35 (2011) 425-432 © TÜBİTAK
doi:10.3906/biy-1001-22
Changes in the hemolymph total protein of Galleria mellonella
(Lepidoptera: Pyralidae) aft er parasitism and envenomation by
Pimpla turionellae (Hymenoptera: Ichneumonidae)
Olga SAK1, Ekrem ERGİN2, Fevzi UÇKAN3, David B. RIVERS4, Aylin ER1 1
Department of Biology, Faculty of Science and Literature, Balıkesir University, 10145 Balıkesir - TURKEY 2
Nursing School, Gülhane Military Medical Academy, 06018 Ankara - TURKEY 3
Department of Biology, Faculty of Science and Literature, Kocaeli University, 41300 İzmit, Kocaeli - TURKEY 4
Department of Biology, Loyola University Maryland, Baltimore, MD 21210 - USA
Received: 20.01.2010
Abstract: Venom from the endoparasitoid Pimpla turionellae L. (Hymenoptera: Ichneumonidae) contains a mixture
of biologically active components, which display potent paralytic, cytotoxic, and cytolytic eff ects towards hosts. Here, we further investigate if parasitism or envenomation by P. turionellae alters total protein of its host Galleria mellonella L. (Lepidoptera: Pyralidae). Various venom concentrations representing doses previously determined to yield host responses yet fall below the calculated LD99 were used for pupae and larvae. Parasitization was only assayed for host pupa since P. turionellae females normally parasitize host prepupae and pupae in nature. Hemolymph total protein concentration remained relatively steady at all doses and at all time points tested in parasitized and venom-injected host pupae and larvae. Th e only exception to this trend was with the highest dose of venom (0.5 VRE) at 24 h for larvae that almost 2 times higher amount of protein were detected with regard to untreated ones. It is likely that the increase in protein concentration in a non-permissive host stage in the present study was induced by venom and/or general injury because the same trend was also observed in null- and PBS-injected larvae. However, neither of the treatments increased the protein concentration of G. mellonella larvae to the same extent that 0.5 VRE injection did, indicating that the increase observed in the latter treatment was not simply the result of wounding or injection of fl uid. Th us, we favor the possibility that stress proteins may play a role in this event.
Key words: Wasp venom, parasitism, hemolymph, total protein
Pimpla turionellae (Hymenoptera: Ichneumonidae) parazitlemesi ve zehir
enjeksiyonu sonrası konak Galleria mellonella (Lepidoptera: Pyralidae) hemolenf
toplam proteinindeki değişiklikler
Özet: Endoparazitoid Pimpla turionellae L. (Hymenoptera: Ichneumonidae) zehiri konak türü üzerinde felç edici,
sitotoksik ve sitolitik etkiler gösteren biyolojik olarak aktif bileşenlerin karışımıdır. Bu çalışmada, P. turionellae dişilerinden elde edilen zehir salgısının ve doğal parazitlemenin konak tür, Galleria mellonella L. (Lepidoptera: Pyralidae) hemolenfi toplam protein miktarına etkileri belirlendi. Konak pupa ve larvaları için daha önce tepki verdikleri hesaplanan LD99 dozu altındaki farklı zehir dozları kullanıldı. P. turionellae dişileri doğada sadece konak prepupa ve
Introduction
Insect parasitoids are highly effi cient at manipulating the physiology, metabolism, and endocrinological state of their hosts. Host conditioning can result from injection of factors of maternal origin derived from ovarian secretions (1-4) or venom glands (5-9), and/or rely on cells and fl uids released from eggs and developing parasitoid progeny (10-12). Endoparasitic koinobiont parasitoids regulate the nutritional and physiological states of their hosts to ensure the successful development of eggs and larvae (13-15). Th e precise mechanisms used to alter the hosts have not been fully revealed, but for many koinobiont species, venom and viruses (e.g., polydnaviruses, entomopox virus) are used alone or synergistically to alter the nutritional content of the host (13,15).
