Durable remissions in
TCF3-HLF positive acute
lymphoblastic leukemia with blinatumomab and
stem cell transplantation
TCF3-HLF-positive leukemia represents a rare subtype
of childhood acute lymphoblastic leukemia (ALL), char-acterized by a high rate of treatment failure despite treat-ment intensification and allogeneic stem cell transplanta-tion (SCT). Given the high and homogeneous expression of CD19 on blast cells of this leukemia subtype, these patients may benefit from CD19-directed immunothera-py. Here, we report the experience on nine
TCF3-HLF-positive ALL patients, most of whom were
treated early in first consolidation with blinatumomab as a bridge to SCT between 2015 and 2018. Treatment with blinatumomab was generally well tolerated; reversible neurotoxicity was observed in two patients. All nine patients achieved molecular remission after blinatu-momab treatment; seven underwent SCT and for one patient SCT is planned. Median follow up after start of blinatumomab treatment was 342 days, and four patients remain in molecular remission after a follow up of 1317, 1292, 1245, and 342 days, respectively. Three patients died because of infectious complications not directly related to blinatumomab, because they occurred either after SCT or after emergence of a CD19-negative leukemia clone. In the light of these encouraging obser-vations, CD19-directed immunotherapy should be con-sidered early after induction chemotherapy in
TCF3-HLF-positive ALL children and patients’ outcome
monitored systematically by study groups.
The chromosomal translocation t(17;19), resulting in the oncogenic fusion transcription factor TCF3-HLF,1
defines a rare cytogenetic subtype of B-cell precursor (BCP) ALL occurring in children and young adults that is associated with a dismal outcome.2Major leukemia study
groups consider TCF3-HLF-positive ALL patients eligible for addition of experimental therapies in first line. Functional screening of patient-derived xenografts
revealed a dependence on BCL2 with promising response to a combination of venetoclax with vincristine and dex-amethasone,3
which motivated the inclusion of a stratum allowing for combination of venetoclax with standard ALL therapy in the setting of a pediatric phase I/II study
(clinicaltrials.gov identifier: 03236857).4
Moreover, given the strong homogeneous expression of CD19 in
TCF3-HLF-positive ALL, and the impressive responses to
CD19-directed immunotherapeutic approaches, these patients may benefit from CD19-directed immunothera-py.5
Blinatumomab is a bispecific T-cell engager (BiTE®) antibody simultaneously binding CD3-positive cytotoxic T cells and CD19-positive B cells, resulting in a T-cell mediated serial lysis of B cells. Based on promising clini-cal activity with effective responses in heavily pretreated patients (clinicaltrials.gov identifier: 01466179),6
blinatu-momab gained accelerated approval by the US Food and Drug Administration (FDA), the European Medicines Agency (EMA), and Swissmedic agencies for treatment of both children and adults with relapsed/refractory Philadelphia chromosome-negative BCP-ALL. Treatment with blinatumomab in adults with minimal residual dis-ease-positive (MRD+)-ALL, mostly used as a bridge to
SCT, resulted in complete molecular response with MRD negativity in 80% of patients.7,8Similarly, high rates of
molecular remission were confirmed in a larger clinical study enrolling adults patients in second or later morpho-logical complete remission (CR).9 Extensive safety and
efficacy data are available from pediatric patients includ-ed into a phase I/II study and an expandinclud-ed access study (RIALTO) at a recommended phase II dose of 15 µg/m2/day continuous infusion (clinicaltrials.gov identifier: 02187354).10 In this first study, treatment of pediatric
BCP-ALL patients with relapsed/refractory disease was initiated with high disease burden, given the end point requirements of the trial, and the fact that only 16 out of 62 patients for whom MRD data were available achieved molecular CR, with a 24-month Kaplan-Meier estimate for overall survival of 25%.11The toxicity profile of
blina-haematologica 2019; 104:e244
L
ETTERS TO THE
E
DITOR
Table 1. Clinical data of nine patients with TCF3-HLF-positive acute lymphoblastic leukemia treated with blinatumomab.
N.
