Contents lists available atScienceDirect
Industrial Crops & Products
journal homepage:www.elsevier.com/locate/indcropPhytochemical characterization and bioactivities of five Apiaceae species:
Natural sources for novel ingredients
Gokhan Zengin
a,⁎,1, Mohamad Fawzi Mahomoodally
b,1, Mehmet Yavuz Paksoy
c,
Carene Picot-Allain
b, Jasmina Glamocilja
d, Marina Sokovic
d, Alina Diuzheva
e, József Jekő
f,
Zoltán Cziáky
f, Maria João Rodrigues
g, Kouadio Ibrahime Sinan
a, Luisa Custodio
gaDepartment of Biology, Science Faculty, Selcuk University, Campus, Konya, Turkey
bDepartment of Health Sciences, Faculty of Science, University of Mauritius, 230 Réduit, Mauritius cDepartment of Environmental Engineering, Faculty of Engineering, University of Munzur, Tunceli, Turkey dInstitute for Biological Research, “Siniša Stanković” University of Belgrade, Belgrade, Serbia
eDepartment of Analytical Chemistry, Pavol Jozef Šafárik University in Košice, Košice, Slovakia
fAgricultural and Molecular Research and Service Institute, University of Nyíregyháza, Nyíregyháza, Hungary
gCentre of Marine Sciences, University of Algarve, Faculty of Sciences and Technology, Ed. 7, Campus of Gambelas, 8005-139, Faro, Portugal
A R T I C L E I N F O Keywords: Apiaceae Lipase Antibacterial Cytotoxicity Antioxidant Enzymes A B S T R A C T
Several species of the Apiaceae family have been employed in traditional cultures for their curative virtues. The present study focused on five Apiaceae species, (Falcaria vulgaris (FV), Smyrniopsis aucheri (SA), Smyrniopsis munzurdagensis (SM), Smyrnium cordifolium (SC), and Actinolema macrolema (AM)). The antioxidant, enzyme inhibitory (α-amylase, α-glucosidase, acetyl- and butyrylcholinesterase, lipase, and tyrosinase), antimicrobial, phytochemical, and cytotoxicity profiles of the methanol extracts of the selected Apiaceae species were de-termined. SC extract (35.68 mg gallic acid equivalent/g extract) possessed the highest phenolic content while the AM extract (56.79 mg rutin equivalent/g extract) had the highest flavonoid content. HPLC-ESI-MS (High per-formance liquid chromatography-electrospray tandem mass spectrometry) analyses showed presence of ferulic acid in all the five species. SC extract exhibited high radical scavenging (59.28 and 94.31 mg Trolox equivalent [TE]/g extract, DPPH (1,1-diphenyl-2-picrylhydrazyl) and ABTS (2,2′-azino-bis(3-ethylbenzothiazoline)-6-sul-fonic acid), respectively) and reducing activity (161.44 and 113.62 mg TE/g extract, for CUPRAC (cupric re-ducing antioxidant capacity) and FRAP (ferric rere-ducing antioxidant power), respectively). SM extract exhibited the highest cholinesterase’s inhibitory action (3.82 and 4.76 mg galantamine equivalent/g extract, for acetyl-and butyrylcholinesterase, respectively). The extracts showed higher inhibition against α-glucosidase (7.32-11.99 mmol acarbose equivalent [ACAE]/g extract) compared to α-amylase (0.51-0.55 mmol ACAE/g extract). SC extract was the most active (137.54 mg kojic acid equivalent/g extract) tyrosinase inhibitor and FV extract (113.75 mg Orlistat equivalent/g) the best lipase inhibitor. SM extract showed potent antibacterial effect against B. cereus (MIC (minimum inhibitory concentration) 0.180 mg/mL), P. mirabilis (MIC 0.180 mg/mL), M. flavus (MIC 0.560 mg/mL), P. aeruginosa (MIC 0.275 mg/mL), and S. typhimurium (MIC 1.500 mg/mL). FV extract (MIC 0.140 mg/mL) suppressed A. fumigatus growth. Cytotoxicity was assessed on murine macrophage (RAW 264.7), human embryonic kidney (HEK 293), and human hepatocellular carcinoma (HepG2) cell lines. FV (60.3%) and SM (57.4%) showed the highest reduction on RAW 264.7 cellular viability, whereas SM (74.1%) showed toxicity against HepG2. This study supports that the Apiaceae species could be considered as promising candidates for the development of novel pharmacophores for the management of several human ailments.
1. Introduction
Plants possessing therapeutic virtues are used by millions of people
across the globe and include not only people with poor access to allo-pathic healthcare systems, but also consumers of developing and de-veloped countries (Polat, 2018). Knowledge of plant therapeutic uses
https://doi.org/10.1016/j.indcrop.2019.04.033
Received 6 March 2019; Received in revised form 8 April 2019; Accepted 15 April 2019 ⁎Corresponding author.
E-mail address:gokhanzengin@selcuk.edu.tr(G. Zengin). 1These authors contributed equally.
Available online 22 April 2019
0926-6690/ © 2019 Elsevier B.V. All rights reserved.
has been transmitted over centuries from one generation to the next and rationalise pharmacological studies. The exponential surge in the use of herbal products as complementary and/or alternative therapeutic strategy is mainly due to the perception that herbal products are safe and thus devoid of side effects (Mahomoodally et al., 2018). It is worth mentioning that the use of plants for therapeutic applications necessi-tates robust pharmacological evaluation of the toxicity and required dosage (Picot-Allain, 2018).
The Apiaceae family is one of the most important families of flow-ering plants, consisting of 3780 species in 434 genera. Species of the Apiaceae family are distributed across the world and are more com-monly found in northern temperate areas and high altitudes in the tropics (Sayed-Ahmad et al., 2017). Ethnobotanical evidences report the use of several species of the Apiaceae family to relieve various human ailments. In traditional African and Asian societies, different plant parts of species belonging to the Apiaceae family have been used to treat stomach ailments, nausea, appendicitits, ingestion, hyperten-sion, cardiovascular problems, constipation, and mosquito bites (Saleem et al., 2017).
However, there is a paucity of scientific information with respect to the biological activity of Falcaria vulgaris, Smyrniopsis aucheri, Smyrniopsis munzurdagensis, Smyrnium cordifolium, and Actinolema macrolema, belonging to the Apiaceae family. In this sense, the present study was geared towards assessing the antioxidant, enzyme inhibitory (α-amylase, α-glucosidase, acetyl cholinesterase, butyryl cholines-terase, lipase, and tyrosinase), antimicrobial, phytochemical, and cy-totoxicity profiles of the methanol extracts of the selected Apiaceae species.
2. Materials and methods
2.1. Plant material and preparation of extracts
The Apiaceae species were collected from different regions of Turkey in June 2018. The taxonomical classification was performed by a local botanist (Dr. Mehmet Yavuz Paksoy from Munzur University, Tunceli). The location details are given inTable 1. The aerial parts including flowers were divided and dried for 10 days at room tem-perature (RT) (25 °C). Then, the samples were powdered with a la-boratory mill. The plant names abbreviations in the paper are FV (Falcaria vulgaris), SA (Smyrniopsis aucheri), SM (Smyrniopsis
munzurdagensis), SC (Smyrnium cordifolium), and AM (Actinolema mac-rolema).
To prepare methanol extracts, dried samples were stirred overnight (24 h) at RT (5 g in 100 mL solvent). After filtration, the extracts were concentrated using a rotary evaporator under vacuum at 40 °C, and the completely dried extracts were then stored at + 4 °C until further ana-lysis.
2.2. Profile of bioactive compounds and HPLC-MS/MS analysis
Assays were conducted as elaborated in detail in our previous study (Uysal et al., 2017;Zengin et al., 2017b). The total amount of phenolics (TPC) (by standard Folin-Ciocalteu method) and flavonoids (TFC) (by AlCl3method) were determined. Standard compounds (gallic acid (mg GAE/g) for TPC and rutin (mg RE/g) for TFC, respectively) were used to express the obtained results.
All HPLC-MS/MS experiments were carried out on a Dionex Ultimate 3000RS UHPLC instrument coupled to a Thermo Q Exactive Orbitrap mass spectrometer. A chromatographic method was developed using a Thermo Accucore C18 (100 mm x 2.1, mm i. d., 2.6 μm) column (Zengin et al., 2018b). All analytical details were given in supplemen-tary material.
2.3. Assays for enzyme inhibition and antioxidant capacity
Lipase, tyrosinase, α-amylase, α-glucosidase and cholinesterases were selected as target enzymes and the procedures of these assays are described in our earlier work (Grochowski et al., 2017;Mollica et al., 2019;Uysal et al., 2017). Standard inhibitors (acarbose (ACAE) [for α-amylase and α-glucosidase], galantamine (GALAE) [for AChE and BChE], kojic acid (KAE) [for tyrosinase]) and orlistat (OE) [for lipase] were used to express the enzyme inhibitory properties.
The antioxidant capacity of the extracts was spectrophotometrically screened by different experiments, namely the ferrozine assay (for chelating abilities), phosphomolybdenum, reduction potentials (by FRAP and CUPRAC assays) and radical scavenging (using DPPH and ABTS radicals). Standard compounds (trolox equivalent (TE)/g and EDTA equivalent (EDTAE)/g) were used to express the antioxidant properties. The procedures of assays are reported in our earlier work (Mollica et al., 2017;Uysal et al., 2017).
Table 1
Locations, total phenolic and flavonoid content of the studied Apiaceae species.
Species/Abbreviations Locations Total phenolic content
(mg GAE/g extract) Total flavonoid content (mg RE/gextract)
Falcaria vulgaris Bernh. (FV) Tunceli; between Tunceli and Nazımiye, Quercus opening,
1500 m, Paksoy 2887 20.25 ± 0.29
d 19.69 ± 0.31d
Smyrniopsis aucheri Boiss (SA) Muş; Malazgirt, Kuruca village, steppes, 1700 m, Paksoy 3020 24.18 ± 0.67b 24.85 ± 0.29c
Smyrniopsis munzurdagensis Yıldırımlı (SM) Tunceli; Ovacık, Karagöl valley, 1350 m, Paksoy 3018 21.82 ± 0.22c 8.36 ± 0.16e
Smyrnium cordiifolium Boiss. (SC) Tunceli; Nazımiye, Düzgünbaba mount, 1550 m, Paksoy 3008 35.68 ± 0.46a 42.39 ± 0.50b
Actinolema macrolema Boiss. (AM) Tunceli; between Tunceli and Pertek, steppes, 1500 m, Paksoy
3016 24.45 ± 0.17
b 56.79 ± 0.80a
Values expressed are means ± S.D. of three parallel measurements. GAE: Gallic acid equivalent; RE: Rutin equivalent. Different superscript indicate differences in the tested extracts (p < 0.05).