Parasitism-mediated manipulation of the host nutritional condition is frequently manifested through changes in the hemolymph content of the host. More specifi cally, host plasma commonly displays quantitative and qualitative changes in protein and amino acid profi les when endoparasitic wasps parasitize their insect hosts (16,17). In several instances, the eff ects of parasitism and venom on the host hemolymph protein profi le are species-specifi c. For example, the concentration of several host hemolymph proteins decreased when Hyposoter exiguae (Viereck) (Hymenoptera: Ichneumonidae) parasitized larvae of the cabbage looper, Trichoplusia ni (Hübner) (Lepidoptera: Noctuidae) (18,19). Similarly, parasitism of larvae of Pieris rapae (L.) (Lepidoptera: Pieridae) by Apanteles glomeratus L. (Hymenoptera: Braconidae) (20) resulted in a decrease in the concentration of host hemolymph storage proteins. When Cotesia (= Apanteles)
congregata (Say) (Hymenoptera: Braconidae)
parasitizes Manduca sexta (L.) (Lepidoptera: Sphingidae), expression of several hemolymph proteins is dramatically altered, including a decrease in the storage protein arylphorin (21). By contrast, arylphorin levels increased in T. ni parasitized by Chelonus sp. (Hymenoptera: Braconidae) (22,23). Parasitism of Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) by C. marginiventris (Cresson) (Hymenoptera: Braconidae) also caused elevations in hemolymph proteins as evidenced by the early production of several high molecular weight proteins (24). In the C. kariyai Watanabe (Hymenoptera: Braconidae) – Pseudaletia separata Walker (Lepidoptera: Noctuidae) system, the protein concentration in the hemolymph of larvae injected with calyx fl uid and venom increased, yet in parasitized hosts, protein levels dropped (25). Th e free amino acid profi le of Lymantria dispar L. (Lepidoptera: Lymantriidae) larvae parasitized by Glyptapanteles liparidis Bouche (Hymenoptera: Braconidae) did not change qualitatively; however, levels of some single amino acids were reduced and those of others were elevated (26). Th ompson and Lee (1993) (27) reported no eff ect on amino acid concentration in M. sexta parasitized by C. congregata. It is clear from these observations that, though the wasps induce an array of changes in the host hemolymph content, the alterations in host condition depend on multiple factors being injected or secreted into the host.
Venoms from these koinobiont species are frequently associated with temporary paralysis, and parasitized hosts continue to grow and develop even aft er parasitization (28). By contrast, most idiobiont parasitoids paralyze their hosts permanently, and
pupalarını parazitlediğinden doğal parazitleme sadece konak pupalarında çalışıldı. Hemolenf toplam protein miktarı parazitlenen ve zehir enjekte edilen tüm konak pupa ve larvalarında tüm dozlarda ve tüm zamanlarda fazla değişiklik göstermedi. Sadece, larvalarda 24 saat sonunda en yüksek zehir dozunda (0,5 kese eşdeğeri zehir) hiçbir işleme tabi tutulmayan kontrol grubundakilere oranla protein miktarında iki kata varan bir artış görüldü. Protein miktarındaki bu artış eğilimi boş enjeksiyon ve sadece fi zyolojik su enjekte edilen larvalarda da görüldüğünden hedef konak evresi olmayan larva evresinde bu artışın zehir ve/veya yaralanmadan kaynaklanmış olabileceği düşünülmektedir. Ancak, diğer hiçbir uygulamada larva protein miktarının 0,5 kese eşdeğeri zehirdekine benzer derecede artış göstermemesi bu artışın sadece yaralanma ve/veya zehir enjeksiyonundan kaynaklanmadığını göstermektedir. Protein artışına konağa enjeksiyon sonucu salgılanan stres proteinlerinin yol açabileceği olasılığı değerlendirilmektedir.
thus preserve the hosts while the parasitoid progeny feed and develop (29). Such diff erences in the action of venoms from koinobiont and idiobiont wasps argue that changes in the nutritional content of the hosts (i.e. hemolymph proteins and amino acids) are more likely to be associated with hosts that continue to feed and grow during parasitism, and not when the host is paralyzed. Despite this prediction, almost nothing is known about the role of idiobiont endoparasitoid venoms in altering the hemolymph profi le of proteins of their hosts. In this study, the solitary idiobiont pupal endoparasitoid Pimpla turionellae L. (Hymenoptera: Ichneumonidae) was used to examine the impact of venom on the host nutritional condition. Changes in the hemolymph total protein were examined in pupae and larvae of Galleria mellonella L. (Lepidoptera: Pyralidae) following parasitism and envenomation.