Patients’ characteristics
First-line
Blinatumomab treatment
Age
Sex
WBC
Calcium
EMD
treatment Prednisone
Disease
Disease
Disease
SAEs
Molecular
(y)
(nL)
(mmol/L)
protocol
response
burden
status involvement
remission
3post
prior to
induction
blina
1 13 F 13.8 2.42 None AALL 1131 N/A FACS 27.5% RD BM Depressed LOC Yes
2 14 F 6.15 3.65 None AIEOP-BFM PPR < 10-3> 10-4 Relapse BM Bilateral hip
Yes osteonecrosis
3 8 M 14.49 2.57 CNS2b AIEOP-BFM PGR 10-2
MRD BM None Yes
4 7 F 5.26 3.2 CNS3 AIEOP-BFM PGR 6 x 10-4 MRD BM Convulsion Yes
CNS pleocytosis1
5 10 M 4.6 2.46 None FRALLE PGR 5 x 10-4 MRD BM
None Yes
6 3 F N/A N/A CNS2 AIEOP-BFM PGR Negative Relapse2 BM None Yes
7 8 F 11.3 N/A None AIEOP-BFM PGR 7.5 x 10-3 MRD BM None Yes
8 5 F 25.7 2.63 None UKALL N/A 3 x 10-5 MRD BM None
Yes
9 7 M 8.2 5.03 None AIEOP-BFM PGR 3.2 x 10-3 MRD
BM CRS Yes4
Blina: blinatumomab; WBC: white blood cell count; EMD: extramedullary disease; RD: refractory disease; BM: bone marrow; SAE: serious adverse events; y: years; LOC: loss of consciousness; CRS: cytokine release syndrome. 1Central nervous system (CNS) pleocytosis without disease involvement. 2Initial diagnosis was c-acute lymphoblastic leukemia (ALL), isolated relapse under maintenance TCF3-HLF positive. 3Molecular remission defined as per Online Supplementary Table S1.4One marker positive non-quantifiable; one marker negative after one cycle blinatumomab.
tumomab is well established, including cytokine release syndrome (CRS) and reversible neurological events, such as ataxia, seizures, and encephalopathy. The mechanisms leading to neurotoxicity remain unclear and may relate to variable expression of CD19 within the brain. Given the good tolerability and promising efficacy data, the poten-tial benefit of blinatumomab in improving outcome is being investigated in patients experiencing high-risk ALL first relapse (clinicaltrials.gov identifier: 02393859) and will be evaluated in first-line treatment in patients with inter-mediate and high-risk BCP-ALL by the Italian Association of Pediatric Hematology and Oncology-Berlin-Frankfurt-Muenster (AIEOP-BFM) study group (clinicaltrials.gov
identifier: 02393859).
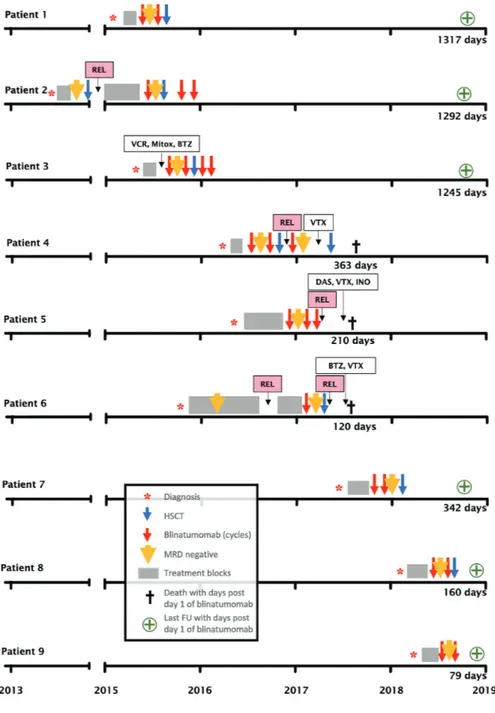
Here, we report the European experience with blinatu-momab using retrospectively collected data from nine patients with TCF3-HLF-positive ALL treated with blina-tumomab between 2015 and 2018 by members of the international BFM study group after approval by the competent ethics committee; two of these nine patients were enrolled in the expanded access RIALTO protocol into Relapsed/Refractory B-precursor ALL. An overview of patients' data and clinical courses is provided in Tables 1 and 2, and Figure 1.
