Contents lists available atScienceDirect
Journal of Hazardous Materials
journal homepage:www.elsevier.com/locate/jhazmatHydrogen sul
fide (H
2
S) and nitric oxide (NO) alleviate cobalt toxicity in
wheat (Triticum aestivum L.) by modulating photosynthesis, chloroplastic
redox and antioxidant capacity
Ceyda Oz
fidan-Konakci
a, Evren Yildiztugay
b, Fevzi Elbasan
b, Mustafa Kucukoduk
c,
Ismail Turkan
d,*
aDepartment of Molecular Biology and Genetics, Faculty of Science, Necmettin Erbakan University, Meram, 42090, Konya, Turkey bDepartment of Biotechnology, Faculty of Science, Selcuk University, Selcuklu, 42250, Konya, Turkey
cDepartment of Biology, Faculty of Science, Selcuk University, Selcuklu, 42250, Konya, Turkey dDepartment of Biology, Faculty of Science, Ege University, Bornova, 35100, Izmir, Turkey
G R A P H I C A L A B S T R A C T A R T I C L E I N F O Editor: R. Debora Keywords: Antioxidant enzyme Chloroplast Hydrogen sulfide Nitric oxide Triticum aestivum A B S T R A C T
The role of hydrogen sulfide (H2S)/nitric oxide (NO) in mitigating stress-induced damages has gained interest in
the past few years. However, the protective mechanism H2S and/or NO has towards the chloroplast system
through the regulation of redox status and activation of antioxidant capacity in cobalt-treated wheat remain largely unanswered. Triticum aestivum L. cv. Ekiz was treated with alone/in combination of a H2S donor (sodium
hydrosulfide (NaHS,600μM)), a NO donor (sodium nitroprusside (SNP,100μM)) and a NO scavenger (rutin hydrate (RTN,50μM)) to assess how the donors affect growth, water relations, redox and antioxidant capacity in chloroplasts, under cobalt (Co) concentrations of 150-300μM. Stress decreased a number of parameters (growth, water content (RWC), osmotic potential (ΨΠ), carbon assimilation rate, stomatal conductance, intercellular CO2
https://doi.org/10.1016/j.jhazmat.2020.122061
Received 9 August 2019; Received in revised form 12 October 2019; Accepted 8 January 2020
Abbreviations: AsA, ascorbate; A, carbon assimilation rate; Ci, intercellular CO2concentrations; DHAR, dehydroascorbate reductase; E, transpiration rate; gs,
stomatal conductance; H2O2, hydrogen peroxide; MDHAR, monodehydroascorbate reductase; NaHS, sodium hydrosulfide NO, Nitric oxide; POX, peroxidase; RGR,
growth; RWC, relative water content; SNP, sodium nitroprusside; SOD, superoxide dismutase
⁎Corresponding author.
E-mail addresses:cozfidan@erbakan.edu.tr(C. Ozfidan-Konakci),eytugay@selcuk.edu.tr(E. Yildiztugay),fevzi.elba@gmail.com(F. Elbasan), mkucukoduk@selcuk.edu.tr(M. Kucukoduk),ismail.turkan@ege.edu.tr(I. Turkan).
Available online 09 January 2020
0304-3894/ © 2020 Elsevier B.V. All rights reserved.
concentrations, transpiration rate and the transcript levels of rubisco, which subsequently disrupt the photo-synthetic capacity). However, SNP/NaHS counteracted the negative effects of stress on these aforementioned parameters and RTN application with stress/non-stress was reversed these effects. Hydrogen peroxide (H2O2)
and TBARS were induced under stress in spite of activated ascorbate peroxidase (APX). SNP/NaHS under stress increased activation of superoxide dismutase (SOD), peroxidase (POX), APX, glutathione reductase (GR), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), ascorbate (tAsA) and glu-tathione (GSH). In conclusion, NaHS/SNP are involved in the regulation and modification of growth, water content, rubisco activity and up-regulation of ascorbate-glutathione cycle (AsA-GSH) in chloroplast under stress.