Materials and methods Parasitoid and host rearing
Pimpla turionellae were reared on pupae (1- or 2-day-old) of G. mellonella at 25 ± 1 °C, 60 ± 5% RH, and with a photoperiod of 12:12 h, L:D. Adult parasitoids were fed a 30% (v/v) honey solution and provided with host pupae (4 pupae for every 10 female wasps once every 3 days). Host colony was maintained by feeding the insects with natural blackened comb (30) to maintain similarity to their natural media in bee hives.
Preparation of P. turionellae venom and injection into G. mellonella
Venom reservoir contents were isolated from honey- and host-fed 15- to 20-day-old females as described (31). Following centrifugation (3000 ×g for 10 min at 25 ± 1 °C) to remove cell debris, fi nal venom concentrations were adjusted to 0.05, 0.02, 0.01, and 0.005 venom reservoir equivalents (VREs) for pupae and 0.5, 0.1, 0.05, and 0.02 VREs for larvae with PBS (0.138 M NaCl and 0.0027 M KCl in 0.01 M PBS, pH 7.4). Th ese venom concentrations represent doses previously determined to yield host responses yet fall below the calculated LD99 for pupae and larvae (32), respectively. A 5 μL solution of the venom preparation was injected between the last 2 lateral abdominal segments of 1- to 2-day-old
host pupae (140 ± 20 mg) and on the fi rst hind leg of last instar larvae (260 ± 10 mg) by using a 10 μL Hamilton microsyringe (Hamilton, Reno, NV, USA). Vaseline was applied to the injection area to prevent hemolymph loss. Controls consisted of pupae and larvae untreated, null-injected, and those injected with only 5 μL of PBS.
Parasitization of G. mellonella pupae
Parasitization was performed on 1- or 2-day-old host pupae by exposing an individual host pupa (140 ± 20 mg) to an individual 15- to 20-day-old wasp female. Parasitized pupae were held at 25 ± 2 °C, 60 ± 5% RH under a photoperiod of 12:12 h LD, as were the controls and venom-treated pupae, until hemolymph collection. P. turionellae females normally parasitize host prepupae and pupae in nature (33); therefore, parasitization was not used as an experimental assay for larvae of G. mellonella.
Hemolymph collection and total protein determination
Hemolymph collection was performed at 4-, 8-, and 24-h post-treatments from venom-injected, parasitized and control host pupae and larvae. Pupae were bled by piercing the cuticle at the abdomen and larvae on the fi rst hind leg with a sterile 19-gauge needle. Five microliters of hemolymph from each individual pupa and larva were collected at each time period and for each treatment with a glass microcapillary tube (Sigma Chemical Co., St. Louis, MO, USA) and ejected into an ice cold Eppendorf tube containing 1 mg of phenylthiourea (Sigma Chemical) to prevent melanization (34). Th e hemolymph was spun at 3000 rpm for 10 min at 4 °C to remove hemocytes. Th e supernatant was transferred to a clean Eppendorf tube and vortexed with a pipette. Th ree microliters of hemolymph suspension was used for total protein analysis and the remaining sample was kept at –20 °C for further analyses. Th e resultant supernatant containing plasma was diluted 1:500 with distilled water. Total protein concentration in hemolymph was measured according to the Lowry method (35) using an UNICAM Heλios-α spectrophotometer (Cambridge, UK) at 750 nm wavelength. A standard curve was prepared by using bovine serum albumin (BSA, Merck). Protein determinations were repeated 3 times for each experimental and control group.
Statistical analysis
Means were compared using one- or two-way analysis of variance (ANOVA) and subsequently, means were separated using Tukey’s Honestly Signifi cant Diff erence (HSD) post hoc test. SPSS (version 15.0 for Windows, SPSS Science, Chicago, IL, USA) was used for data analysis. Results were considered statistically signifi cant when P < 0.05.