Blinatumomab was initiated for disease refractory to induction chemotherapy (patient 1), for persistent MRD after consolidation chemotherapy (patients 3, 4, 5, 7, 8, 9), and after first relapse (patients 2, 6). Blinatumomab was administered at 15 µg/m2/day for 2-4 cycles. All nine
patients responded, obtaining molecular CR after either the first cycle (8 out of 9) or the second cycle (patient 7). Seven out of nine patients underwent allogeneic SCT
after blinatumomab treatment. Details on the type of donor are shown in Table 2. Four patients developed mild to moderate acute graft-versus-host disease (GvHD). No suitable donor could be found in time for patient 5, and this patient relapsed with CD19-negative leukemia blasts.
Four patients achieved a long-term remission and are still MRD negative after a follow up of 1317, 1292, 1245, and 342 days, respectively (Figure 1). Given the expected risk for subsequent relapse, additional therapy was pro-vided after SCT with one (patient 4) or two (patients 2 and 3) cycles of blinatumomab. Three patients died from infectious complications: patient 4 due to a transplant-related adenoviral infection while in second molecular remission after her second SCT, patient 5 from a fungal infection while in CD19-negative relapse, and patient 6 from transplant-related aspergillus infection while in molecular relapse. Significant neurotoxicity occurred in two patients (patients 1 and 4). For patient 1, blinatu-momab had to be interrupted due to a state of mental confusion and a decreased level of consciousness lasting 24 hours; it was resumed with a reduced dose of 5 µg/m2/day, and was finally stopped because of seizures.
This patient is currently still in molecular remission after SCT. Similar complications have previously been report-ed in preport-ediatric patients.12,13 Disseminated intravascular
coagulation was reported during treatment in two patients with overt leukemia, but was not considered to be treatment-related because TCF3-HLF-positive leukemia often presents with coagulopathies. Patient 9 developed grade 2 CRS lasting for 48 hours. No other sig-nificant toxicity was reported.
haematologica 2019; 104:e245
L
ETTERS TO THE
E
DITOR
Table 2.Definitive treatment of nine patients with blinatumomab and outcome.
N.
HSCT
Current
FU
status
time (d)
3Donor type
Conditioning
GvHD
GvHD
for HSCT
prophylaxis
1 Matched Bu, Thiotepa, Cy CsA Mild ACR 1317
Unrelated GvHD
2 Matched Bu, Cy, VP16, CsA Mild ACR 1292
Unrelated1 Thymoglobulin GvHD
3 Matched TBI, VP16, ATG CsA, MTX Moderate ACR 1245
Unrelated GvHD
4 Haploidentical2 Melphalan, Alemtuzumab, No Died from infection 363
Thiotepa, MM under remission
Fludarabine
5 No HSCT – – – Died from infection 210
under CD19-neg relapse
6 Matched TBI, VP16 CsA, MTX Moderate Relapse; died from 120
Related GvHD infection after HSCT
7 Haploidentical TBI, Thiotepa, None No ACR 342
Fludarabine
8 Matched TBI, Cy Alemtuzumab, CsA, MTX No ACR 160
Unrelated
9 No HSCT – – – ACR 79
FU: follow up; neg: negative; MM: mycophenolate mofetil; ACR: alive and in complete remission. 1Patient underwent prior transplantation after induction but relapsed shortly after.2Patient underwent prior transplantation after 2 cycles blinatumomab, but relapsed shortly after.3Follow up since first day of blinatumomab treatment. HSCT: hematopoietic stem cell transplantation; Bu: buprenorphin; ATG: Antithymocyte globulin; Bu: busulfan; Cy: cyclophosphamide; VP16: etoposide; TBI: total body irradiation CsA: Cyclosporine A; MTX: methotrexate; GvHD: Graft-versus-host disease.