Table 2 Chemical composition of Falcaria vulgaris . No Compound Formula Rt [M + H] + [M -H] − Fragment 1 Fragment 2 Fragment 3 Fragment 4 Fragment 5 Literature 1 Uralenneoside C12H14O8 9,94 285.06105 153.0184 152.0105 109.0282 108.0204 2 Kynurenic acid C10H7NO3 13.01 190.05042 162.0553 144.0444 116.0499 89.0391 3 1 Chlorogenic acid (3-O-Caffeoylquinic acid) C16H18O9 14.34 355.10291 163.0393 145.0287 135.0445 117.0340 89.0391 4 Coumaroylhexose C15H18O8 14.73 325.09235 163.0392 119.0490 5 Syringic acid C9H10O5 15.98 197.04500 182.0215 166.9977 153.0551 138.0309 123.0074 6 Feruloylhexose C16H20O9 16.78 355.10291 295.0830 235.0614 193.0502 149.0598 7 Coumaroylquinic acid isomer 1 C16H18O8 16.93 337.09235 191.0557 173.0448 163.0392 119.0490 93.0332 8 1 4-Coumaric acid C9H8O3 17.92 163.03952 119.0490 93.0333 9 5-O-Feruloylquinic acid C17H20O9 18.04 367.10291 193.0502 191.0557 173.0448 134.0363 93.0332 10 Caffeoylshikimic acid C16H16O8 18.10 335.07670 179.0343 161.0236 135.0441 93.0330 11 Tuberonic acid C12H18O4 18.35 225.11269 181.1225 165.0911 147.0806 135.0805 59.0124 12 Scopoletin (7-Hydroxy-6-methoxycoumarin) C10H8O4 18.65 193.05009 178.0264 165.0550 149.0601 137.0602 133.0288 13 Coumaroylquinic acid isomer 2 C16H18O8 19.22 337.09235 191.0556 173.0448 163.0390 119.0490 93.0331 14 Ferulic acid C10H10O4 19.42 193.05009 178.0264 149.0599 137.0234 134.0363 121.0282 15 Coumaroylshikimate isomer 1 C16H16O7 19.46 319.08178 163.0392 155.0345 119.0489 111.0437 93.0330 16 Coumaroylshikimate isomer 2 C16H16O7 20.48 319.08178 163.0391 155.0338 119.0490 111.0437 93.0332 17 Quercetin-O-(pentosyl)hexoside isomer 1 C26H28O16 21.20 595.12991 301.0353 300.0281 271.0255 255.0307 18 Quercetin-O-(pentosyl)hexoside isomer 2 C26H28O16 21.85 595.12991 301.0376 300.0283 271.0255 255.0303 19 Dicaffeoylquinic acid isomer 1 C25H24O12 22.41 515.11896 353.0885 191.0557 179.0343 173.0448 135.0441 20 Coumaroylshikimate isomer 3 C16H16O7 22.42 319.08178 163.0393 155.0338 119.0490 111.0439 93.0331 21 Hyperoside (Quercetin-3-O-galactoside. Hyperin) C21H20O12 22.77 463.08765 301.0360 300.0282 271.0255 255.0303 151.0027 22 Quercetin-O-glucuronide C21H18O13 22.80 477.06692 301.0360 255.0305 163.0028 151.0027 23 Isoquercitrin (Hirsutrin. Quercetin-3-O-glucoside) C21H20O12 23.01 463.08765 301.0360 300.0281 271.0255 255.0302 151.0025 24 Quercetin-O-(deoxyhexosyl)hexoside C27H30O16 23.11 609.14557 301.0360 300.0281 271.0254 255.0302 25 Kaempferol-O-(pentosyl)hexoside C26H28O15 23.22 579.13500 447.0957 285.0412 284.0333 255.0302 227.0349 26 Isorhamnetin-O-(pentosyl)hexoside C27H30O16 23.38 609.14557 477.1055 315.0521 314.0441 299.0205 271.0254 27 Quercetin-O-pentoside isomer 1 C20H18O11 23.59 433.07709 301.0371 300.0281 271.0247 255.0298 151.0027 28 Dicaffeoylquinic acid isomer 2 C25H24O12 24.22 515.11896 353.0884 191.0559 179.0344 173.0449 135.0442 29 Quercetin-O-pentoside isomer 2 C20H18O11 24.33 433.07709 301.0358 300.0281 271.0257 255.0306 151.0028 30 Kaempferol-3-O-glucuronide C21H18O12 24.67 461.07200 285.0413 229.0505 175.0241 113.0232 31 Astragalin (Kaempferol-3-O-glucoside) C21H20O11 24.77 447.09274 285.0412 284.0334 255.0302 227.0349 32 Kaempferol-O-pentoside isomer 1 C20H18O10 24.95 417.08218 285.0407 284.0332 255.0301 227.0347 33 1 Isorhamnetin-3-O-glucoside C22H22O12 24.99 477.10330 315.0522 314.0441 285.0412 271.0255 243.0301 34 Isorhamnetin-O-glucuronide C22H20O13 25.23 491.08257 315.0519 300.0281 271.0253 243.0299 35 Kaempferol-O-pentoside isomer 2 C20H18O10 25.32 417.08218 285.0409 284.0334 255.0303 227.0349 36 Kaempferol-O-malonylhexoside C24H22O14 25.69 533.09314 489.1049 285.0412 284.0333 255.0302 227.0349 37 Kaempferol-O-(3-hydroxy-3-methylglutaroyl)hexoside C27H28O15 25.92 591.13500 529.1363 489.1047 447.0942 284.0333 255.0302 38 Afzelin (Kaempferol-3-O-rhamnoside) C21H20O10 26.48 431.09782 285.0412 284.0333 255.0302 227.0348 39 Kaempferol-O-caffeoylhexoside C30H26O14 26.85 609.12444 447.0966 285.0414 284.0335 255.0298 227.0347 40 Isorhamnetin-O-methylglucuronide C23H22O13 27.00 505.09822 315.0521 314.0441 299.0205 285.0411 271.0254 41 1 Quercetin C15H10O7 27.04 303.05048 285.0394 257.0452 229.0499 153.0183 137.0237 42 Jasmonic acid C12H18O3 27.77 209.11777 165.1276 109.0647 59.0124 43 Kaempferol-O-feruloylhexoside C31H28O14 28.37 623.14009 447.0951 285.0416 284.0336 255.0304 227.0351 44 Kaempferol-O-coumaroylhexoside isomer 1 C30H26O13 28.40 593.12952 285.0413 284.0335 255.0302 227.0350 45 1 Kaempferol (3,4',5,7-Tetrahydroxyflavone) C15H10O6 29.35 287.05556 258.0525 241.0491 165.0187 153.0185 121.0289 46 γ-Terpinene C10H16 29.63 137.13303 109.1019 95.0862 81.0706 67.0550 ( Jaberian et al., 2013 ) 47 1 Isorhamnetin (3'-Methoxy-3,4',5,7-tetrahydroxyflavone) C16H12O7 29.89 315.05048 300.0282 283.0253 227.0349 164.0105 151.0028 48 Dimethoxy-trihydroxy(iso)flavone C17H14O7 30.23 329.06613 314.1531 299.0570 297.0413 269.0457 49 Kaempferol-O-cinnamoylhexoside isomer 1 C30H26O12 30.69 577.13461 285.0412 284.0335 255.0299 229.0504 50 Kaempferol-O-coumaroylhexoside isomer 2 C30H26O13 31.03 593.12952 285.0412 284.0331 257.0461 229.0505 51 4-Hydroxyphenylethyl trans-ferulate C18H18O5 31.12 313.10760 193.0503 149.0598 134.0363 117.0334 52 Kaempferol-O-cinnamoylhexoside isomer 2 C30H26O12 32.17 577.13461 285.0412 284.0334 255.0303 229.0506 53 Camphene C10H16 33.87 137.13303 109.1018 95.0862 81.0706 67.0550 ( Jaberian et al., 2013 ) 54 α-Pinene C10H16 34.43 137.13303 109.1017 95.0862 81.0706 67.0550 ( Jaberian et al., 2013 ) (continued on next page )
2.4. Antibacterial and antifungal activities
Antimicrobial and antifungal activities were determined using the microdilution method as described previously (Zengin et al., 2017a). The antibacterial activity of the studied extracts was evaluated using several bacterial strains. Escherichia coli (ATCC 35210), Pseudomonas aeruginosa (ATCC 27853) and Salmonella typhimurium (ATCC 13311) were used as Gram-negative bacteria. For Gram-positive bacteria, Pro-teous mirabilis (human isolate), Enterobacter cloacae (ATCC 35030), Bacillus cereus (clinical isolate), Micrococcus flavus (ATCC 10240), and Staphylococcus aureus (ATCC 6538) were used. The results were ex-pressed as minimum inhibitory concentrations (MICs) and minimum bactericidal concentrations (MBCs).
The antifungal activity of the extracts was evaluated using different fungal species, including Aspergillus versicolor (ATCC 11730), A. fumi-gatus (plant isolate), A. terreus (soil isolate), A. niger (ATCC 6275), Penicillium ochrochloron (ATCC 9112), P. funiculosum (ATCC 36839), P. verrucosum (food isolate) and Trichoderma viride (IAM 5061). Antifungal results were expressed by MICs and minimum fungicidal concentrations (MFCs).
2.5. Cell culture
The murine RAW 264.7 macrophages, human embryonic kidney (HEK) 293, and human hepatocellular carcinoma (HepG2) cell lines were provided by the Faculty of Pharmacy and Centre for Neurosciences and Cell Biology (University of Coimbra, Portugal), the Functional Biochemistry and Proteomics, and the Marine Molecular Bioengineering groups (Centre of Marine Sciences, Portugal). RAW 264.7 cells were maintained in RPMI 1640 culture media, while HEK and HepG2 cells were cultured in DMEM culture media, both supple-mented with 10% heat-inactivated FBS, 1%L-glutamine (2 mM), and
1% penicillin (50 U/mL) / streptomycin (50 μg/mL), and were kept at 37 °C in moistened atmosphere with 5% CO2.
2.6. Determination of the cytotoxicity of the extracts
Cells at 80% confluency were seeded in 96-well microplates at a density of 1 × 104cells/well (RAW 264.7) and 5 × 103cells/well (HEK 293 and HepG2) and left to adhere for 24 h. Then, the extracts were applied at the concentration of 100 μg/mL for 72 h, and control cells were treated with DMSO at maximum concentration used in the ex-tracts (0.2%). Cellular viability was determined by the MTT 3-(4,5-di-methylthiazol-2-yl)-2,5-diphenyltetrazolium bromide colorimetric assay, using a microplate reader (Biotek Synergy 4), as described pre-viously (Rodrigues et al., 2014). Results were expressed in terms of cellular viability (%).
2.7. Data evaluation
The obtained results were expressed as mean ± standard deviation (SD) and statistically evaluated by one-way ANOVA (by Tukey test, p < 0.05), to indicate differences among the tested extracts. To further statistical evaluation, Pearson correlation, heat map, Principal compo-nent (PCA) and Sparse Partial Least Squares (sPLS-DA) analysis were carried out to observe variabilities of the tested extracts. The statistical procedures were performed by R software v. 3.5.1.