Results and discussion
Eff ects of parasitization and venom injection on the protein concentration of pupae
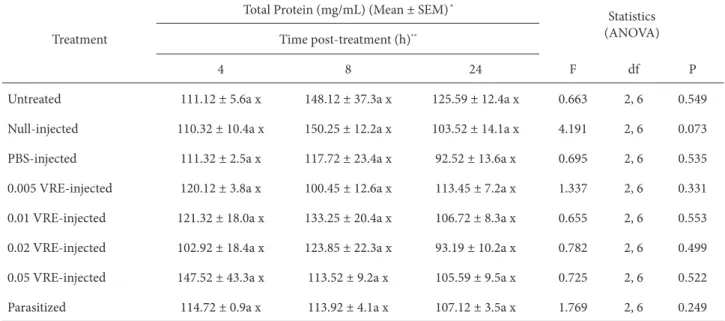
Total protein concentration of G. mellonella pupae did not diff er signifi cantly among treatments at 4 (F = 0.539; df = 7, 16; P = 0.793), 8 (F = 0.754; df = 7, 16; P = 0.632), and 24 (F = 1.049; df = 7, 16; P = 0.437) h post-treatments (Table 1). Similarly, hemolymph total protein concentration remained relatively steady at all time points tested in parasitized and venom-injected host pupae, regardless of the venom concentration injected into G. mellonella (Table 1). Analyses using two-way ANOVA indicated that the
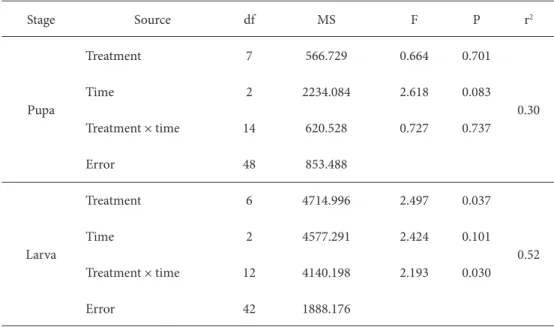
eff ect of venom injection and parasitization on the total protein concentration of host pupae was not treatment (P = 0.701) and time (P = 0.083) dependent, and the relationship between treatment and the total protein concentration was not infl uenced by time (P = 0.737) (Table 2).
Eff ects of venom injection on the protein concentration of larvae
Total protein concentration of G. mellonella larvae did not diff er signifi cantly among treatments at 4 (F = 1.096; df = 6, 14; P = 0.411) and 8 (F = 1.744; df = 6, 14; P = 0.183) h post-treatments, but was diff erent at 24 (F = 2.883; df = 6, 14; P = 0.05) h (Table 3). Hemolymph total protein concentration remained relatively steady at all time points tested in venom-injected host larvae, regardless of the venom concentration injected into G. mellonella except for 0.5 VRE at 24 h (Table 3). Analyses using two-way ANOVA indicated that the eff ect of venom injection on the total protein concentration of host larvae was treatment (P = 0.037) but not time (P = 0.101) dependent, and the relationship between treatment and the total protein concentration was infl uenced by time (P = 0.030) (Table 2).
Table 1. Hemolymph total protein concentration (mg/mL) of G. mellonella pupae experimentally envenomated and parasitized by P.
turionellae.
Treatment
Total Protein (mg/mL) (Mean ± SEM) *
Statistics (ANOVA) Time post-treatment (h)**
4 8 24 F df P
Untreated 111.12 ± 5.6a x 148.12 ± 37.3a x 125.59 ± 12.4a x 0.663 2, 6 0.549 Null-injected 110.32 ± 10.4a x 150.25 ± 12.2a x 103.52 ± 14.1a x 4.191 2, 6 0.073 PBS-injected 111.32 ± 2.5a x 117.72 ± 23.4a x 92.52 ± 13.6a x 0.695 2, 6 0.535 0.005 VRE-injected 120.12 ± 3.8a x 100.45 ± 12.6a x 113.45 ± 7.2a x 1.337 2, 6 0.331 0.01 VRE-injected 121.32 ± 18.0a x 133.25 ± 20.4a x 106.72 ± 8.3a x 0.655 2, 6 0.553 0.02 VRE-injected 102.92 ± 18.4a x 123.85 ± 22.3a x 93.19 ± 10.2a x 0.782 2, 6 0.499 0.05 VRE-injected 147.52 ± 43.3a x 113.52 ± 9.2a x 105.59 ± 9.5a x 0.725 2, 6 0.522 Parasitized 114.72 ± 0.9a x 113.92 ± 4.1a x 107.12 ± 3.5a x 1.769 2, 6 0.249
* Each represents the mean and standard error of mean of 3 replicates with 25 μL hemolymph obtained from 5 individuals (140 ± 20 mg). ** Numbers in rows (a) and columns (x) followed by the same letter are not signifi cantly diff erent (P > 0.05).