This is the first report of clinical activity of CD19-directed immunotherapy in TCF3-HLF-positive ALL, a rare BCP-ALL subtype reported to be almost always fatal even with the most intensive conventional treatment reg-imens.2Although the number of patients treated is small
and follow-up time limited, these results provide a strong rationale for rapid intervention with immunotherapy for this ALL subtype. The response to blinatumomab has been reported to be less effective in patients with higher disease burden, suggesting that this approach may be more effective in the MRD setting.6 Current ongoing
ran-domized pediatric studies will hopefully provide more evidence to answer this question. Until then, we recom-mend using a different treatment modality to reduce the leukemia burden before initiating blinatumomab. A con-ventional cytoreduction will also be required if immunotherapy with autologous chimeric antigen recep-tor T (CAR-T) cell is considered, allowing lymphaphere-sis and CAR-T-cell manufacturing. Based on the current
evidence, blinatumomab appears to be a promising therapeutic element to improve the quality of remission in TCF3-HLF-positive BCP-ALL patients as a bridge to SCT. In contrast, CAR-T might provide an attractive stand-alone treatment for definitive therapy without SCT. In general, this approach will have to be validated in clinical studies. Given the strong preclinical evidence for sensitivity to the BCL2 inhibitor venetoclax in most cases, including relapsing patients, combination of vene-toclax with standard ALL chemotherapy may provide an additional treatment element to improve outcome in these patients. Patients could be evaluated by functional screening using drug response profiling3,14
and BH3 profil-ing15 at diagnosis. Three patients in this cohort have
received additional courses of blinatumomab post trans-plant for further consolidation of the leukemia remission, since even with MRD-negative remissions prior to and after allogeneic HSCT relevant rates of subsequent relapses are observed in TCF3-HLF-positive ALL.2
haematologica 2019; 104:e246
L
ETTERS TO THE
E
DITOR
Figure 1. Time course of nine patients with TCF3-HLF-positive acute lymphoblastic leukemia treated with blinatumomab. REL: relapse; VCR: vincristine; Mitox: mitoxantrone; BTZ: bortezomib; VTX: venetoclax; DAS: dasatinib; INO: inotuzumab; HSCT: hematopoietic stem cell transplan-tation; MRD: minimal residual dis-ease; FU: follow up.
Persisting clones in compartments other than bone mar-row may not be covered by MRD quantification but could still be responsive to blinatumomab therapy. The responses detected in this TCF3-HLF-positive ALL cohort are encouraging and suggest that the application of immunotherapy prior to extensive clonal selection sec-ondary to intensive chemotherapy may be beneficial. As data are updated, the true value of this approach can be assessed. The benefit of adding blinatumomab to front-line ALL chemotherapy will be addressed in an interna-tional prospective clinical trial (clinicaltrials.gov identifier:
03643276). Taken together, our results indicate that
immunotherapy may improve the outcome of
TCF3-HLF-positive ALL.
Brice Mouttet,1Luciana Vinti,2Philip Ancliff,3
Nicole Bodmer,1 Benoît Brethon,4Gunnar Cario,5
Christiane Chen-Santel,6Sarah Elitzur,7Volkan Hazar,8
Joachim Kunz,9Anja Möricke,5Jerry Stein,10Ajay Vora,3
Yöntem Yaman,8Martin Schrappe,5,11Sema Anak,8
André Baruchel,4Franco Locatelli,2Arend von Stackelberg,6
Martin Stanulla12 and Jean-Pierre Bourquin1
1Pediatric Oncology, University Children’s Hospital Zurich,
Switzerland; 2Department of Pediatric Haemato-Oncology, IRCCS
Ospedale Bambino Gesù, Sapienza University of Rome, Italy;
3Haematology and Oncology Department, Great Ormond Street
Hospital, London, UK; 4Department of Pediatric Hematology, Hôpital
Robert Debré, Assistance Publique – Hôpitaux de Paris, France;
5Department of Pediatrics I, ALL-BFM Study Group,
Christian-Albrechts University Kiel and University Medical Center Schleswig-Holstein, Kiel, Germany; 6Department of Pediatric
Oncology/Hematology, Charité Universitätsmedizin, Berlin, Germany;
7Pediatric Hematology-Oncology, Schneider Children’s Medical Center,
Sackler Faculty of Medicine, Tel Aviv University, Israel; 8Department
of Pediatric Hematology, Medipol University Hospital, Istanbul, Turkey; 9Pediatric Oncology, Hematology and Immunology, University
of Heidelberg, Germany; 10Bone Marrow Transplant Unit, Schneider
Children's Medical Center, Sackler Faculty of Medicine, Tel Aviv University, Israel; 11Pediatrics, Christian Albrechts University Kiel,
University Medical Center, Kiel, Germany and 12Pediatric Hematology
and Oncology, Hannover Medical School, Germany Correspondence: JEAN-PIERRE BOURQUIN. jean-pierre.bourquin@kispi.uzh.ch
doi:10.3324/haematol.2018.210104
Acknowledgments: the authors thank Yun Huang (University Children’s Hospital Zurich) for careful review of the manuscript and Renate Siegenthaler (University Children’s Hospital Zurich) to provide data.