Table 2 (continued ) No Compound Formula Rt [M + H] + [M -H] − Fragment 1 Fragment 2 Fragment 3 Fragment 4 Fragment 5 Literature 55 Unidentified saponin C48H76O19 36.16 955.49026 793.4359 731.4392 613.3752 497.3661 455.3537 56 Limonene C10H16 36.64 137.13303 109.1017 95.0862 81.0706 67.0550 ( Jaberian et al., 2013 ) 57 β-Pinene C10H16 37.74 137.13303 109.1017 95.0862 81.0706 67.0550 ( Jaberian et al., 2013 ) 58 Falcarinol C17H24O 38.76 245.19055 175.1485 161.0966 147.0808 105.0706 91.0549 ( Fujita et al., 1995 ) 59 Stearidonic acid C18H28O2 39.79 275.20111 231.2107 59.0144 60 Stearidonic acid methyl ester C19H30O2 41.76 291.23241 259.2062 241.0961 175.1485 135.1173 93.0705 61 1 α-Linolenic acid C18H30O2 44.76 277.21676 259.2064 59.0139 62 Hydroxypalmitic acid C16H32O3 44.98 271.22732 253.2170 225.2222 223.2065 1Confirmed by standard.
Table 3 Chemical composition of Smyrniopsis aucheri . No. Compound Formula Rt [M + H] + [M -H] − Fragment 1 Fragment 2 Fragment 3 Fragment 4 Fragment 5 Literature 1 Caffeoylhexose isomer 1 C15H18O9 12.12 341.08726 179.0342 161.0233 135.0441 113.0227 89.0231 2 Coumaroylquinic acid isomer 1 C16H18O8 12.55 337.09235 191.0556 173.0446 163.0391 119.0489 93.0331 3 Chlorogenic acid (3-O-Caffeoylquinic acid) C16H18O9 14.23 355.10291 163.0393 145.0288 135.0450 117.0340 89.0392 4 Caffeoylhexose isomer 2 C15H18O9 14.32 341.08726 179.0342 161.0225 135.0440 113.0235 89.0231 5 trans-Melilotoside C15H18O8 14.63 325.09235 163.0391 119.0489 6 Coumaroylquinic acid isomer 2 C16H18O8 16.84 337.09235 191.0555 173.0447 163.0391 119.0489 93.0331 7 Coumaroylquinic acid isomer 3 C16H18O8 17.53 337.09235 191.0556 173.0447 163.0391 119.0489 93.0331 8 Feruloylquinic acid C17H20O9 17.97 367.10291 193.0502 191.0555 173.0446 134.0362 93.0331 9 Caffeoylshikimic acid C16H16O8 18.02 335.07670 179.0342 161.0234 135.0440 10 Scopoletin (7-Hydroxy-6-methoxycoumarin) C10H8O4 18.57 193.05009 178.0266 165.0550 149.0596 137.0601 133.0288 11 Apigenin- O-hexoside-O-glucuronide C27H28O16 19.13 607.12992 445.0771 431.0988 269.0458 268.0382 12 Coumaroylquinic acid isomer 4 C16H18O8 19.16 337.09235 191.0555 173.0445 163.0389 119.0488 93.0331 13 Ferulic acid C10H10O4 19.35 193.05009 178.0264 149.0598 137.0234 134.0363 121.0283 14 Luteolin-di-O-hexoside C27H30O16 19.65 609.14557 447.0942 285.0411 284.0332 15 Quercetin-O-(hexosyl)hexoside C27H30O17 20.01 625.14048 301.0359 300.0280 271.0253 16 Tetrahydroxyflavanone-O-hexoside C21H22O11 20.38 449.10839 287.0567 151.0027 135.0441 107.0126 17 Coumaroylshikimate C16H16O7 20.40 319.08178 163.0391 155.0340 119.0489 18 Luteolin-O-(glucuronyl)hexoside C27H28O17 20.73 623.12483 447.0945 285.0410 175.0394 151.0025 19 Scoparone (6,7-Dimethoxycoumarin) C11H10O4 21.19 207.06574 191.0346 179.0710 163.0391 151.0757 136.0522 20 Cnidioside B isomer C18H22O10 21.53 397.11348 235.0610 217.0501 191.0710 176.0470 21 Apigenin-O-(glucuronyl)hexoside C27H28O16 22.03 607.12992 269.0460 225.0548 22 Tetrahydroxyflavone-O-glucuronide C21H18O12 22.25 461.07201 285.0411 217.0506 199.0393 175.0394 151.0027 23 Smyrinol (Decursinol) isomer O-hexoside isomer 1 C20H24O9 22.34 409.14986 247.0967 229.0862 214.0632 175.0392 24 Prunin (Naringenin-7-O-glucoside) C21H22O10 22.40 433.11347 271.0619 177.0184 151.0027 119.0489 107.0124 25 Luteolin-7-O-glucoside (Cynaroside) C21H20O11 22.44 447.09274 327.0520 285.0410 284.0331 256.0381 151.0023 26 Hyperoside (Hyperin, Quercetin-3-O-galactoside) C21H20O12 22.73 463.08765 301.0357 300.0281 271.0253 255.0300 151.0026 27 Quercetin-O-glucuronide C21H18O13 22.74 477.06692 301.0367 255.0297 178.9977 151.0027 28 Nakhsmyrin isomer O-hexoside C20H22O9 22.78 407.13421 273.0762 245.0811 227.0704 217.0862 175.0392 29 1 Isoquercitrin (Hirsutrin, Quercetin-3-O-glucoside) C21H20O12 22.95 463.08765 301.0359 300.0280 271.0253 255.0301 151.0026 30 Luteolin-7-O-rutinoside (Scolymoside) C27H30O15 23.11 593.15065 447.0932 285.0410 284.0330 31 Smyrinol (Decursinol) isomer O-hexoside isomer 2 C20H24O9 23.33 409.14986 247.0969 229.0864 214.0628 175.0393 32 Smyrinol (Decursinol) isomer O-hexoside isomer 3 C20H24O9 23.67 409.14986 247.0970 229.0865 214.0628 175.0393 33 Smyrindiol C14H14O5 23.79 263.09195 245.0813 203.0707 175.0393 ( Fujita et al., 1995 ) 34 1 Cosmosiin (Apigetrin, Apigenin-7-O-glucoside) C21H20O10 24.02 433.11348 271.0606 153.0183 145.0293 119.0496 35 Dicaffeoylquinic acid C25H24O12 24.18 515.11896 353.0883 191.0556 179.0342 173.0446 135.0441 36 Methoxy-trihydroxyflavone-O-glucuronide C22H20O12 24.27 475.08766 299.0566 284.0331 175.0239 113.0231 37 Chrysoeriol-O-hexoside C22H22O11 24.31 461.10839 446.0860 299.0566 298.0488 283.0253 255.0301 38 Smyrinol (Decursinol) isomer 1 C14H14O4 24.57 247.09704 229.0864 214.0628 175.0393 ( Fujita et al., 1995 ) 39 Methoxy-trihydroxyflavone-O-(deoxyhexosyl)hexoside C28H32O15 24.80 609.18195 463.1231 301.0710 286.0476 85.0290 71.0499 40 Luteolin-O-(malonyl)hexoside C24H22O14 25.25 533.09314 489.1046 447.0931 285.0411 284.0331 133.0286 41 Smyrinol (Decursinol) isomer 2 C14H14O4 25.36 247.09704 229.0865 214.0635 175.0393 ( Fujita et al., 1995 ) 42 Smyrinol (Decursinol) isomer 3 C14H14O4 26.21 247.09704 229.0864 214.0628 175.0393 ( Fujita et al., 1995 ) 43 Apigenin-O-(malonyl)hexoside C24H22O13 26.78 517.09822 473.1077 269.0459 268.0381 44 1 Naringenin C15H12O5 27.23 271.06065 177.0184 165.0183 151.0026 119.0489 107.0126 45 Dimethoxy-furocoumarin C13H10O5 27.27 247.06065 232.0370 217.0135 189.0196 161.0236 95.0133 46 Luteolin-O-(acetyl)hexoside C23H22O12 27.29 489.10331 285.0410 284.0332 47 Dihydroxy-methoxyflavone-O-glucuronide C22H20O11 27.57 459.09274 283.0617 268.0380 48 Methoxy-furocoumarin C12H8O4 27.71 217.05009 202.0265 174.0317 146.0367 131.0497 49 1 Luteolin (3',4',5,7-Tetrahydroxyflavone) C15H10O6 27.85 285.03991 217.0501 199.0395 175.0391 151.0025 133.0283 50 Apigenin-O-(acetyl)hexoside C23H22O11 28.78 473.10839 269.0459 268.0381 51 1 Apigenin (4',5,7-Trihydroxyflavone) C15H10O5 29.68 269.04500 225.0557 201.0553 151.0026 149.0234 107.0126 52 Chrysoeriol (Scoparol, 3'-Methoxy-4',5,7-trihydroxyflavone) C16H12O6 29.90 299.05556 284.0331 256.0380 227.0346 151.0025 107.0123 53 Angelicin C11H6O3 30.50 187.03952 159.0445 143.0495 131.0496 115.0546 103.0546 54 Quercetin-O-(coumaroyl)deoxyhexoside C30H26O13 30.80 593.12952 447.0940 301.0359 300.0280 299.0205 271.0252 (continued on next page )
3. Results and discussion 3.1. Phytochemical profile
The biochemical evaluation of phytochemicals underpins the quest for novel pharmacophores used to design potent drugs. In this study, the total phenolic and total flavonoid contents of five species of the Apiaceae family were assessed. As presented inTable 1, SC (35.68 mg GAE/g extract) possessed the highest TPC while the TPC values re-corded for other Apiaceae species ranged between 20.25–24.45 mg GAE/g extract. As to TFC, the highest value recorded was from AM (56.79 mg RE/g extract) while the lowest value was recorded for SM (8.36 mg RE/g extract). Results indicated that the studied Apiaceae species possessed distinct TPC and TFC. In addition to spectro-photometric determinations, phytochemicals occurring in the studied Apiaceae species were characterised by HPLC-ESI-MS. The profile of FV, SA, SM, SC, and AM are presented inTables 2–6, respectively. Ferulic acid ([M−H]−at m/z 193.05) was identified in the studied Apiaceae species. Ferulic acid was first isolated from Ferula foetida, belonging to the Apiaceae family (de Oliveira Silva and Batista, 2017). Besides, previous studies have reported the occurrence of ferulic acid in several Apiaceae species (Abas et al., 2014;Babu et al., 2017;Ezzat et al., 2012; Martins et al., 2016) (Tables 3–6).
3.2. Antioxidant activity
The constant production and removal of reactive oxygen species orchestrates cellular homeostasis (Uysal et al., 2019). However, im-balances lead to oxidative stress, a condition characterised by a surplus of reactive oxygen species which impairs normal cellular activity. Un-healthy life style factors, such as tobacco smoking, stress, pollution, exposure to chemicals and radiations, have contributed to boost the prevalence of oxidative stress related complications. Exogenous sources of antioxidants play a crucial defensive role against oxidative damage and thus assist in maintaining optimum health. Interestingly, phyto-chemicals naturally occurring in plants have demonstrated superior efficacy as antioxidants. In order to provide a comprehensive prediction of the antioxidant capacity of the studied Apiaceae species different antioxidant assays were used.
