Comparison of
Angiotensin-Converting Enzyme,
Malonaldehyde, Zinc, and Copper
Levels in Preeclampsia
S
EREFDENA
ÇIKGOZ,
1M
UGEH
ARMA,
2M
EHMETH
ARMA,
2G
ORKEMM
UNGAN,
1M
URATC
AN,*
,1 ANDS
ELDAD
EMIRTAS31
Department of Bıochemıstry, Faculty of Medicine, Karaelmas
University, Zonguldak, Turkey;
2Department of Obstetrics
and Gynaecology, Faculty of Medicine, Harran University, Urfa,
Turkey; and
3Department of Biochemistry, Faculty of Medicine,
Ufuk University, Ankara, Turkey
Received December 12, 2005; Revised January 25, 2006; Accepted February 29, 2006
ABSTRACT
Preeclampsia is a syndrome of unknown etiopathogenesis. Recent studies carried out on preeclampsia have focused on the increase in free radicals in the feto-placental unit with poor perfusion. It is believed that the renin–angiotensin system (RAS) has a role in the poor perfusion of the placenta. It is uncertain whether there is a pre-existing impairment in RAS in pre-eclamptic pregnant women or not. In the present study, we meas-ured angiotensin-converting enzyme (ACE), malonaldehyde (MDA), zinc, and copper levels in the placental tissue of 16 pre-eclamptic pregnant women and compared them with those in 20 healthy pregnant women.
Whereas ACE activity and MDA were found to be high in the placen-tas of pre-eclamptic patients, zinc and copper levels were low and there was a negative correlation between ACE activity and zinc concentration. These findings suggest that high ACE activity might play a role in the increase in tissue hypoxia and consequent lipid peroxidation through vasoconstriction; zinc deficiency in the placental tissue might cause insufficiency of superox-ide dismutase, an antioxidant enzyme. Furthermore, deficiency in placental zinc also plays a role in the biosynthesis of connective tissue, maintaining its integrity, which might have an impact on the structure of the spiral arteries.
Index Entries: Preeclampsia; angiotensin-converting enzyme; mal-onaldehyde, zinc, copper.
0163-4984/(Online) 1559-0720/06/11301–0001 $30.00
INTRODUCTION
Preeclampsia is a clinical condition that can lead to maternal and fetal morbidity and mortality (1). The etiopathogenesis of preeclampsia has not been fully understood to date. One of the hypotheses proposes that utero-placental hypoperfusion causes modifications to nutrient molecules as well as oxygen balance (2,3). It is believed that in preeclampsia, the physiologi-cal remodeling of the uterine spiral arteries into dilated utero-placental ves-sels observed in normal pregnancies is disrupted (2,3). Indeed, medial hyperplasia and atherosis of the spiral arteries have been reported (3).
Angiotensin-converting enzyme (ACE) is released from the vascular endothelial cells as an ectoenzyme. It not only converts angiotensin I into angiotensin II, a vasoconstricting peptide, but also inactivates bradykinin, a vasodilating peptide present on the surface of endothelial cells of blood vessels (2,4). The feto-placental unit is the location of much of the conver-sion of angiotensin I into angiotensin II by ACE takes place (2).
Impairment of perfusion of the feto-placental unit is the cause of increase in free-oxygen radicals and therefore lipid peroxidation (5). Previ-ous studies have demonstrated that lipid peroxidation is increased (5–9) and superoxide dismutase (SOD) concentration is decreased (5–7,10,11) in pre-eclampsia. SOD is one of the enzymes that inactivate the free radicals found to be increased in the hypoperfused placenta. It contains zinc and copper.
Zinc is an essential element required for activating enzymes that con-tribute to antioxidant defense mechanisms, protein synthesis, and nucleic acid replication. It is also required for the integrity and biosynthesis of connective tissue (12). Pregnant women are at risk for zinc deficiency because there is high demand by the fetus for the element (12).
