff μ ρ μ ρ μ ρ . / / / 100 WinXCom MNCPX WinXCom (8)
According toTable 2, the calculated Diff. values in WinXCom and MCNPX results are in the range of 0.13–2.63%, 0.10–4.16%, 0.13–2.63%, 0.07–5.60%, 0.19–6.60%, 0.13–5.81%, 0.10–4.69%, 0.24–5.21% and 0.34–5.55% for BaMoP1, BaMoP2, BaMoP3, BaMoP4, BaMoP5, BaMoP6, BaMoP7 and BaMoP8 glasses, respectively. The difference between both WinXcom and MCNPX results is less than 7% and this validate the obtainedμ/ρ values.
Effective atomic numbers (Zeff) and electron densities (Ne,eff)
Considering the values ofμ/ρ, the Zeffand Ne,effabsorption
para-meters for BaO, MoO3and P2O5based glass systems under investigation
have been calculated. Detailed data for the atomic numbers and masses of the elements can be found in IUPAC technical report[34,35].Figs. 3 and 4 represent the variations of Zeff and Ne,eff values of all glass
samples, respectively.
Thefluctuations observed inFig. 3can be easily described basing on the dominance of different photon interaction mechanisms as detailed above mentioned. Similar to variation ofμ/ρ results, the trend of Zeff
with photon energy exhibits discontinuous jumps at low energies (E < 0.04 MeV) as shown inFig. 3. The energy of these jumps corre-sponds to photoelectric absorption edges of barium (Ba), molybdenum (Mo) and phosphate (P) elements as given inTable 3. Among these elements, the valence electron of Ba which is heavy element occupies at n = 6 (s-shell) from electron configuration and therefore, its absorption edges of K, L and M are higher than 1 keV energy. On the other hand,
the trend in Zefffluctuations becomes nearly independent of energy for
all investigated glasses in incident photon energy region between 0.6 and 10 MeV where may be due to dominance of the Compton scattering process.Fig. 4indicates the dependence of the electron density values (Ne,eff) of the investigated glass systems with photon energy. It can be
clearly seen from this figure that the fluctuations of observed Ne,eff
represent completely similar trend to that of Zeff. The variation in Neffis
provided by Zefffor compounds and mixtures since atomic number is
related to proton (or electron) numbers in elements.
Half value layer (HVL) and mean free path (MFP)
On the other hand, the HVL values of the studied BaO, MoO3and
P2O5based glass systems have been estimated to evaluate the photon
attenuation characteristics directly. It is well-known that the smaller HVL values are the better the glass system considered is, for gamma rays radiation shielding applications. HVL results of the present glass samples for the energy range in this study are illustrated inFig. 5. Al-though all the BaMoP1–BaMoP8 glass systems appears to have nearly the same HVL values at low photon energies than 0.1 MeV, it is clear that BaMoP8 glass sample (which possesses the highest density) among the glass samples has lowest values over energy range of 0.01–15 MeV as analyzed in detail, then we can conclude that this sample can at-tenuate more photons than the rest of the samples, thus has superior shielding properties. Furthermore, we have compared the HVL thick-nesses for the present glass systems with those of different commer-cially shielding glasses (RS-253 G18, RS-360) developed by SCHOTT company [36] and three types of concretes (ordinary, hematite –-serpentine, ilmenite–limonite concretes) reported by Bashter[37]for specific energy range as shown inFig. 5b andFig. 5c, respectively. It is evident that the HVL thicknesses of all the selected glasses are lower than those of both RS-253 G18 glass sample (see Fig. 5b) and the concretes used in the comparison (seeFig. 5c) at all energies except for result of RS-360 glass in medium energy region of 0.1–1 MeV. These results point out that the selected glasses exhibited good performance in terms of photon shielding when compared to both various types of concretes and some commercial glasses. The MFP variation of the stu-died BaO, MoO3and P2O5based glass samples with photon energies is
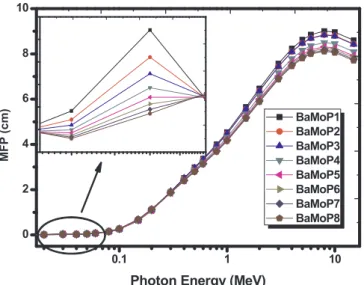
shown inFig. 6. The MFP for all samples increases with the increase in the energy, which means that high energy photons can penetrate the absorber easily while low energy photons are more attenuated. Ad-ditionally, there is a significant reduction in the HVL and MFP results from BaMoP1 with ρ = 3.63 g/cm3 glass sample to BaMoP8 with
3.99 g/cm3fromFigs. 5a and6. It can be seen that HVL and MFP values decrease as MoO3mol% increase owing to the gradual increase of the
glass density (seeTable 1) as well as theμ/ρ for the present glass sys-tems. Therefore, it indicates the higher shielding effectiveness of BaMoP8 glass sample with MoO3of 70% mol among the studied
sam-ples.