Table 3. Hemolymph total protein concentration (mg/mL) of G. mellonella larvae experimentally envenomated by P. turionellae. Treatment Total Protein (mg/mL) (Mean ± SEM) * Statistics (ANOVA) Time post-treatment (h)** 4 8 24 F df P
Untreated 225.2 ± 19.8a x 187.3 ± 31.7a x 155.1 ± 11.3a xy 2.417 2, 6 0.170
Null-injected 205.9 ± 36.0a x 172.6 ± 17.1a x 202.9 ± 29.8a xy 0.411 2, 6 0.680
PBS-injected 176.1 ± 9.3a x 229.4 ± 17.1a x 199.7 ± 21.7a xy 2.508 2, 6 0.162
0.02 VRE-injected 203.1 ± 5.9a x 203.6 ± 2.0a x 207.3 ± 31.0a xy 0.016 2, 6 0.984
0.05 VRE-injected 207.8 ± 14.4a x 213.9 ± 10.8a x 265.3 ± 37.4a xy 1.741 2, 6 0.253
0.1 VRE-injected 226.9 ± 20.1a x 227.6 ± 17.5a x 257.4 ± 44.1a xy 0.351 2, 6 0.717
0.5 VRE-injected 173.1 ± 21.0a x 239.6 ± 18.6ab x 330.1 ± 47.0b y 6.215 2, 6 0.035
* Each represents the mean and standard error of mean of 3 replicates with 25 μL hemolymph obtained from 5 individuals (260 ± 10 mg).
** Numbers in rows (a-b) and columns (x-y) followed by the same letter are not signifi cantly diff erent (P > 0.05). Table 2. ANOVAs of the eff ects of diff erent treatments, time, and their interactions on the hemolymph
protein concentration of G. mellonella pupae and larvae.
Stage Source df MS F P r2 Pupa Treatment 7 566.729 0.664 0.701 0.30 Time 2 2234.084 2.618 0.083 Treatment × time 14 620.528 0.727 0.737 Error 48 853.488 Larva Treatment 6 4714.996 2.497 0.037 0.52 Time 2 4577.291 2.424 0.101 Treatment × time 12 4140.198 2.193 0.030 Error 42 1888.176
Parasitism- and venom-related changes in hemolymph protein profi les and total or specifi c protein levels have been detected in numerous parasitoid-host model systems. Not surprisingly, the impact of the parasitoids on the host hemolymph has been variable, and in many cases, the hemolymph protein changes seem to be part of the host conditioning necessary for the parasitoid’s larvae to successfully complete development (2,5,25,26). Altering the host nutritional condition for the benefi t of wasp off spring is generally thought to be most common for koinobionts, and would presumably not be expected for a solitary idiobiont like P. turionellae (36). Consistent with this prediction are the observations in this study that protein concentrations of hemolymph from G. mellonella pupae did not diff er among controls, parasitized, or those injected with isolated venom.
However, the present result contradicts an earlier study (37) describing a dramatic decrease in the total protein concentration of G. mellonella pupae parasitized by P. turionellae at 1 and 6 h, and a signifi cant decrease at 12 and 36 h. On the other hand, the authors reported considerable increases at 48 and 72 h post-parasitism, whereas no signifi cant changes were observed at 3, 24, and 60 h between total protein levels of parasitized and unparasitized hosts (37). We do not expect diff erences in protein quantity since idiobiont parasitoids like P. turionellae usually paralyze their host, inhibiting continued host growth and lacking the ability to regulate host metabolism. Venom from this idiobiotic wasp contains several mid to high range molecular weight proteins, as well as noradrenalin, apamin, and melittin (31), phospholipase B, histamine, and serotonin (38). Th e last of these are also consistent with the apparent nonspecifi c paralytic action of the venom (39).