In the present investigation, the phosphomolybdenum, metal che-lating, reducing power (CUPRAC, FRAP) and radical scavenging (ABTS, DPPH) assays were employed to assess the antioxidant activity of the plant extracts. As seen inTable 7, SC extract exhibited high radical scavenging (59.28 and 94.31 mg TE/g extract, DPPH and ABTS, re-spectively) and reducing activity (161.44 and 113.62 mg TE/g extract, for CUPRAC and FRAP, respectively). These values were also close to that of AM reported. The radical scavenging and reducing activities of SC extract were related to the phenolic content since the extract pos-sessed the highest TPC. Indeed, several studies have reported positive correlation between phenolic content and radical and reducing abilities of plant extracts (Smilin et al., 2019;Zhou et al., 2019). Another group of authors (Tabaraki and Ghadiri, 2013) previously described the DPPH scavenging (71.91% inhibition), FRAP (115.80 μmol Fe2+/g dry weight), and phenolic content (22.16 mg GAE/g dry weight) for the methanol extract from Smyrnium cordifolium leaves. It is worth noting that indicate some of the phytochemicals present in SC with high an-tioxidant properties. SM extract was also found to exhibit high total antioxidant activity in the phosphomolybdenum assay. However, SM extract showed low antioxidant activity. For instance, the DPPH scavenging activity recorded for SM was 2.29 mg TE/g extract, whereas
Table 3 (continued ) No. Compound Formula Rt [M + H] + [M -H] − Fragment 1 Fragment 2 Fragment 3 Fragment 4 Fragment 5 Literature 55 Luteolin-O-(cinnamoyl)hexoside C30H26O12 32.12 577.13461 285.0411 284.0334 56 Nakhsmyrin isomer 1 C14H12O4 32.22 245.08139 227.0703 217.0865 203.0707 175.0393 ( Jaberian et al., 2013 ) 57 Nakhsmyrin isomer 2 C14H12O4 32.84 245.08139 227.0706 217.0862 203.0707 175.0393 ( Jaberian et al., 2013 ) 58 Nakhsmyrin isomer 3 C14H12O4 34.43 245.08139 227.0706 217.0862 203.0706 175.0392 ( Jaberian et al., 2013 ) 59 Xanthyletin C14H12O3 35.71 229.08647 214.0628 187.0395 175.0393 159.0444 131.0495 60 Selinidin C19H20O5 35.81 329.13890 229.0863 201.0914 187.0394 175.0393 159.0443 1Confirmed by standard.
Table 4
Chemical composition of Smyrniopsis munzurdagensis.
No. Compound Formula Rt [M + H]+ [M - H]− Fragment 1 Fragment 2 Fragment 3 Fragment 4 Fragment 5 Literature 1 Neochlorogenic acid (5-O-Caffeoylquinic
acid) C16H18O9 9.08 355.10291 163.0393 145.0288 135.0450 117.0340 89.0392
2 Caffeoylhexose isomer 1 C15H18O9 12.19 341.08726 179.0342 161.0236 135.0441 113.0231 89.0231 3 Coumaroylquinic acid isomer 1 C16H18O8 12.63 337.09235 191.0557 173.0447 163.0391 119.0490 93.0332 4 Kynurenic acid C10H7NO3 12.98 190.05042 162.0553 144.0446 116.0498 89.0391
5 Chlorogenic acid (3-O-Caffeoylquinic
acid) C16H18O9 14.35 355.10291 163.0393 145.0288 135.0445 117.0340 89.0392
6 Caffeoylhexose isomer 2 C15H18O9 14.38 341.08726 179.0342 161.0225 135.0440 113.0227 89.0230 7 Feruloylquinic acid isomer 1 C17H20O9 14.59 367.10291 193.0502 191.0560 173.0447 134.0363 93.0331 8 trans-Melilotoside C15H18O8 14.70 325.09235 163.0392 119.0490
9 Chryptochlorogenic acid
(4-O-Caffeoylquinic acid) C16H18O9 15.66 355.10291 163.0393 145.0288 135.0445 117.0341 89.0393 10 Coumaroylquinic acid isomer 2 C16H18O8 15.68 337.09235 191.0556 173.0446 163.0391 119.0489 93.0331 11 Coumaroylquinic acid isomer 3 C16H18O8 16.91 337.09235 191.0555 173.0447 163.0390 119.0489 93.0331 12 Quercetin-di-O-hexoside C27H30O17 17.27 625.14048 463.0891 462.0814 301.0359 299.0202 271.0254 13 Coumaroylquinic acid isomer 4 C16H18O8 17.59 337.09235 191.0556 173.0447 163.0391 119.0490 93.0331 14 Feruloylquinic acid isomer 2 C17H20O9 18.04 367.10291 193.0503 191.0555 173.0446 134.0362 93.0331 15 Caffeoylshikimic acid C16H16O8 18.07 335.07670 179.0342 161.0234 135.0440
16 Tetrahydroxyflavone-O-(glucuronyl)
hexoside C27H28O17 18.30 623.12483 447.0941 285.0409 284.0332 255.0302 227.0348 17 Feruloylquinic acid isomer 3 C17H20O9 18.57 367.10291 193.0501 191.0557 173.0447 134.0362 93.0331 18 Scopoletin
(7-Hydroxy-6-methoxycoumarin) C10H8O4 18.64 193.05009 178.0265 165.0550 149.0604 137.0602 133.0288 19 Apigenin-O-hexoside-O-glucuronide C27H28O16 19.16 607.12992 445.0770 431.0988 269.0459 268.0380
20 Coumaroylquinic acid isomer 5 C16H18O8 19.22 337.09235 191.0556 173.0450 163.0391 119.0489 93.0331 21 Ferulic acid C10H10O4 19.40 193.05009 178.0264 149.0598 137.0233 134.0362 121.0281 22
Tetrahydroxyflavone-O-glucuronide-O-hexoside isomer 1 C27H28O17 19.48 623.12483 461.0748 447.0933 285.0412 284.0332 23 Quercetin-O-(hexosyl)hexoside C27H30O17 20.05 625.14048 301.0363 300.0280 271.0253
24 Tetrahydroxyflavanone-O-hexoside C21H22O11 20.41 449.10839 287.0567 151.0027 135.0441 107.0126 25 Coumaroylshikimate isomer 1 C16H16O7 20.46 319.08178 163.0391 155.0339 119.0489
26 Quercetin-O-(deoxyhexosyl)hexoside C27H30O16 21.13 609.14557 301.0351 300.0280 271.0253 255.0301
27 Scoparone (6,7-Dimethoxycoumarin) C11H10O4 21.26 207.06574 191.0342 179.0710 163.0393 151.0758 136.0526 28
Tetrahydroxyflavone-O-glucuronide-O-hexoside isomer 2 C27H28O17 21.35 623.12483 461.0750 447.0943 285.0411 284.0328
29 Tetrahydroxyflavone-O-glucuronide C21H18O12 22.30 461.07201 285.0411 217.0500 199.0391 175.0391 151.0027 30 Coumaroylshikimate isomer 2 C16H16O7 22.39 319.08178 163.0391 155.0340 119.0489
31 Smyrinol (Decursinol) isomer O-hexoside
isomer 1 C20H24O9 22.41 409.14986 247.0970 229.0864 214.0604 175.0394
32 Prunin (Naringenin-7-O-glucoside) C21H22O10 22.45 433.11347 271.0619 177.0168 151.0026 119.0490 107.0126 33 Luteolin-7-O-glucoside (Cynaroside) C21H20O11 22.48 447.09274 327.0506 285.0410 284.0332 256.0382 151.0027 34
Tetrahydroxyflavanone-O-(deoxyhexosyl)hexoside C27H30O15 22.55 593.15065 447.0945 285.0411 284.0332 255.0302 227.0347 35 Quercetin-O-glucuronide C21H18O13 22.79 477.06692 301.0359 255.0304 178.9978 151.0026
36 Nakhsmyrin isomer O-hexoside C20H22O9 22.82 407.13421 273.0761 245.0812 227.0706 217.0863 175.0393 371 Isoquercitrin (Hirsutrin,
Quercetin-3-O-glucoside) C21H20O12 23.01 463.08765 301.0358 300.0280 271.0254 255.0300 151.0026 38 Rutin (Quercetin-3-O-rutinoside) C27H30O16 23.12 611.16122 465.1026 303.0506 129.0551 85.0291 71.0499 39 Luteolin-7-O-rutinoside (Scolymoside) C27H30O15 23.14 593.15065 447.0910 285.0411 284.0332
40 Smyrinol (Decursinol) isomer O-hexoside
isomer 2 C20H24O9 23.74 409.14986 247.0970 229.0862 214.0628 175.0394
41 Smyrindiol C14H14O5 23.85 263.09195 245.0812 203.0708 175.0394
42
Quercetin-O-(3-hydroxy-3-methylglutaroyl)hexoside C27H28O16 24.00 607.12992 545.1295 505.0997 463.0895 301.0360 300.0280 431 Cosmosiin (Apigetrin,
Apigenin-7-O-glucoside) C21H20O10 24.09 433.11348 271.0606 153.0185 145.0282 119.0497
44 Dicaffeoylquinic acid C25H24O12 24.23 515.11896 353.0884 191.0556 179.0342 173.0446 135.0441 45
Methoxy-trihydroxyflavone-O-glucuronide C22H20O12 24.31 475.08766 299.0567 284.0332 175.0241 113.0231
46 Chrysoeriol-O-hexoside C22H22O11 24.35 461.10839 446.0861 299.0566 298.0489 283.0254 255.0301 47 Smyrinol (Decursinol) isomer O-hexoside
isomer 3 C20H24O9 24.50 409.14986 247.0971 229.0864 214.0604 175.0394
48 Smyrinol (Decursinol) isomer 1 C14H14O4 24.60 247.09704 229.0865 214.0630 175.0394 49
Methoxy-trihydroxyflavone-O-(deoxyhexosyl)hexoside C28H32O15 24.64 609.18195 463.1245 301.0712 286.0478 85.0291 71.0499 50 Tetrahydroxyflavone-O-hexoside C21H20O11 24.77 447.09274 327.0499 285.0411 284.0332 255.0301 227.0347 511 Isorhamnetin-3-O-glucoside C22H22O12 25.01 477.10330 315.0518 314.0440 299.0205 285.0412 243.0299
52 Luteolin-O-(malonyl)hexoside C24H22O14 25.31 533.09314 489.1046 447.0962 285.0410 284.0332 133.0280 53 Methoxy-furocoumarin isomer 1 C12H8O4 25.36 217.05009 202.0266 174.0313 146.0361 131.0491
54 Smyrinol (Decursinol) isomer 2 C14H14O4 26.25 247.09704 229.0865 214.0627 175.0394 55 Apigenin-O-(malonyl)hexoside C24H22O13 26.82 517.09822 473.1084 269.0460 268.0373
561 Quercetin C15H10O7 27.07 303.05048 285.0396 257.0450 229.0502 153.0181 137.0237
571 Naringenin C15H12O5 27.27 271.06065 177.0186 165.0182 151.0026 119.0489 107.0126
58 Dimethoxy-furocoumarin C13H10O5 27.32 247.06065 232.0370 217.0135 189.0196 161.0236 95.0133
59.28 mg TE/g extract for SC. The low antioxidant activity of SM for the radical scavenging and reducing power determinations tend to corro-borate with the low level of TFC recorded for SM extract. It is worth highlighting that phytochemicals present in plants might act in syner-gism, producing a higher activity as against the activity observed for a simple compound. This might explain the higher metal chelating ac-tivity recorded for SA extract. Phytochemicals identified in SA extract might act in combination to produce the high metal chelating activity observed (Tsao, 2015;Zeljković et al., 2015).