Conclusions
The obtained results revealed that BaMoP8 glass sample with MoO3
of 70% mol among the studied samples is observed to possess superior gamma-ray shielding performance because of its both higherμ/ρ and lower HVL and MFP values. Besides, all the selected glass samples (BaMoP1–BaMoP8) at all energies have lower values of HVL than dif-ferent concrete types and commercial glass sample and so, these sam-ples are a better replacement for ordinary concrete. In addition, The MCNPX Monte Carlo simulation code has been employed for the as-sessment ofμ/ρ values for all the studied glasses to validate the theo-retical data. The results displayed that the μ/ρ values of WinXCom software and MNCPX code are quite compatible. Finally, these glasses possess the advantage of being transparent to visible light. It is in particular useful for various shielding purposes in which being in view of the gamma ray sources are favorable.
Acknowledgement
Authors would like gratefully acknowledge use of the services and facilities of Universiti Putra Malaysia (UPM), Malaysia where this work was supported by UPM under GP-IPM/2016/9484400 grant.
Appendix A. Supplementary data
Supplementary data to this article can be found online athttps:// doi.org/10.1016/j.rinp.2018.12.003.
References
[1] Jalali M, Mohammadi A. Gamma ray attenuation coefficient measurement for neutron-absorbent materials. Radiat Phys Chem 2008.https://doi.org/10.1016/j. radphyschem.2007.12.014.
[2] Chanthima N, Kaewkhao J. Investigation on radiation shielding parameters of bis-muth borosilicate glass from 1 keV to 100 GeV. Ann Nucl Energy 2013;55:23–8. https://doi.org/10.1016/j.anucene.2012.12.011.
[3] Alfassi ZB, Chung C. Prompt gamma neutron activation analysis. Boca Raton, FL, USA: CRC Press; 1995.
[4] Singh VP, Badiger NM, Chanthima N, Kaewkhao J. Evaluation of gamma-ray ex-posure buildup factors and neutron shielding for bismuth borosilicate glasses. Radiat Phys Chem 2014;98:14–21.https://doi.org/10.1016/j.radphyschem.2013. 12.029.
[5] Sayyed MI, Lakshminarayana G. Structural, thermal, optical features and shielding parameters investigations of optical glasses for gamma radiation shielding and de-fense applications. J Non Cryst Solids 2018;487:53–9.https://doi.org/10.1016/j. jnoncrysol.2018.02.014.
[6] Chanthima N, Kaewkhao J, Limsuwan P. Study of photon interactions and shielding properties of silicate glasses containing Bi2O3, BaO and PbO in the energy region of
1 keV to 100 GeV. Ann Nucl Energy 2012;41:119–24.https://doi.org/10.1016/j. anucene.2011.10.021.
[7] Saeed A, Elbashar YH, El Shazly RM. Optical properties of high density barium borate glass for gamma ray shielding applications. Opt Quantum Electron 2016;48:1–10.https://doi.org/10.1007/s11082-015-0274-3.