An alternative explanation for the absence of variation in the hemolymph protein concentration may be associated with the host stage being attacked since the pupae cannot feed and represent a closed nutritional container for the parasitoid progeny. On the other hand, protein concentration of hemolymph from G. mellonella larvae showed an extensive increase at all venom doses and was considerably higher at the end of 24 h at the highest dose of 0.5 VRE with respect to untreated larvae. Previous reports have
also documented the production of storage proteins and an increase of the titer of those storage proteins in the hemolymph of parasitized lepidopteran larvae (25,40,41). Th ese types of host alterations are perhaps more likely to occur when host larvae are attacked by ectoparasitoids that rely entirely on venom to alter the development of their insect hosts and cause an arrestment of the larval-larval molting process in the host (42). It would seem, however, that P. turionellae would be predicted to induce similar host changes, unless those factors other than venom also participate in host conditioning, or the venom operates by a diff erent mode of action than these ectoparasitic species. Considering that the composition of venom from P. turionellae appears to be unique from that reported for other parasitic wasps (31,43,44), the latter scenario is a likely explanation for the diff erential venom eff ects on host hemolymph protein content.
It is likely that the increase in protein concentration in a non-permissive host stage in the present study was induced by venom and/or general injury since the same trend was also observed in null- and PBS-injected larvae. However, neither of the treatments increased the protein concentration of G. mellonella larvae to the same extent that 0.5 VRE injection did, indicating that the increase observed in the latter treatment was not simply the result of wounding or injection of fl uid. Although, at present, there are insuffi cient data to determine which of these scenarios is correct, we favor the possibility that stress proteins may play a role in this event.
Acknowledgements
Th is research was supported by a grant (2006-106T255) from the Scientifi c and Technological Research Council of Turkey (TÜBİTAK).
Corresponding author:
Fevzi UÇKAN Kocaeli University,
Faculty of Science and Literature, Department of Biology,
41300 Umuttepe, İzmit, Kocaeli - TURKEY E-mail: fevzi.uckan@kocaeli.edu.tr
1. Schepers EJ, Dahlman DL, Zhang D. Microplitis croceipes teratocytes: in vitro culture and biological activity of teratocyte secreted protein. J Insect Physiol 44: 767-777, 1998.
2. Bae S, Kim Y. Host physiological changes due to parasitism
of a braconid wasp, Cotesia plutellae, on diamondback moth, Plutella xylostella. Comp Biochem Physiol A 138: 39-44, 2004.
3. Kaeslin M, Pfi ster-Wilhelm R, Molina D et al. Changes in
the haemolymph proteome of Spodoptera littoralis induced by the parasitoid Chelonus inanitus or its polydnavirus and physiological implications. J Insect Physiol. 51: 975-988, 2005.
4. Li Y, Lu J, Feng C et al. Role of venom and ovarian proteins
in immune suppression of Ostrinia furnacalis (Lepidoptera: Pyralidae) larvae parasitized by Macrocentrus cingulum (Hymenoptera: Braconidae), a polyembryonic parasitoid. Insect Sci 14: 93-100, 2007.
5. Nakamatsu Y, Tanaka T. Venom of ectoparasitoid, Euplectrus
sp. near plathypenae (Hymenoptera: Eulophidae) regulates the physiological state of Pseudaletia separata (Lepidoptera: Noctuidae) host as a food resource. J Insect Physiol 49: 149-159, 2003.
6. Rivers DB, Zdarek J, Denlinger DL. Disruption of pupariation
and eclosion behavior in the fl esh fl y, Sarcophaga bullata Parker (Diptera: Sarcophagidae), by venom from the ectoparasitic wasp Nasonia vitripennis (Walker) (Hymenoptera: Pteromalidae). Arch Insect Biochem Physiol 57: 78-91, 2004.
7. Suzuki M, Tanaka T. Virus-like particles in venom of Meteorus
pulchricornis induce host hemocyte apoptosis. J Insect Physiol 52: 602-613, 2006.
8. Asgari S. Venom proteins from polydnavirus-producing
endoparasitoids: Th eir role in host-parasite interactions. Arch
Insect Biochem Physiol 61:146-156, 2006.