Various studies have explored ACE, malonaldehyde (MDA), zinc, and copper levels in placental tissue and blood, erythrocyte zinc, and leuko-cyte zinc and copper concentrations but have not evaluated the renin–angiotensin system (RAS), trace elements, and lipid peroxidation together in placental tissue. The objective of this study was to measure the levels of ACE, MDA, zinc, and copper in the placental tissue of pre-eclamptic pregnant women and to compare them with those in healthy pregnant women.
MATERIALS AND METHODS
The study population, treated in Harran University Hospital, com-prised 17 women with preeclampsia and 20 women with normotensive, uncomplicated pregnancies. ACE, MDA, zinc, and copper levels were measured at the Laboratory of the Department of Biochemistry of Zongul-dak Karaelmas University School of Medicine. The diagnosis of preeclamp-sia was established in accordance with the definition of the American College of Obstetricians and Gynecologists (13). Infants in both
pre-eclamp-tic and normal pregnancy groups were delivered by elective cesarean sec-tion and none of the women went through labor. Elective caesarean secsec-tions were performed in the control group as a result of cephalo-pelvic dispro-portion, repeated caesarean section, or breech presentation. Caesarean sec-tion was performed in women with preeclampsia because of deteriorasec-tion in fetal or maternal condition. None of the patients had pre-existing hyper-tensive disorders or renal, hepatic, or hematological diseases and they received no medication or vitamin supplementation before the samples were obtained. None of them were smokers. Informed written consent was obtained from all subjects. The healthy group of subjects showed no signs of pregnancy complication, and all gave birth to healthy infants.
Fresh placental tissues were dissected, selected, and stored at –80°C. Samples were transported in dry ice. One of the samples was excluded from the study. Samples were weighed using an analog scale, and a 10% homogenate was prepared for ACE, MDA, zinc, copper, and protein meas-urements. MDA was assayed in tissue homogenate; ACE, zinc, copper, and protein levels were assayed in the supernatant. Tissue ACE concentration is expressed in units per milligram of tissue protein, and tissue MDA, zinc, and copper levels expressed as gram of tissue were determined and evaluated.
Angiotensin-Converting Enzyme
The ACE activity was determined with an ACE diagnostic kit (Sigma Diagnostic Inc, St. Louis, MO). Measurements in this and other assays were carried out using a Shimadzu UV 1601 spectrophotometer (Shimadzu Co., Kyoto, Japan). An ACE calibrator (Sigma Diagnostics, Inc., St. Louis, MO, cat. no. 305-50) was used for calibration. Tissue ACE concentrations were expressed as per tissue protein.
Malonaldehyde Assay
The tissue MDA level was assessed according to the method described by Uchiyama and Mihara (14). Three milliliters of 1% phos-phoric acid and 1 mL of 0.6% thiobarbituric acid (TBA) aqueous solution were added to 0.5 mL of 10% homogenate. The mixture was heated for 45 min in a boiling water bath. After cooling, 4 mL of n-butanol was added and mixed vigorously. The butanol phase was separated by centrifugation and absorbance was measured at 535 and 520 nm on a Shimadzu UV 1601 spectrophotometer. The difference was used as the TBA value.
As a standard, 1,1,3,3-tetraetoxypropane was used. MDA concentra-tion was calculated per gram of tissue.
Zinc
The zinc level was determined using the Globe Diagnostic kit (Globe Diagnostic S.r.l., Milan, Italy). Zinc nitrate at a concentration of 200 µg/dL was used as standard. Tissue zinc concentration was expressed on a per tissue weight basis.
Copper
Copper levels were measured using the Globe Diagnostics kit (Globe Diagnostic S.r.l., Milan, Italy); 200 µg/dL copper sulfate was used as a standard. Tissue copper content was expressed per gram of tissue.