3.3. Enzyme inhibitory activity
Modulation of enzymatic activity plays a pivotal role in the treat-ment and/or managetreat-ment of several chronic complications (Rauf and Jehan, 2017). As such, the management of Alzheimer’s disease is greatly dependent on the inhibition of AChE and BChE. These enzymes hydrolyse acetylcholine, a neurotransmitter responsible for nerve transmission in central and peripheral cholinergic synapses, thereby regulating cognition and learning. Upregulation of AChE and BChE cause a deficiency in acetylcholine in the brain. Therefore the inhibition of these enzymes is a key strategy to alleviate symptoms of Alzheimer’s disease (Hassan et al., 2019). Nonetheless, low efficacy of currently used drugs has fuelled the need for new cholinesterase inhibitors and plants has proven to be an interesting source of pharmacophores. In the present investigation, the studied Apiaceae species, except AM, in-hibited AChE and BChE. SM extract exin-hibited the highest cholines-terases inhibition (3.82 and 4.76 mg GALAE/g extract, for AChE and BChE, respectively).
The inhibition of carbohydrate hydrolysing enzymes, namely α-amylase and α-glucosidase, plays a crucial role in the regulation of
hyperglycaemia, the hallmark of type 2 diabetes mellitus. Currently used hypoglycaemic drugs carry several side effects, including gastro-intestinal disturbances. It was found that excessive inhibition of α-amylase disturbed normal digestion process and resulted in large amounts of undigested food in the colon causing gastrointestinal in-conveniences, such as flatulence, incontinence, diarrhoea, and ab-dominal pain (Alam et al., 2019). Consequently, novel pharmacophores showing none or mild inhibitory action on α-amylase and significant inhibition on α-glucosidase, are in need (Etxeberria et al., 2012). Data presented inTable 8, showed that the studied Apiaceae species (0.51-0.55 mmol ACAE/g extract) were poor inhibitors of α-amylase. In contrast, higher inhibition was observed against α-glucosidase (7.32-11.99 mmol ACAE/g extract).
The ability of the selected Apiaceae species to inhibit tyrosinase was also evaluated. Tyrosinase, a copper-containing enzyme, plays a key role in the synthesis of melanin, by catalysing the hydroxylation ofL
-tyrosine toL-DOPA, which is subsequently oxidised to dopaquinone.
Considering that hyperpigmentation conditions occur due to over-production of melanin, tyrosinase inhibitors have proven to be valuable therapeutic tools (Zolghadri et al., 2019). In this study, FV, SA, SM, SC, and AM showed inhibitory action on tyrosinase, SC extract being the most active (137.54 mg KAE/g extract) as against SM (125.14 mg KAE/ g extract).
Lipase is a crucial target in the management of obesity. In fact, the digestion of fat to fatty acids and glycerides by lipase is an imperative to its uptake. Therefore, the inhibition of lipase is determinant in the management of obesity (Picot and Mahomoodally, 2017). Findings gathered in the present study revealed that the studied Apiaceae species suppressed lipase action. FV extract (113.75 mg OE/g) showed the highest lipase inhibitory action while AM extract (81.85 mg OE/g) was
Table 4 (continued)
No. Compound Formula Rt [M + H]+ [M - H]− Fragment 1 Fragment 2 Fragment 3 Fragment 4 Fragment 5 Literature 59 Luteolin-O-(acetyl)hexoside C23H22O12 27.33 489.10331 285.0411 284.0332
60
Dihydroxy-methoxyflavone-O-glucuronide C22H20O11 27.61 459.09274 283.0619 268.0381
61 Methoxy-furocoumarin isomer 2 C12H8O4 27.77 217.05009 202.0265 174.0315 146.0367 131.0493
621 Luteolin (3',4',5,7-Tetrahydroxyflavone) C15H10O6 27.91 285.03991 217.0505 199.0396 175.0391 151.0027 133.0283
63 Pabulenol C16H14O5 28.05 287.09195 269.0808 243.0654 203.0342 173.0234 147.0441 64 Apigenin-O-(acetyl)hexoside C23H22O11 28.83 473.10839 269.0459 268.0381
65 Chrysoeriol-O-(acetyl)hexoside C24H24O12 28.85 503.11896 488.0969 299.0577 298.0483 283.0255 255.0300 661 Apigenin (4',5,7-Trihydroxyflavone) C15H10O5 29.72 269.04500 225.0552 201.0551 151.0027 149.0234 107.0125
67 Tetrahydroxyflacone-O-(crotonyl)
hexoside C25H24O12 29.77 515.11896 447.0956 285.0410 284.0332
681 Isorhamnetin
(3'-Methoxy-3,4',5,7-tetrahydroxyflavone) C16H12O7 29.89 315.05048 300.0281 271.0269 164.0103 151.0026 69 Chrysoeriol (Scoparol,
3'-Methoxy-4',5,7-trihydroxyflavone) C16H12O6 29.95 299.05556 284.0331 256.0379 227.0346 151.0027 107.0125
70 Angelicin C11H6O3 30.53 187.03952 159.0444 143.0495 131.0496 115.0548 103.0546
71 Quercetin-O-(coumaroyl)deoxyhexoside C30H26O13 30.86 593.12952 447.0937 301.0357 300.0280 299.0216 271.0256 72 Luteolin-O-(cinnamoyl)hexoside C30H26O12 32.19 577.13461 285.0410 284.0333
73 Nakhsmyrin isomer 1 C14H12O4 32.27 245.08139 227.0706 217.0867 203.0708 175.0393 74 Nakhsmyrin isomer 2 C14H12O4 32.87 245.08139 227.0707 217.0862 203.0708 175.0394 75 Nakhsmyrin isomer 3 C14H12O4 34.50 245.08139 227.0707 217.0861 203.0707 175.0393 76 Dihydroxy-methoxy(iso)flavone C16H12O5 34.56 283.06065 268.0377 239.0342 211.0388
77 Xanthyletin C14H12O3 35.76 229.08647 214.0626 187.0396 175.0394 159.0445 131.0496 78 Selinidin C19H20O5 35.87 329.13890 229.0865 201.0916 187.0396 175.0394 159.0445
Table 5
Chemical composition of Smyrnium cordiifolium.
No. Compound Formula Rt [M + H]+ [M - H]− Fragment 1 Fragment 2 Fragment 3 Fragment 4 Fragment 5 Literature 1 Quinic acid C7H12O6 1.21 191.05557 173.0446 127.0388 111.0439 93.0331 85.0280
2 Pantothenic acid C9H17NO5 5.44 220.11850 202.1079 184.0974 174.1127 116.0347 90.0556 3 Kynurenic acid C10H7NO3 12.95 190.05042 162.0553 144.0448 116.0501 89.0391
41 Chlorogenic acid
(3-O-Caffeoylquinic acid) C16H18O9 14.25 355.10291 163.0393 145.0288 135.0445 117.0339 89.0391 5 Coumaroylhexose C15H18O8 14.68 325.09235 163.0391 119.0489
6 Quercetin-di-O-hexoside isomer 1 C27H30O17 16.79 625.14048 463.0888 462.0815 301.0359 299.0202 271.0255 7 Coumaroylquinic acid isomer 1 C16H18O8 16.89 337.09235 191.0556 173.0446 163.0391 119.0489 93.0331 8 Fraxetin
(7,8-Dihydroxy-6-methoxycoumarin) C10H8O5 17.14 207.02935 192.0060 164.0100
9 Caffeoylshikimic acid isomer 1 C16H16O8 17.22 335.07670 179.0343 161.0234 135.0442 111.0438 93.0331 10 Quercetin-di-O-hexoside isomer 2 C27H30O17 17.26 625.14048 463.0890 462.0814 301.0359 299.0202 271.0253 11 Coumaroylquinic acid isomer 2 C16H18O8 17.57 337.09235 191.0556 173.0447 163.0391 119.0489 93.0331 12 Caffeoylshikimic acid isomer 2 C16H16O8 17.63 335.07670 179.0340 161.0234 135.0441 111.0438 93.0331 131 4-Coumaric acid C9H8O3 17.88 163.03952 119.0489 93.0332
14 5-O-Feruloylquinic acid C17H20O9 18.01 367.10291 193.0501 191.0555 173.0447 134.0362 93.0331 15 Caffeoylshikimic acid isomer 3 C16H16O8 18.06 335.07670 179.0342 161.0234 135.0441 111.0438 93.0331 16
Quercetin-O-hexoside-O-malonylhexoside C30H32O20 18.26 711.14087 667.1522 505.1010 463.0887 462.0813 299.0201 17 Scopoletin
(7-Hydroxy-6-methoxycoumarin) C10H8O4 18.60 193.05009 178.0265 165.0551 149.0600 137.0602 133.0289 18 Caffeoylshikimic acid isomer 4 C16H16O8 18.90 335.07670 179.0344 161.0234 135.0442 111.0438 93.0333 19 Coumaroylquinic acid isomer 3 C16H18O8 19.17 337.09235 191.0556 173.0447 163.0388 119.0487 93.0331 20 Ferulic acid C10H10O4 19.39 193.05009 178.0263 149.0599 137.0232 134.0363 121.0282 21 Methyl-O-caffeoylquinate C17H20O9 19.63 367.10291 191.0560 179.0342 161.0234 135.0440
22
Kaempferol-O-deoxyhexoside-O-hexoside C27H30O15 20.07 593.15065 447.0944 446.0858 431.1005 285.0411 283.0252 23 Coumaroylshikimic acid C16H16O7 20.44 319.08178 163.0390 155.0339 119.0489 111.0437 93.0331 24 Dicaffeoylquinic acid isomer 1 C25H24O12 22.37 515.11896 353.0885 335.0784 191.0556 179.0343 135.0441 25 Quercetin-O-hexoside C21H20O12 22.72 463.08765 301.0360 300.0281 271.0254 255.0300 151.0026 26 Quercetin-O-(deoxyhexosyl)
hexoside C27H30O16 23.12 609.14557 301.0356 300.0282 271.0249 255.0296 151.0027 27 Quercetin-O-pentoside isomer 1 C20H18O11 23.29 433.07709 301.0358 300.0280 271.0257 255.0304 151.0026 28 Quercetin-O-pentoside isomer 2 C20H18O11 23.56 433.07709 301.0359 300.0281 271.0252 255.0301 151.0026 29 Quercetin-O-malonylhexoside C24H22O15 23.79 549.08805 505.0997 301.0359 300.0280 271.0254 255.0301 30 Caffeoyl-coumaroylquinic acid
isomer 1 C25H24O11 23.84 499.12404 353.0877 337.0938 191.0555 173.0445 163.0391 31 Eugenol (4-Allyl-2-methoxyphenol) C10H12O2 23.85 165.09156 137.0602 133.0653 124.0523 109.0289 105.0705 32 Caffeoyl-coumaroylquinic acid