9. Keenan B, Uçkan F, Ergin E et al. Morphological and
biochemical changes in cultured cells induced by venom from the endoparasitoid, Pimpla turionellae, In: Rivers D, Yoder J. eds. Recent Advances in the Biochemistry, Toxicity, and Mode of Action of Parasitic Wasp Venoms. Research Signpost; 2007: pp. 75-92.
10. Pfi ster-Wilhelm R, Lanzrein B. Precocious induction of metamorphosis in Spodoptera litoralis (Noctuidae) by the parasitic wasp Chelonus inantius (Braconidae): identifi cation of the parasitoid larva as the key element and the host corpora allata as a main target. Arch Insect Biochem Physiol 32: 511-525, 1996.
11. Reed DA, Brown JJ. Host/parasitoid interactions: critical timing of parasitoid-derived products. J Insect Physiol 44: 721-732, 1998.
12. Richards EH, Edwards JP. Proteins synthesized and secreted by larvae of the ectoparasitic Wasp, Eulophus pennicornis. Arch Insect Biochem Physiol 46: 140-151, 2001.
13. Vinson SB, Iwansch GF. Host regulation by insect parasitoids. Q Rev Biol 55: 143-165, 1980.
14. Beckage NE. Games parasites play: Th e dynamic roles of
proteins and peptides in the relationship between parasite
and host. In: Beckage NE, Th ompson SN, Federici BA. eds.
Parasites and Pathogens of Insects. Academic Press; 1993: pp. 25-57.
15. Th ompson SN. Redirection of host metabolism and eff ects on
parasite nutrition. In: Beckage NE, Th ompson SN, Federici BA.
eds. Parasites and Pathogens of Insects. Academic Press; 1993: pp. 125-144.
16. Th ompson SN, Lee RWK. Glucose metabolism in an insect,
Manduca sexta, and eff ects of parasitism. Biochim Biophys Acta 1200: 322-330, 1994.
17. Richards EH, and Edwards JP. Parasitism of Lacanobia oleracea (Lepidoptera, Noctuidae) by the ectoparasitoid wasp Eulophus pennicornis, results in the appearance of a 27 kDa parasitism-specifi c protein in host plasma. Insect Biochem. Molec. Biol. 29: 557-569, 1999.
18. Smilowitz Z. Electrophoretic patterns in hemolymph protein of cabbage looper during development of the parasitoid Hyposoter exiguae. Ann Entomol Soc Am 66: 93-99, 1973.
19. Th ompson SN. Immediate eff ects of parasitization by the insect
parasitoid Hyposoter exiguae on the nutritional physiology of it’s host Trichoplusia ni. J Parasitol 68: 936-941, 1982.
20. Smilowitz A, Smith CL. Hemolymph proteins of developing Pieris rapae larvae parasitized by Apanteles glomeratus. Ann Entomol Soc Am 70: 447-454, 1977.
21. Beckage NE, Templeton TJ. Physiological eff ects of parasitism by Apanteles congregatus in terminal stage tobacco hornworm larvae. J Insect Physiol 32: 299-314, 1986.
22. Kunkel JG, Grossniklaus-Burgin C, Karpells ST et al.
Arylphorin of Trichoplusia ni: characterization and parasite induced precocious increase in titre. Arch Insect Biochem Physiol 13: 117-125, 1990.
23. Jones D, Jones G, Rudnicka M et al. Precocious expression of the fi nal larval instar developmental program in larvae of Trichoplusia ni pseudoparasitized by Chelonus spp. Comp Biochem Physiol B 83: 339-346, 1985.
24. Ferkovich SM, Greary PD, Dillard C. Changes in haemolymph proteins of the fall armyworm, Spodoptera frugiperda (JE Smith) associated with parasitization by the braconid parasitoid Cotesia marginiventris (Cresson). J Insect Physiol 29: 933-942, 1983.
25. Nakamatsu Y, Gyotoku Y, Tanaka T. Th e endoparasitoid Cotesia
kariyai (Ck) regulates the growth and metabolic effi ciency of Pseudaletia separata larvae by venom and Ck polydnavirus. J Insect Physiol 47: 573-584, 2001.
26. Bischof C, Ortel J. Th e eff ects of parasitism by Glyptapanteles
liparidis (Braconidae: Hymenoptera) on the hemolymph and total body composition of gypsy moth larvae (Lymantria dispar, Lymantriidae: Lepidoptera). Parasitol Res 82: 687-692, 1996.