[8] Lakshminarayana G, Baki SO, Kaky KM, Sayyed MI, Tekin HO, Lira A, et al. Investigation of structural, thermal properties and shielding parameters for multi-component borate glasses for gamma and neutron radiation shielding applications. J Non Cryst Solids 2017.https://doi.org/10.1016/j.jnoncrysol.2017.06.001. [9] Sayyed MI, Lakshminarayana G, Mahdi MA. Evaluation of radiation shielding
parameters for optical materials. Chalcogenide Lett 2017;14:43–7.
[10] El-Mallawany R, Sayyed MI, Dong MG. Comparative shielding properties of some tellurite glasses: Part 2. J Non Cryst Solids 2017;474:16–23.https://doi.org/10. 1016/j.jnoncrysol.2017.08.011.
[11] Çelikbilek Ersundu M, Ersundu AE, Sayyed MI, Lakshminarayana G, Aydin S. Evaluation of physical, structural properties and shielding parameters for K2O–WO3–TeO2glasses for gamma ray shielding applications. J Alloys Compd
2017;714:278–86.https://doi.org/10.1016/j.jallcom.2017.04.223.
[12] Sayyed MI. Half value layer, mean free path and exposure buildup factor for tell-urite glasses with different oxide compositions. J Alloys Compd 2017;695:3191–7. https://doi.org/10.1016/j.jallcom.2016.11.318.
[13] Kaewkhao J, Limsuwan P. Mass attenuation coefficients and effective atomic numbers in phosphate glass containing Bi2O3, PbO and BaO at 662 keV. Nucl
Instruments Methods Phys Res Sect A Accel Spectrom Detect Assoc Equip 2010;619:295–7.https://doi.org/10.1016/j.nima.2009.11.033.
[14] El-bashir BO, Sayyed MI, Zaid MHM, Matori KA. Comprehensive study on physical, elastic and shielding properties of ternary BaO-Bi2O3-P2O5glasses as a potent
ra-diation shielding material. J Non Cryst Solids 2017.https://doi.org/10.1016/j. jnoncrysol.2017.04.031.
[15] Kharita MH, Jabra R, Yousef S, Samaan T. Shielding properties of lead and barium phosphate glasses. Radiat Phys Chem 2012;81:1568–71.https://doi.org/10.1016/j. radphyschem.2012.05.002.
[16] Singh S, Kumar A, Singh D, Thind KS, Mudahar GS. Barium-borate-flyash glasses: as
radiation shielding materials. Nucl Instrum Methods Phys Res Sect B Beam Interact with Mater Atoms 2008.https://doi.org/10.1016/j.nimb.2007.10.018. [17] Sayyed MI. Bismuth modified shielding properties of zinc boro-tellurite glasses. J
Alloys Compd 2016;688:111–7.https://doi.org/10.1016/j.jallcom.2016.07.153. [18] Dong MG, Sayyed MI, Lakshminarayana G, Çelikbilek Ersundu M, Ersundu AE,
Nayar P, et al. Investigation of gamma radiation shielding properties of lithium zinc bismuth borate glasses using XCOM program and MCNP5 code. J Non Cryst Solids 2017.https://doi.org/10.1016/j.jnoncrysol.2017.04.018.
[19] Ravi Kumar AV, Srinivasa Rao C, Murali Krishna G, Ravi Kumar V, Veeraiah N. Structural features of MoO3doped sodium sulpho borophosphate glasses by means
of spectroscopic and dielectric dispersion studies. J Mol Struct 2012.https://doi. org/10.1016/j.molstruc.2012.02.039.
[20] Rada M, Rada S, Pascuta P, Culea E. Structural properties of molybdenum-lead-borate glasses. Spectrochim Acta Part A Mol Biomol Spectrosc 2010.https://doi. org/10.1016/j.saa.2010.08.014.
[21] El Batal FH, Abo-Naf SM, Marzouk SY. Gamma ray interactions with MoO3-doped
lead phosphate glasses. Philos Mag 2011;91:341–56.https://doi.org/10.1080/ 14786435.2010.521491.