isomer 2 C25H24O11 23.99 499.12404 353.0884 337.0935 191.0556 179.0343 163.0392 33 Quercetin-O-methylglucuronide C22H20O13 24.02 475.08766 463.0890 301.0360 300.0281 271.0253 255.0299 34 Dicaffeoylquinic acid isomer 2 C25H24O12 24.17 515.11896 353.0884 335.0766 191.0556 179.0342 173.0447 35 Kaempferol-O-hexoside C21H20O11 24.19 447.09274 285.0410 284.0332 255.0301 227.0347 36 Chrysoeriol-O-glucuronide C22H20O12 24.29 475.08766 299.0567 284.0332 256.0377 37 Chrysoeriol-O-hexoside C22H22O11 24.32 461.10839 446.0866 299.0566 298.0494 283.0254 255.0301 38 Caffeoyl-methylcaffeoylquinic acid isomer 1 C26H26O12 24.60 529.13460 367.1039 179.0342 173.0447 161.0234 135.0441 391 Isorhamnetin-3-O-glucoside C22H22O12 24.79 477.10330 315.0518 314.0439 285.0411 271.0253 243.0299 40 Caffeoyl-coumaroylquinic acid isomer 3 C25H24O11 25.72 499.12404 337.0936 191.0557 173.0446 163.0391 41 Caffeoyl-coumaroylquinic acid isomer 4 C25H24O11 26.03 499.12404 353.0882 337.0939 191.0556 179.0343 173.0447 42 Afzelin (Kaempferol-3-O-rhamnoside) C21H20O10 26.46 431.09782 285.0410 284.0332 255.0300 227.0347 43 Caffeoyl-methylcaffeoylquinic acid isomer 2 C26H26O12 26.55 529.13460 367.1038 179.0342 173.0446 161.0234 135.0441 44 Isorhamnetin-O-deoxyhexoside C22H22O11 26.60 461.10839 315.0519 314.0439 299.0204 285.0412 271.0252 451 Quercetin C15H10O7 27.03 303.05048 285.0402 257.0449 229.0498 165.0187 153.0186 46 Methoxymellein C11H12O4 27.73 209.08139 191.0708 181.0865 163.0758 135.0809 105.0706 47 Jasmonic acid C12H18O3 27.75 209.11777 165.1267 109.0640 59.0123
481 Luteolin (3',4',5,7-Tetrahydroxyflavone) C15H10O6 27.88 285.03991 217.0504 199.0399 175.0390 151.0026 133.0284 49 Methoxy-tetrahydroxy(iso)flavone C16H12O7 27.99 315.05048 300.0280 283.0258 243.0298 227.0352 164.0105 50 Dimethoxy-tetrahydroxy(iso) flavone C17H14O8 28.32 345.06105 330.0387 315.0155 287.0206 511 Kaempferol (3,4',5,7-Tetrahydroxyflavone) C15H10O6 29.35 285.03991 257.0452 229.0503 187.0393 185.0597 52 Chrysoeriol (Scoparol,
3'-Methoxy-4',5,7-trihydroxyflavone) C16H12O6 29.85 299.05556 284.0332 256.0383 227.0342 151.0020 531 Isorhamnetin (3'-Methoxy-3,4',5,7-tetrahydroxyflavone) C16H12O7 29.86 315.05048 300.0280 283.0260 227.0349 164.0104 151.0026 54 Methyl-trihydroxyxanthone C14H10O5 29.87 257.04500 215.0348 213.0555 189.0549 55 Dimethoxy-trihydroxy(iso)flavone isomer 1 C17H14O7 30.06 329.06613 314.0439 313.0355 299.0201
the least active. It is important to highlight that the enzyme inhibitory activity of the studied plant extracts was not representative of the TPC and TFC values recorded for the different extracts. For instance, SM extract possessing low TFC showed pronounced AChE and BChE in-hibition. Again, this could be attributed to the synergistic action of phytochemicals identified in this extract.
3.4. Multivariate analysis
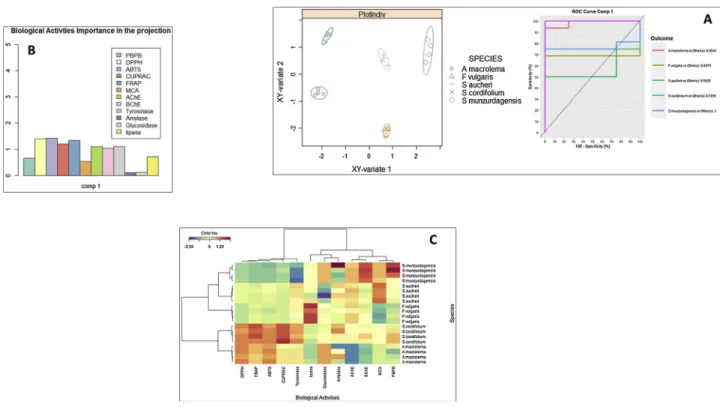
To provide a comprehensive insight on the tested extracts, we per-formed further statistical analysis. The statistical results were given Figs. 1 and 2. Apparently, we observed linear correlation between total bioactive compounds (TPC and TFC) and radical scavenging (0.73 for TPC and 0.95 for TFC in DPPH; 0.71 for TPC and 0.96 for TFC in ABTS) as well as reducing power (0.93 for TPC and 0.72 for TFC in CUPRAC; 0.83 for TPC and 0.85 for TFC in FRAP). However, a good correlation was not observed between enzyme results and the bioactive compo-nents. Also, principal component analysis (PCA) was performed to classify the tested extracts and biological activity assays (PC1: 48.3%; PC2: 19.1%). In PCA; the extracts were clearly classified. On the other hand, Sparse Partial Least Squares (sPLS-DA) analysis could provide a certain classification and this results are shown inFig. 2. In fact, the VIP analysis showed that the small subset of biological activities that better explain the differences across the tested extracts was DPPH and ABTS scavenging abilities (Fig. 2).
3.5. Antimicrobial activity
Significant advancements have been accomplished in micro-organism control over the past decades. Still, the emergence of drug-resistant strains threatening public health is a major public health concern representing a major challenge as well as a drive for the de-velopment of new classes of antimicrobial agents. In the current in-vestigation, the antibacterial activity of the selected Apiaceae species was evaluated against 3 g-positive and 5 g-negative bacteria. The minimum concentration of extract required to stop visible growth of bacteria (MIC) and to kill the bacteria (MBC) were determined. From Table 9, SA and SM extracts suppressed growth of S. aureus with an MIC value of 0.560 mg/mL but a concentration of 0.750 mg/mL was re-quired to produce a bactericidal effect. SM extract showed potent an-tibacterial effect against B. cereus (MIC 0.180 mg/mL), P. mirabilis (MIC 0.180 mg/mL), M. flavus (MIC 0.560 mg/mL), P. aeruginosa (MIC
0.275 mg/mL), S. typhimurium (MIC 1.500 mg/mL). As can be seen from Table 9, the recorded MBC values for these bacteria were higher as compared to the MIC values. FV extract (MIC 0.370 mg/mL) exhibited highest inhibitory action against E. coli. A group of researchers (Foroughi et al., 2018) published the inhibitory activity of FV extract against E. coli but reported a higher MIC value (240 mg/mL). Indeed, FV has been used in traditional medicine as antibacterial and antifungal agent (Zangeneh et al., 2018). Eight fungal strains were used as can-didate fungi for antifungal evaluation of the selected Apiaceae species. Antifungal assessment demonstrated that FV extract (MIC 0.140 mg/ mL) suppressed A. fumigatus growth. A. fumigatus has been identified as the most prevalent airborne fungal pathogen, responsible for severe and lethal infections in immunocompromised hosts, such as cancer patients, in developed countries (Latgé, 1999). SA extract showed even higher activity against A. fumigatus, with a MIC value of 0.035 mg/mL and a MFC value of 0.070 mg/mL. SA has been reported to exhibit antifungal action and in line with the present study, coumarins were identified from the Apiaceae species (Faridi et al., 2008). SM extract showed antifungal activity against A. terreus (MIC 0.070 mg/mL), T. viride (MIC 0.135 mg/mL), P. ochrochloron (MIC 0.370 mg/mL), and P. veruccosum (MIC 0.135 mg/mL) with MIC values lower than the positive control, ketoconazole (0.150, 1.000, 1.000, and 0.200 mg/mL for A. terreus, T. viride, P. ochrochloron, and P. veruccosum, respectively). It is good to note that the studied Apiaceae species showed antibacterial and anti-fungal activity towards all the tested strains, exhibiting variable degree of effectiveness.
3.6. Cytotoxicity
Despite the common perception that herbal products have reduced toxicity, it is a great importance to assess their safety for human utili-zation, as for example through cytotoxicity determination against mammalian cell lines (Mahomoodally et al., 2018). In this sense, the effect of the selected Apiaceae extracts on mammalian cells viability was investigated. Different cell lines, murine macrophages (RAW 264.7), human embryonic kidney (HEK 293), and human hepatocel-lular carcinoma (HepG2) cells were treated with the plant extracts in order to assess the cytotoxicity, and a cellular viability above 80% was considered as non-toxic (Zengin et al., 2018a). As can be seen in Table 10, the most part of the extracts showed to be non-toxic against all the cell lines, except the samples FV (60.3%) and SM (57.4%) that significantly reduced the cellular viability of RAW 264.7 macrophages,
Table 5 (continued)
No. Compound Formula Rt [M + H]+ [M - H]− Fragment 1 Fragment 2 Fragment 3 Fragment 4 Fragment 5 Literature 56 Dimethoxy-trihydroxy(iso)flavone
isomer 2 C17H14O7 30.41 329.06613 314.0437 313.0351 299.0205
57
1β-Acetoxyeudesman-4(15),7(11)-dien-8α,12-olide C17H22O4 30.98 291.15964 231.1385 213.1279 185.1330 170.1095 157.1016 (et al., 1985Ulubelen ) 58 Kaempferol-O-cinnamoylhexoside C30H26O12 32.13 577.13461 285.0410 284.0331 255.0300 227.0347
59 Hydroxy-trimethoxyxanthone C16H14O6 34.11 303.08686 288.0633 271.0609 243.0656 60
Methyl-dihydroxy-methoxyxanthone C15H12O5 35.02 271.06065 256.0380 255.0297 228.0429 1Confirmed by standard.