27. Th ompson SN, Lee RWK. Metabolic fate of alanine in an insect, Manduca sexta: eff ects of starvation and parasitism. Biochim Biophys Acta 1157: 259-293, 1993.
28. Gauld ID. Evolutionary patterns of host utilization by
ichneumonoid parasitoids (Hymenoptera: Ichneumonidae and Braconidae). Biol J Linnean Soc 35: 351-377, 1988.
29. Wharton RA. Bionomics of the braconidae. Annu Rev Entomol 38: 121-143, 1993.
30. Uçkan F, Ergin E. Eff ect of host diet on the immature developmental time, fecundity, sex ratio, adult longevity, and size of Apanteles galleriae (Hymenoptera: Braconidae). Environ Entomol 31: 168-171, 2002.
31. Uçkan F, Sinan S, Savaşçı Ş et al. Determination of venom components from the endoparasitoid wasp Pimpla turionellae L. (Hymenoptera; Ichneumonidae). Ann Entomol Soc Am 97: 775-780, 2004.
32. Ergin E, Uçkan F, Rivers DB et al. In vivo and in vitro activity of venom from the endoparasitic wasp Pimpla turionellae (L.) (Hymenoptera: Ichneumonidae). Arch Insect Biochem Physiol 61: 87-97, 2006.
33. Kansu İA, Uğur A. Pimpla turionellae (L.) (Hym.,
Ichneumonidae) ile konukçusu bazı Lepidopter pupaları arasındaki biyolojik ilişkiler üzerinde araştırmalar. Doğa Bil Derg 8: 160-173, 1984.
34. Zupko K, Sklan D, Lensky Y. Proteins of the honey bee (Apis mellifera L.) body surface and exocrine glands. J Insect Physiol 39: 41-46, 1993.
35. Lowry OH, Rosebbrough NJ, Farr AL et al. Protein
measurement with the folin phenol reagent. J Biol Chem 193: 265- 275, 1951.
36. Jervis MA, Kidd NA. Insect Natural Enemies: Practical Approaches to their Study and Evaluation. Chapman and Hall. London; 1996.
37. Kaleli S, Aksoylar MY, Aktümsek A et al. Eff ects of parasitism by endoparasitoid Pimpla turionellae on hemolymph proteins of host Galleria mellonella. J Sci Technol 1: 146-156, 2007. 38. Uçkan F, Ergin E, Rivers DB et al. Age and diet infl uence the
composition of venom from the endoparasitic wasp Pimpla turionellae L. (Hymenoptera: Ichneumonidae). Arch Insect Biochem Physiol 63: 177-187, 2006.
39. Piek T, Spanjier W. Chemistry and pharmacology of solitary wasp venoms. In: Piek T. ed. Venoms of the Hymenoptera. Academic Press; 1986, pp 161-307.
40. Coudron TA, Jones D, Jones G. Premature production of late larval storage proteins in larvae of Trichoplusia ni parasitized by Euplectrus comstockii. Arch Insect Biochem Physiol 26: 97-109, 1994.
41. Coudron TA, Brandt SL, Raqib A. Comparison of the response of Heliothis virescens to parasitism by Euplectrus comstockii and Euplectrus plathypenae. Comp Biochem Physiol B 116: 197-202, 1997.
42. Coudron TA, Raqib A, Brandt SL et al. Comparison of the hemolymph proteins in permissive and non-permissive hosts of Euplectrus comstockii. Comp Biochem Physiol B 120: 349-357, 1998.
43. Ergin E, Uçkan F, Rivers DB. Biochemical characterization and mode of action of venom from the endoparasitoid wasp Pimpla turionellae. In: Rivers D, Yoder J. eds. Recent Advances in the Biochemistry, Toxicity, and Mode of Action of Parasitic Wasp Venoms. Research Signpost; 2007: pp. 129-160.
44. Rivers DB, Ergin E, Uçkan F.Cell death in the host-parasitoid
relationship. In: Corvin AJ. ed. New Developments in Cell Apoptosis Research. Nova Science Publishers Inc; 2007: pp. 69-96.