[22] Koudelka L, Kalenda P, Holubová J, Mošner P, Montagne L, Revel B. Structural study of BaO-MoO3-P2O5glasses by Raman and NMR spectroscopy. J Non Cryst
Solids 2017;476:114–21.https://doi.org/10.1016/j.jnoncrysol.2017.09.040. [23] Akman F, Durak R, Turhan MF, Kaçal MR. Studies on effective atomic numbers,
electron densities from mass attenuation coefficients near the K edge in some sa-marium compounds. Appl Radiat Isot 2015.https://doi.org/10.1016/j.apradiso. 2015.04.001.
[24] Kaur P, Singh D, Singh T. Heavy metal oxide glasses as gamma rays shielding material. Nucl Eng Des 2016;307:364–76.https://doi.org/10.1016/j.nucengdes. 2016.07.029.
[25] Gerward L, Guilbert N, Jensen KB, Levring H. WinXCom– A program for calculating X-ray attenuation coefficients. Radiat Phys Chem 2004.https://doi.org/10.1016/j. radphyschem.2004.04.040.
[26] Sayyed MI, Qashou SI, Khattari ZY. Radiation shielding competence of newly de-veloped TeO2-WO3glasses. J Alloys Compd 2017.https://doi.org/10.1016/j.
jallcom.2016.11.160.
[27] Akman F, Geçibesler IH, Sayyed MI, Tijani SA, Tufekci AR, Demirtas I. Determination of some useful radiation interaction parameters for waste foods. Nucl Eng Technol 2018.https://doi.org/10.1016/j.net.2018.05.007.
[28] Eke C, Agar O, Segebade C, Boztosun I. Attenuation properties of radiation shielding materials such as granite and marble againstγ-ray energies between 80 and 1350 keV. Radiochim Acta 2017.https://doi.org/10.1515/ract-2016-2690. [29] Agar O. Study on gamma ray shielding performance of concretes doped with natural
sepiolite mineral 2018.
[30] Agar O, Khattari ZY, Sayyed MI, Tekin HO, Al-Omari S, Maghrabi M, et al. Evaluation of the shielding parameters of alkaline earth based phosphate glasses using MCNPX code. Results Phys 2019;12:101–6.https://doi.org/10.1016/j.rinp. 2018.11.054.
[31] Sayyed MI, Dong MG, Tekin HO, Lakshminarayana G, Mahdi MA. Comparative investigations of gamma and neutron radiation shielding parameters for different borate and tellurite glass systems using WinXCom program and MCNPX code. Mater Chem Phys 2018;215:183–202.https://doi.org/10.1016/j.matchemphys.2018.04. 106.
[32] Gaikwad DK, Sayyed MI, Obaid SS, Issa SAM, Pawar,. Gamma ray shielding prop-erties of TeO2-ZnF2-As2O3-Sm2O3glasses. J Alloys Compd 2018:pp.https://doi.org/
10.1016/j.jallcom.2018.06.240.
[33] Sayyed MI, Tekin HO, Altunsoy EE, Obaid SS, Almatari M. Radiation shielding study of tellurite tungsten glasses with different antimony oxide as transparent shielding materials using MCNPX code. J Non Cryst Solids 2018.https://doi.org/10.1016/j. jnoncrysol.2018.06.022.
[34] Wieser ME, Coplen TB. Atomic weights of the elements 2009 (IUPAC technical report). Pure Appl Chem 2009;2010.https://doi.org/10.1351/PAC-REP-10-09-14. [35] Sayyed MI. Investigation of shielding parameters for smart polymers. Chin J Phys
2016.https://doi.org/10.1016/j.cjph.2016.05.002.
[36] SCHOTT.http://www.schott.com/advanced_optics/english/products/ opticalmaterials/ special-materials/radiation-shielding-glasses/index.html. [Accessed 03 September 2018]; 2018.
[37] Bashter II. Calculation of radiation attenuation coefficients for shielding concretes. Ann Nucl Energy 1997;24:1389–401.https://doi.org/10.1016/S0306-4549(97) 00003-0.