Information on authorship, contributions, and financial & other disclo-sures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
1. Inaba T, Roberts WM, Shapiro LH, et al. Fusion of the leucine zipper gene HLF to the E2A gene in human acute B-lineage leukemia. Science. 1992;257(5069):531-534.
2. Minson KA, Prasad P, Vear S, et al. t(17;19) in Children with Acute Lymphocytic Leukemia: A Report of 3 Cases and a Review of the Literature. Case Rep Hematol. 2013;2013(4):563291-563294. 3. Fischer U, Forster M, Rinaldi A, et al. Genomics and drug profiling of
fatal TCF3-HLF-positive acute lymphoblastic leukemia identifies recurrent mutation patterns and therapeutic options. Nat Genet. 2015;47(9):1020-1029.
4. Place AE, Goldsmith K, Bourquin J-P, et al. Accelerating drug devel-opment in pediatric cancer: a novel Phase I study design of veneto-clax in relapsed/refractory malignancies. Future Oncol. 2018;14(21):2115-2129.
5. Velasquez MP, Bonifant CL, Gottschalk S. Redirecting T cells to hematological malignancies with bispecific antibodies. Blood. 2018;131(1):30-38.
6. Topp MS, Gökbuget N, Stein AS, et al. Safety and activity of blina-tumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16(1):57-66.
7. Topp MS, Kufer P, Gökbuget N, et al. Targeted Therapy With the T-Cell–Engaging Antibody Blinatumomab of Chemotherapy-Refractory Minimal Residual Disease in B-Lineage Acute Lymphoblastic Leukemia Patients Results in High Response Rate and Prolonged Leukemia-Free Survival. J Clin Oncol. 2011;29(18):2493-2498.
8. Topp MS, Gökbuget N, Zugmaier G, et al. Long-term follow-up of hematologic relapse-free survival in a phase 2 study of blinatu-momab in patients with MRD in B-lineage ALL. Blood. 2012;120(26):5185-5187.
9. Gökbuget N, Dombret H, Bonifacio M, et al. Blinatumomab for min-imal residual disease in adults with B-cell precursor acute lym-phoblastic leukemia. Blood. 2018;131(14):1522-1531.
10. Stackelberg von A, Locatelli F, Zugmaier G, et al. Phase 1/2 Study in Pediatric Patients with Relapsed/Refractory B-Cell Precursor Acute Lymphoblastic Leukemia (BCP-ALL) Receiving Blinatumomab Treatment. Blood. 2014;124(21):2292.
11. Gore L, Locatelli F, Zugmaier G, et al. Survival after blinatumomab treatment in pediatric patients with relapsed/refractory B-cell precur-sor acute lymphoblastic leukemia. Blood Cancer J. 2018;8(9):80. 12. Teachey DT, Rheingold SR, Maude SL, et al. Cytokine release
syn-drome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed ther-apy. Blood. 2013;121(26):5154-5157.
13. Stackelberg von A, Locatelli F, Zugmaier G, et al. Phase I/Phase II Study of Blinatumomab in Pediatric Patients With Relapsed/Refractory Acute Lymphoblastic Leukemia. J Clin Oncol 2016;34(36):4381-4389.
14. Frismantas V, Dobay MP, Rinaldi A, et al. Ex vivo drug response pro-filing detects recurrent sensitivity patterns in drug-resistant acute lymphoblastic leukemia. Blood. 2017;129(11):e26-e37.
15. Deng J, Carlson N, Takeyama K, Dal Cin P, Shipp M, Letai A. BH3 Profiling Identifies Three Distinct Classes of Apoptotic Blocks to Predict Response to ABT-737 and Conventional Chemotherapeutic Agents. Cancer Cell. 2007;12(2):171-185.
haematologica 2019; 104:e247