Tissue Protein Assay
Protein concentrations in the supernatant fraction was determined by the method of Lowry et al. (15). Folin–Ciocalteau reagent was obtained from Sigma Diagnostics. Absorbance was measured at 750 nm. Bovine albumin (Sigma Diagnostics) was used as a standard.
Statistical Analyses
The significance of differences between the pre-eclamptic and normal control groups was tested using Mann–Whitney U-test. Correlation analy-sis was carried out using Spearman correlation. The SPSS 11.0 (SPSS Inc, Chicago, IL, USA) statistical package was used for all data analyses.
RESULTS
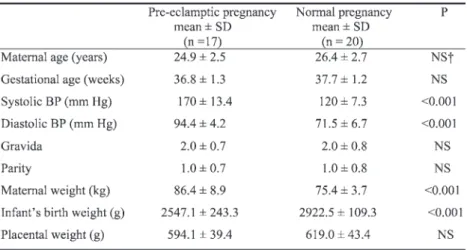
Table 1 summarizes the clinical characteristics of the patients. The sys-tolic and diassys-tolic blood pressures of the pre-eclamptic pregnant women were higher than those of healthy pregnant women (p < 0.001) and the infants of pre-eclamptic women had lower birth weight compared to those of normal women (p < 0.001). We also found that placental weights of
pre-Table 1
Demographic and Clinical Data of Subjects
eclamptic women were less than those of healthy pregnant women, although the difference was not significant.
The ACE and MDA levels of the placental tissue in pre-eclamptic patients were higher than in healthy pregnant women (p < 0.01 and p < 0.05, respectively). On the other hand, zinc and copper levels were lower than in the healthy group. Whereas the difference in zinc was statistically significant (p < 0.05), the difference in copper concentration was not (p > 0.05) (Table 2). There was a significant negative correlation between ACE and zinc (r = –0.678, p < 0.05).
DISCUSSION
The ACE is produced by the venous endothelial cells within the pla-cental stem villous tissues and the umbilicus. The placenta is one of the locations where ACE converts angiotensin I into angiotensin II (2). We found increased ACE activity in the placental tissues of pre-eclamptic women compared to that in the control group. This finding is consistent with that of Mitsuaki et al. (2). Our result also supports the argument that the RAS plays a role in preeclampsia (16) and that there is a disruption in the circadian rhythm of serum ACE activity (17).
There is a debate about why ACE is increased in preeclampsia and whether there is a genetic predisposing factor, and these questions are being explored (3,4,16,18–20). Pre-eclamptic patients included in our study did not have high blood pressure or renal, hepatic, or hematological dis-orders and were not under any medication or vitamin supplementation before they became pregnant.
It has been revealed in previous studies that increased ACE activity gives rise to increased production of angiotensin II and inhibition of bradykinin synthesis, resulting in vasospasm (2,18), and one should expect to observe tissue hypoxia and an increase in free radicals and lipid perox-idation following vasospasm. Previous studies demonstrated an increase in lipid peroxidation products (7–9) and a decrease in SOD activity (6,7) during normal pregnancy. Numerous studies have reported an increase in lipid peroxidation and a decrease in antioxidant capacity in preeclampsia compared to normal pregnancies (5–7,11). Another significant fact is that
Table 2
ACE, MDA, Zn, and Cu Concentrations in the Placentas of Pre-eclamptic and Normal Pregnant Women
antioxidant capacity in pre-eclamptic patients does not reach a magnitude sufficient to eliminate oxidative stress. The high lipid peroxidation in pla-cental tissue in preeclampsia observed in the present study (p < 0.05) lends support to these studies.
Several studies have disclosed changes in the levels of trace elements with pregnancy. Superoxide dismutase is an antioxidant enzyme that tains the trace elements zinc and copper. We found zinc and copper con-centrations in the placental tissues of pre-eclamptic women to be lower than those in healthy pregnant women (zinc, p < 0.05; copper, p > 0.05). Pregnant women are at risk for zinc deficiency resulting from consump-tion of zinc by the fetus (12).