Table 6 Chemical composition of Actinolema macrolema. No. Compound Formula Rt [M + H] + [M -H] − Fragment 1 Fragment 2 Fragment 3 Fragment 4 Fragment 5 Literature 1 1 Gallic acid (3,4,5-Trihydroxybenzoic acid) C7H6O5 2.37 169.01370 125.0231 97.0282 81.0331 69.0331 2 Neochlorogenic acid (5-O-Caffeoylquinic acid) C16H18O9 8.86 355.10291 163.0394 145.0288 135.0445 117.0340 107.0497 3 Coumaroylhexose isomer 1 C15H18O8 14.21 325.09235 265.0729 187.0396 163.0390 145.0284 119.0489 4 Chlorogenic acid (3-O-Caffeoylquinic acid) C16H18O9 14.23 355.10291 163.0393 145.0288 135.0445 117.0339 107.0497 5 Biflorin or isobiflorin C16H18O9 14.54 353.08726 263.0565 245.0450 233.0453 205.0502 191.0556 6 3-O-Feruloylquinic acid C17H20O9 14.56 367.10291 193.0501 191.0555 173.0448 134.0362 93.0330 7 Coumaroylhexose isomer 2 C15H18O8 14.67 325.09235 265.0731 187.0401 163.0391 145.0284 119.0489 8 Biflorin or isobiflorin C16H18O9 15.33 353.08726 263.0563 245.0453 233.0453 205.0503 191.0557 9 Feruloylhexose C16H20O9 15.66 355.10291 295.0830 235.0615 193.0502 175.0392 160.0156 10 Apigenin- C-hexoside- C-pentoside-O-hexoside C32H38O19 17.43 727.20856 565.1569 403.1036 367.0820 313.0713 283.0606 11 5-O-Feruloylquinic acid C17H20O9 18.01 367.10291 193.0503 191.0556 173.0447 134.0363 93.0331 12 Caffeoylshikimic acid C16H16O8 18.06 335.07670 179.0341 161.0236 135.0441 13 Luteolin- C-hexoside-O-hexoside C27H30O16 18.52 609.14557 447.0940 357.0621 327.0515 313.0360 299.0566 14 4-O-Feruloylquinic acid C17H20O9 18.54 367.10291 193.0501 191.0555 173.0446 134.0363 93.0331 15 Vicenin-2 (Apigenin-6,8-di-C-glucoside) C27H30O15 19.03 593.15065 575.1429 503.1209 473.1096 383.0777 353.0672 16 Ferulic acid C10H10O4 19.37 193.05009 178.0263 149.0597 137.0233 134.0362 121.0281 17 Luteolin- C- hexoside-C-pentoside isomer 1 C26H28O15 19.51 579.13500 519.1192 489.1039 459.0940 399.0727 369.0623 18 Luteolin- C-hexoside-C-pentoside isomer 2 C26H28O15 19.67 579.13500 519.1149 489.1049 459.0939 399.0727 369.0621 19 Apigenin- C-hexoside-O-hexoside C27H30O15 19.85 595.16630 433.1140 415.1033 337.0712 313.0713 283.0606 20 1 Sinapic acid (Sinapinic acid) C11H12O5 19.89 225.07630 207.0659 192.0419 175.0394 147.0442 119.0494 21 Vicenin-1 (Apigenin-8- C-glucoside-6-C-xyloside) C26H28O14 20.08 563.14009 503.1192 473.1096 443.0992 383.0779 353.0672 22 N-(2-Phenylethyl)acetamide C10H13NO 20.13 164.10754 122.0969 105.0705 103.0549 90.9484 23 Schaftoside (Apigenin-8- C-arabinoside-6-C-glucoside) C26H28O14 20.34 563.14009 503.1212 473.1095 443.0991 383.0778 353.0672 24 Isoschaftoside (Apigenin-6- C-arabinoside-8-C-glucoside) C26H28O14 20.74 563.14009 503.1170 473.1097 443.0989 383.0780 353.0671 25 Isoorientin (Homoorientin, Lespecapitoside, Lutonaretin, Luteolin-6-C-glucoside) C21H20O11 20.77 449.10839 431.0984 413.0881 353.0664 329.0663 299.0556 26 1 Vitexin (Orientoside, Apigenin-8-C-glucoside) C21H20O10 21.42 433.11347 415.1033 397.0928 367.0818 313.0713 283.0607 27 Luteolin-C-pentoside isomer 1 C20H18O10 22.13 419.09783 401.0875 383.0770 365.0664 329.0663 299.0556 28 Isovitexin (Avroside, Homovitexin, Saponaretin, Apigenin-6-C-glucoside) C21H20O10 22.32 433.11347 415.1035 397.0928 367.0822 313.0714 283.0608 29 Dimethoxy-trihydroxy(iso)flavone-C-acetylhexoside isomer 1 C25H26O13 22.68 535.14517 517.1362 499.1247 481.1140 379.0820 349.0712 30 Apigenin- C-hexoside-O-deoxyhexoside C27H30O14 22.70 579.17139 433.1138 415.1032 337.0711 313.0712 283.0606 31 Scoparin (Chrysoeriol-8-C-glucoside) or Isoscoparin (Chrysoeriol-6-C-glucoside) C22H22O11 22.75 463.12404 445.1139 427.1031 409.0926 343.0820 313.0713 32 Quercetin-O-glucuronide C21H18O13 22.78 477.06692 301.0358 255.0291 178.9973 151.0027 33 Scoparin (Chrysoeriol-8-C-glucoside) or Isoscoparin (Chrysoeriol-6-C-glucoside) C22H22O11 23.11 463.12404 445.1135 427.1030 409.0926 343.0819 313.0712 34 Apigenin- C-hexoside-O-feruloylhexoside C37H38O18 23.55 771.21364 433.1133 415.1032 313.0711 283.0606 177.0550 35 Luteolin-C-pentoside isomer 2 C20H18O10 23.65 419.09783 401.0877 383.0769 365.0663 329.0662 299.0555 36 Apigenin- C-hexoside-O-coumaroylhexoside C36H36O17 24.02 741.20307 433.1140 379.0823 313.0713 283.0606 147.0444 37 Apigenin- C-pentoside-O-deoxyhexoside isomer 1 C26H28O13 24.15 549.16082 403.1032 385.0925 367.0819 313.0712 283.0606 38 Rosmarinic acid (Labiatenic acid) C18H16O8 24.29 359.07670 197.0451 179.0342 161.0234 135.0441 133.0284 39 Apigenin-C-pentoside isomer 1 C20H18O9 24.30 403.10291 385.0925 367.0821 337.0712 313.0712 283.0607 40 Apigenin- C- pentoside-O-feruloylhexoside C36H36O17 24.40 741.20307 403.1036 385.0925 367.0820 283.0607 177.0551 41 Dimethoxy-trihydroxy(iso)flavone-C-acetylhexoside isomer 2 C25H26O13 24.45 535.14517 517.1350 499.1244 481.1139 379.0820 349.0713 42 1 Eriodictyol C15H12O6 24.94 287.05556 151.0026 135.0440 107.0121 83.0122 43 Apigenin-C-pentoside isomer 2 C20H18O9 25.28 403.10291 385.0924 367.0821 337.0713 313.0713 283.0607 44 Bergapten (5-Methoxypsoralen) C12H8O4 25.30 217.05009 202.0266 189.0552 185.0241 174.0314 161.0602 45 Abscisic acid C15H20O4 25.40 263.12834 219.1387 204.1152 201.1280 152.0832 151.0754 46 Apigenin- C-pentoside-O-deoxyhexoside isomer 1 C26H28O13 25.53 549.16082 403.1033 385.0926 367.0820 313.0713 283.0607 (continued on next page )
Table 6 (continued ) No. Compound Formula Rt [M + H] + [M -H] − Fragment 1 Fragment 2 Fragment 3 Fragment 4 Fragment 5 Literature 47 Dihydroxy-dimethoxy(iso)flavone-C-hexoside C23H24O11 25.68 477.13969 445.1118 427.1043 409.0928 349.0715 295.0606 48 Dihydroxy-methoxy(iso)flavone- C-hexoside-O-deoxyhexoside C28H32O14 26.47 593.18703 447.1295 429.1189 351.0869 327.0869 297.0762 49 Dihydroxy-methoxy(iso)flavone-C-hexoside C22H22O10 26.67 447.12913 429.1187 411.1083 351.0870 327.0869 297.0763 50 Dihydroxy-methoxy(iso)flavone- C-hexoside-O-feruloylhexoside C38H40O18 27.06 785.22929 447.1290 429.1187 327.0867 297.0762 177.0550 51 1 Naringenin C15H12O5 27.25 271.06065 177.0187 151.0027 119.0488 107.0125 93.0332 52 Isopimpinellin C13H10O5 27.27 247.06065 232.0368 217.0134 189.0194 53 Methoxsalen (Xanthotoxin, 8-Methoxypsoralen) C12H8O4 27.71 217.05009 202.0265 174.0316 161.0600 131.0496 54 1 Luteolin (3',4',5,7-Tetrahydroxyflavone) C17H21NO3 27.88 285.03991 217.0500 199.0390 175.0391 151.0027 133.0283 55 Methoxy-tetrahydroxy(iso)flavone C16H12O7 28.00 315.05048 300.0279 56 1 Apigenin (4',5,7-Trihydroxyflavone) C15H10O5 29.70 269.04500 225.0549 201.0560 151.0028 149.0231 117.0331 57 1 Isorhamnetin (3'-Methoxy-3,4',5,7-tetrahydroxyflavone) C16H12O7 29.85 315.05048 300.0282 151.0027 107.0125 58 Chrysoeriol (Scoparol, 3'-Methoxy-4',5,7-trihydroxyflavone) C16H12O6 29.91 299.05556 284.0331 256.0382 59 Apigenin-C-deoxyhexoside isomer 1 C21H20O9 30.43 417.11856 399.1077 381.0975 363.0869 313.0713 283.0606 60 Angelicin C11H6O3 30.52 187.03952 159.0444 143.0497 131.0497 115.0550 103.0552 61 Apigenin-C-deoxyhexoside isomer 2 C21H20O9 31.20 417.11856 399.1072 381.0974 363.0871 313.0715 283.0604 62 Tetrahydroxyflavone-O-cinnamoylhexoside C30H26O12 32.11 577.13461 285.0405 284.0323 255.0298 63 Dodecanedioic acid C12H22O4 33.33 229.14399 211.1337 167.1433 64 Furocoumarine derivative C14H10O3 34.14 227.07082 212.0467 199.0395 187.0393 159.0445 115.0546 65 Xanthyletin C14H12O3 35.69 229.08647 214.0628 187.0396 175.0393 159.0445 131.0495 66 Hydroxylauric acid C12H24O3 39.32 215.16473 197.0543 169.1588 67 Hexadecanedioic acid C16H30O4 40.36 285.20659 267.1969 223.2065 68 1 α-Linolenic acid C18H30O2 44.73 277.21676 259.2084 233.2246 127.0754 59.0125 69 Hydroxypalmitic acid C16H32O3 44.97 271.22732 253.2185 225.2221 223.2063 70 1 Linoleic acid C18H32O2 45.75 279.23241 97.0659 71.0139 59.0139 71 1 Oleic acid C18H34O2 46.84 281.24806 195.0444 1Confirmed by standard.
and SM (74.1%) that had low cytotoxicity (> 70% of cell viability) against HepG2 cells. However, it is important to note that this is a preliminary experiment before future testing on mammalian animal models.