Zinc deficiency in placental tissue found in our study is consistent with the studies of Brophy et al. (21), Adeniyi (22), and Diaz et al. (23). Ilhan et al.
(7) and Atamer et al. (6) found the zinc concentration in serum to be low as
well. Whereas Diaz et al. (23) and Harma et al. (24) found an elevated serum zinc concentration, Adeniyi (22), Ajayi (25), and Borella et al. (26) found a high plasma concentration. Mahomed and colleagues (27), on the other hand, reported an increase in leukocyte zinc. Adeniyi (22) reported decreased zinc in placental tissue but an increase in plasma zinc concentration.
We found a significant negative correlation between ACE activity and zinc concentration in the placental tissue (r = –0.678, p < 0.05). Tamura and colleagues (20) found no significant correlation between ACE activity and plasma zinc concentration among pregnant women with high blood pres-sure, regardless of their ACE genotype. Adeniyi (22) detected increased plasma and decreased placental zinc concentration. Therefore, placental zinc concentration is more important than plasma zinc concentration because zinc is required in the biosynthesis and maintenance of the integrity of connective tissue, and its deficiency in placental tissue might lead to a defect in the remodeling of the spiral arteries and atherosis. A negative correlation between zinc and ACE at the significance level of 0.05 suggests that alterations in the spiral artery structure might stimulate ACE synthesis from the vascular endothelium. The fact that pre-eclamptic patients did not have previous high blood pressure or renal, hepatic, or hematological disorders and were not on any medication or vitamin sup-plementation also supports this notion.
As a result, ACE activity is affected by zinc deficiency, and the increase in ACE in placental tissue in preeclampsia suggests that the RAS plays a role in preeclampsia and tissue hypoxia and, consequently, in lipid peroxidation.
REFERENCES
1. F. B. Pipkin and P. C. Rubin, Preeclampsia: the disease of theories, Br. Med. Bull. 50,
381–396 (1994).
2. I. Mitsuaki, A. Itakura, Y. Ohno, et al., Possible activation of the Renin–angiotensin system
3. T. Morgan, C. Craven, and K. Ward, Human spiral artery renin–angiotensin system, Hypertension 32, 683–687 (1998).
4. F. Gurdol, E. Isbilen, H. Yilmaz, T. Isbir, and A. Dirican, The association between
preeclampsia and angiotensin-converting enzyme insertion/deletion polymorphism,
Clin. Chim. Acta 341, 127–131 (2004).
5. S. Aydin, A. Benian, R. Madazli, S. Uludag, H. Uzun, and S. Kaya, Plasma
malondialde-hyde, superoxide dismutase, sE-selectin, fibronectin, endothelin 1 and nitric oxide lev-els in women with preeclampsia, Eur. J. Obstet. Gynecol. Reprod. Biol. 113, 21–25 (2004).
6. Y. Atamer, Y. Kocyigit, B. Yokus, A. Atamer, and A. C. Erden, Lipid peroxidation,
antioxidant defense, status of trace metals and leptin levels in preeclampsia, Eur. J.
Obstet. Gynecol. Reprod. Biol. 119, 60–66 (2005).
7. N. Ilhan, N. Ilhan, and M. Simsek, The changes of trace elements, malondialdehyde
lev-els and superoxide dismutase activities in pregnancy with or without preeclampsia,
Clin. Biochem. 35, 393–397 (2002).
8. S. Kharb, Lipid peroxidation in pregnancy with preeclampsia and diabetes, Gynecol. Obstet. Invest. 50, 113–116 (2000).
9. J. J. Wu, Lipid peroxidation in preeclamptic and eclamptic pregnancies, Eur. J. Obstet. Gynecol. 64, 51–54 (1996).
10. J. E. Vaughan and S. W. Walsh, Oxidative stress reproduces placental abnormalities of
preeclampsia, Hypertens. Pregn. 21, 205–223 (2002).
11. Y. Wang and S. W. Wals, Antioxidant activities and mRNA expression of superoxide
dismutase, catalase and glutathione peroxidase in normal and preeclamptic placentas,
J. Soc. Gynecol. Invest. 3, 179–184 (1996).
12. C. A. Burtis and E. R. Ashwood, Tietz Textbook of Clinical Chemistry, 3rd ed., W. B,
Saun-ders Company, Philadelphia, (1999).
13. ACOG, Hypertension in pregnancy. Committee on Technical Bulletins of the American
College of Obstetricians and Gynecologists, Int. J. Gynaecol. Obstet. 53, 175–183 (1996).
14. M. Uchiyama and M. Mihara, Determination of malonaldehyde precursor in tissues by
thiobarbituric acid test, Anal. Biochem. 86, 271–278 (1978).
15. O. H. Lowry, N. J. Rosebrough, A. L. Farr, and R. J. Randall, Protein estimation with the
Folin–Ciocalteau reagent, J. Biol. Chem. 193, 265 (1951).
16. H. Choi, J. Y. Kang, H. S. Yoon, et al., Association of angiotensin-converting enzyme
and angiotensinogen gene polymorphisms with preeclampsia, J. Kor. Med. Sci., 19, 253–257 (2004).
17. P. Cugini, C. Letizia, L. D. Palma, et al., Serum angiotensin-converting enzyme activity
in pre-eclamptic pregnancy: evidence for a relative hypermesor ACEemia, Enzyme 43, 113–121 (1990).
18. E. Isbilen, Y. Unlucerci, F. Gurdol, et al., The association of preeclampsia with
angiotensin-converting enzyme I7I gene polymorphism and hyperhomocystinemia,
Turk. Klin. Biyokim. Derg. 1, 27–32 (2003).
19. I. Bouba, G. Makrydimas, R. Kalaitzidis, D. E. Lolis, K. C. Siamopoulos, and I.
Geor-giou, Interaction between the polymorphism of the renin–angiotensin system in preeclampsia, Eur. J. Obstet. Gynecol. 110, 8–11 (2003).
20. T. Tamura, G. L. Johanning, R. L. Goldenberg, K. E. Johnston, and M. B. DuBard, Effect
of angiotensin-converting enzyme gene polymorphism on pregnancy outcome, enzyme activity and zinc concentration, Obstet. Gynecol. 88, 497–502 (1996).
21. M. H. Brophy, N. F. Harris, and I. L. Crawford, Elevated copper and lowered zinc in the
placentae of pre-eclamptics, Clin. Chim. Acta 145, 107–112 (1985).
22. F. A. Adeniyi, The implications of hypozincemia in pregnancy, Acta Obstet. Gynecol. Scand. 66, 579–581 (1987).
23. E. Diaz, A. Halhali, C. Luna, L. Diaz, E. Avila, and F. Larrea, Newborn birth weight
cor-relates with placental zinc, umbilical insulin-like growth factor I, and leptin levels in preeclampsia, Arch. Med. Res. 33, 40–47 (2002).
24. M. Harma, M. Harma, and A. Kocyigit, Correlation between maternal plasma
homo-cysteine and zinc levels in preeclamptic women, Biol. Trace Element Res. 104, 97–106 (2005).
25. G. Ajayi, Concentrations of calcium, magnesium, copper, zinc and iron during normal
and EPH-gestosis pregnancy, Trace Elements Med. 10, 151–152 (1993).
26. P. Borella, A. Szilagy, G. Than, I. Csaba, A. Giardiano, and F. Faccinetti, Maternal
plasma concentrations of magnesium, calcium, zinc and copper in normal and patho-logical pregnancies, Sci. Total Environ. 99, 67–76 (1990).
27. K. Mahomed, M. A. Williams, G. B. Woelk, et al., Leukocyte selenium, zinc, and copper
concentrations in preeclamptic and normotensive pregnant women, Biol. Trace Element