4. Conclusion
From the present investigation, Apiaceae species might be regarded
as important medicinal plant species, harbouring several classes of phytochemicals which possess multiple biological activities. Data col-lected from this investigation tend to support the use of Apiaceae spe-cies in traditional medicinal systems for the management of human ailments. SC and AM demonstrated high radical scavenging and redu-cing activity. In general, the studied Apiaceae species were poor in-hibitors of α-amylase and potent inin-hibitors of α-glucosidase. SM, SC, and FV exhibited highest inhibition against cholinesterase enzymes,
Table 7
Antioxidant properties of the Apiaceae species. Samples Phosphomolybdenum
(mmol TE/g extract) DPPH(mg TE/g extract) ABTS(mg TE/g extract) CUPRAC (mg TE/g extract) FRAP(mg TE/g extract) Metal chelatingactivity (mg EDTAE/g extract) FV 1.18 ± 0.06b 10.27 ± 0.48c 38.62 ± 2.90c 85.29 ± 1.41b 62.23 ± 1.31c 23.37 ± 1.18d SA 1.32 ± 0.06a 19.37 ± 1.66b 48.13 ± 3.57b 87.19 ± 3.84b 59.64 ± 2.23d 48.22 ± 1.13a SM 1.68 ± 0.27a 2.29 ± 0.21d 17.94 ± 3.03d 72.40 ± 1.74c 43.91 ± 0.43e 43.35 ± 1.59a SC 1.47 ± 0.16a 59.28 ± 1.27a 94.31 ± 3.84a 161.44 ± 6.23a 113.62 ± 6.69a 35.91 ± 0.57b AM 1.16 ± 0.13b 59.06 ± 1.00a 94.68 ± 4.30a 115.55 ± 2.18a 92.90 ± 2.96b 30.58 ± 0.52c Values expressed are means ± S.D. of three parallel measurements. TE: Trolox equivalent; EDTAE: EDTA equivalent. Different superscript indicate differences in the tested extracts (p < 0.05).
Table 8
Enzyme inhibitory effects of the Apiaceae species. Samples AChE inhibition
(mg GALAE/g extract) BChE inhibition(mg GALAE/g extract) Tyrosinaseinhibition (mg KAE/g extract)
Amylase inhibition (mmol ACAE/g
extract) Glucosidase inhibition(mmol ACAE/g extract) Lipase inhibition (mg OE/g) FV 3.45 ± 0.22b 1.87 ± 0.46c 133.64 ± 0.28b 0.51 ± 0.01a 10.06 ± 0.47b 113.75 ± 3.20a SA 3.59 ± 0.17b 1.80 ± 0.30c 134.64 ± 0.78b 0.54 ± 0.01a 7.32 ± 1.34c 91.57 ± 7.21b SM 3.82 ± 0.08a 4.76 ± 0.19a 125.14 ± 1.15c 0.52 ± 0.07a 11.65 ± 0.01a 92.26 ± 0.36b SC 2.92 ± 0.10c 2.64 ± 0.46b 137.54 ± 0.55a 0.55 ± 0.03a 9.72 ± 0.52b 85.97 ± 3.31c
AM na 0.27 ± 0.09d 133.85 ± 1.96b 0.52 ± 0.05a 11.99 ± 0.05a 81.85 ± 0.93c
Values expressed are means ± S.D. of three parallel measurements. GALAE: Galatamine equivalent; KAE: Kojic acid equivalent; ACAE: Acarbose equivalent; OE: Orlistat equivalent. Different superscript indicate differences in the tested extracts (p < 0.05).
Fig. 1. (A: Relationship between total bioactive compounds and biological activities (Pearson Correlation Coefficient (R), p < 0.05). B: Factorial plan 1–2 of the PCA
tyrosinase, and lipase, respectively. SM suppressed bacterial growth. Most of the extracts were non-toxic against all the cell lines, except FV and SM. This study supports the need for further biological evaluations
to establish the activity of phytochemical or group of phytochemicals occurring in the studied Apiaceae species. Validation using in vivo and in silico models is highly recommended.
Fig. 2. (A: Factorial plan 1–2 of the sPLS-DA results obtained from biologicals activities of five extracts. B: Influence of 12 variables (biologicals activities) for the
total map (VIP variable importance in the prediction. C: Clustering of extracts in according to biological activities based on Heatmap. PBPB: Phosphomolybdenum; MCA: Metal chelating).
Table 9
Antibacterial and antifungal activity (mg/mL) of the Apiaceae species*.
Antibacterial abilities
Apiaceae species S. aureus+ B. cereus+ P. mirabilis M. flavus+ P. aeruginosa E. coli S. typhimurium E. cloacae FV MIC 1.125 ± 0.02c 0.560 ± 0.02e 0.370 ± 0.04e 0.750 ± 0.04d 0.275 ± 0.03b 0.370 ± 0.02b 2.250 ± 0.30c 0.750 ± 0.03b MBC 1.500 ± 0.03c 0.750 ± 0.03d 0.750 ± 0.05d 1.500 ± 0.08c 0.370 ± 0.02b 0.750 ± 0.05b 3.000 ± 0.60b 1.500 ± 0.08b SA MIC 0.560 ± 0.03b 0.370 ± 0.01d 0.275 ± 0.04d 0.750 ± 0.03d 0.275 ± 0.02b 0.560 ± 0.04c 2.250 ± 0.40c 0.750 ± 0.06b MBC 0.750 ± 0.06b 0.750 ± 0.04d 0.370 ± 0.03c 1.500 ± 0.06c 0.370 ± 0.03b 1.125 ± 0.08c 3.000 ± 0.80b 1.500 ± 0.05b SM MIC 0.560 ± 0.04b 0.180 ± 0.03c 0.180 ± 0.06c 0.560 ± 0.03c 0.275 ± 0.03b 0.560 ± 0.03c 1.500 ± 0.50b 1.125 ± 0.04c MBC 0.750 ± 0.05b 0.370 ± 0.03c 0.750 ± 0.04d 0.750 ± 0.05b 0.370 ± 0.02b 1.500 ± 0.06c 3.000 ± 0.30b 1.500 ± 0.06b SC MIC 1.125 ± 0.04c 0.560 ± 0.02e 0.560 ± 0.05f 1.125 ± 0.08e 0.560 ± 0.03c 0.560 ± 0.05c 2.250 ± 0.40c 1.125 ± 0.08c MBC 1.500 ± 0.05c 0.750 ± 0.05d 0.750 ± 0.03d 1.500 ± 0.06c 0.750 ± 0.06d 0.750 ± .0.06b 3.000 ± 0.30b 1.500 ± 0.06b AM MIC 1.125 ± 0.04c 0.560 ± 0.04e 0.275 ± 0.02d 0.560 ± 0.04c 0.275 ± 0.03b 0.560 ± 0.06c 2.250 ± 0.20c 0.750 ± 0.04b MBC 1.500 ± 0.03c 0.750 ± 0.06d 0.370 ± 0.03c 0.750 ± 0.05b 0.370 ± 0.02b 0.750 ± 0.05b 3.000 ± 0.50b 1.500 ± 0.06b Streptomycin MIC 0.100 ± 0.03a 0.025 ± 0.002a 0.100 ± 0.03b 0.050 ± 0.003a 0.100 ± 0.003a 0.100 ± 0.03a 0.100 ± 0.10a 0.100 ± 0.03a MBC 0.200 ± 0.02a 0.050 ± 0.003a 0.200 ± 0.02b 0.100 ± 0.02a 0.200 ± 0.04a 0.200 ± 0.03a 0.200 ± 0.09a 0.200 ± 0.03a Ampicillin MIC 0.100 ± 0.03a 0.100 ± 0.003b 0.100 ± 0.002a 0.100 ± 0.03b 0.300 ± 0.03a 0.150 ± 0.02a 0.100 ± 0.08a 0.150 ± 0.02a MBC 0.150 ± 0.02a 0.150 ± 0.03b 0.012 ± 0.003a 0.150 ± 0.03a 0.500 ± 0.06a 0.200 ± 0.02a 0.200 ± 0.06a 0.200 ± 0.02a Antifungal abilities
Apiaceae species A. fumigatus A. versicolor A. terreus A. niger T. viride P. ochrochloron P. funiculosum P. veruccosum
FV MIC 0.140 ± 0.05b 2.250 ± 0.40e 0.280 ± 0.02c 3.000 ± 0.40d 0.275 ± 0.02b 3.000 ± 0.90f 1.500 ± 0.08d 0.750 ± 0.03c MFC 0.280 ± 0.02b 4.000 ± 0.80d 0.560 ± 0.04c 4.500 ± 0.60f 0.370 ± 0.03b 4.500 ± 0.80d 3.000 ± 0.30d 1.500 ± 0.20b SA MIC 0.035 ± 0.003a 1.500 ± 0.30d 0.220 ± 0.03c 0.750 ± 0.05c 0.370 ± 0.03c 0.560 ± 0.04c 0.750 ± 0.03c 0.750 ± 0.03c MFC 0.070 ± 0.004a 3.000 ± 0.50c 0.280 ± 0.02b 1.500 ± 0.03d 0.750 ± 0.05c 0.750 ± 0.05b 3.000 ± 0.20d 3.000 ± 0.40c SM MIC 0.210 ± 0.03c 1.500 ± 0.20d 0.070 ± 0.005a 0.560 ± 0.03b 0.135 ± 0.02a 0.370 ± 0.02b 0.275 ± 0.02a 0.135 ± 0.03a MFC 0.280 ± 0.02b 3.000 ± 0.40c 0.140 ± 0.02a 0.750 ± 0.04c 0.180 ± 0.02a 0.750 ± 0.04b 0.370 ± 0.03b 0.180 ± 0.02a SC MIC 0.140 ± 0.02b 0.370 ± 0.05b 0.280 ± 0.03c 0.560 ± 0.05b 0.135 ± 0.02a 0.750 ± 0.03d 0.560 ± 0.03b 0.275 ± 0.02b MFC 0.280 ± 0.03b 0.750 ± 0.08b 0.560 ± 0.04c 0.750 ± 0.03c 0.180 ± 0.03a 1.500 ± 0.20c 0.750 ± 0.04c 0.370 ± 0.03b AM MIC 1.125 ± 0.05d 0.750 ± 0.06c 3.000 ± 0.50d 0.750 ± 0.03c 0.277 ± 0.03b 0.560 ± 0.03c 0.560 ± 0.03b 0.750 ± 0.02c MFC 2.250 ± 0.30c 3.000 ± 0.30c 4.500 ± 0.60d 3.000 ± 0.50e 0.370 ± 0.03b 0.750 ± 0.03b 0.750 ± 0.05c 3.000 ± 0.30c Ketoconazole MIC 0.200 ± 0.03c 0.200 ± 0.03a 0.150 ± 0.03bc 0.200 ± 0.02a 1.000 ± 0.04d 1.000 ± 0.20e 0.200 ± 0.02a 0.200 ± 0.02ab MFC 0.500 ± 0.02bc 0.500 ± 0.05b 0.200 ± 0.02b 0.500 ± 0.03b 1.500 ± 0.05c 1.500 ± 0.30c 0.500 ± 0.03bc 0.300 ± 0.03b Bifonazole MIC 0.150 ± 0.02b 0.100 ± 0.02a 0.100 ± 0.01b 0.150 ± 0.02a 0.150 ± 0.02a 0.200 ± 0.03a 0.200 ± 0.02a 0.100 ± 0.02a MFC 0.200 ± 0.02b 0.200 ± 0.03a 0.150 ± 0.02a 0.200 ± 0.02a 0.200 ± 0.02a 0.250 ± 0.03a 0.250 ± 0.02a 0.200 ± 0.02a * Different letters indicate the differences in the these species for each bacterial and fungal strains (p < 0.05).