1. Introduction
Nitric oxide (NO) and carbon monoxide (CO) play fundamental roles as gaseous signal molecules in plants as well as in bacteria, ver-tebrates and mammals. Hydrogen sulfide (H2S), a colorless, soluble and flammable gas, is considered as the third gaseous transmitter (Hancock and Whiteman, 2014). In recent years, the roles of H2S and its inter-action with other molecules in the cells have been an important re-search topic. For instance, a study conducted byMancardi et al. (2009) revealed that H2S is produced fromL-cysteine by cystathionine
β-syn-thase and cystathionine gamma-lyase. Within the last decade, it is ac-knowledged that H2S can act in primary growth and metabolic pro-cesses such as adventitious root and lateral root formation (Fang et al., 2014), seed germination (Dooley et al., 2013), the regulation of post-harvest fruit ripening and senescence (Hu et al., 2014), signaling in guard cells (Hou et al., 2013), the enhancement of photosynthesis (Chen et al., 2011), the delay of gibberellin-mediated programmed cell death (Xie et al., 2014) and hormone-signaling pathway such as abscisic acid, auxin and ethylene signaling (Cheng et al., 2013;Jin et al., 2013). On the other hand, NO, another gaseous transmitter, promotes the photosynthetic rate, growth, stomatal movement, plant senescence, programmed cell death, signal transduction and enzyme activity under stress or non-stress conditions (Fan et al., 2013;Sami et al., 2018). For example, pre-treatment with NO donor promoted the tolerance against cadmium and osmotic stresses in Vigna radiata (Li et al., 2019) and the signaling pathways related to Ca2+, protein kinases and transcription factors (García-Mata and Lamattina, 2013). Accumulating evidence showed that one of the roles of H2S is to serve as an emerging signaling molecule in plants, similar to the NO activation (Chen et al., 2011; Fotopoulos et al., 2015). Additionally, a previous study has noted that there is co-operation and cross-talk between H2S and NO in stress-treated plants (Li et al., 2013). During seed germination, lateral root formation and stress conditions, the cross-talk among NO, Ca2+and H2S has been displayed (Dooley et al., 2013;Li et al., 2014;Shi et al., 2014). However, the interaction of NO and H2S needs to be further dissected, especially in terms of their impact on photosynthesis and their modulating role on redox and antioxidant capacity of chloroplasts. Apart from the roles of H2S on the regulatory and developmental process, there are a number of reports demonstrating the ameliorative effects of H2S against stress conditions such as drought, hypoxia, os-motic, salinity,flooding and extreme temperatures (Corpas et al., 2019; He et al., 2019;Kaya et al., 2019). Furthermore, it has been widely reported that H2S has positive impacts on tolerance in plants grown under metal-contaminated soils caused by elements such as cadmium, nickel, chromium, copper and lead (Kushwaha and Singh, 2019; Rizwan et al., 2019). However, to the best of our knowledge, no evi-dence has yet been reported whether H2S could be involved in the regulation of tolerance mechanisms under excess cobalt conditions.
Cobalt (Co2+), an integral component of the coenzyme cobalamin (vitamin B12 and its derivatives), can play a role in the retardation of leaf senescence via inhibition of ethylene biosynthesis and inhibition uptake of toxic elements (such as cadmium and lead) and nodule de-velopment and nitrogen fixation of leguminous plants (Palit et al., 1994). When a high level of Co2+concentrations has reached in plants, a series of remarkable impacts are noted on chloroplast, namely: (i)
reduction of carbon uptake leading to the disruption of carbon dioxide assimilation, (ii) disturbance of the enzymes involved in chlorophyll biosynthetic pathway, (iii) replacement of magnesium by Co2+in ru-bisco (ribulose-1,5-bisphosphate-carboxylase/oxygenase) that is a key protein for photosynthesis and (iv) the production of reactive oxygen species (ROS) such as superoxide anion radicals (O2%−) and hydrogen peroxide (H2O2) through interaction between molecular oxygen and electrons escaping from the photosynthetic electron transfer system (Foyer et al., 1994;Van Breusegem et al., 2001;Pandey and Sharma, 2002). The accumulation of ROS in chloroplast is minimized by the water-water cycle composed of antioxidants namely superoxide dis-mutase (SOD), ascorbate peroxidase (APX), glutathione reductase (GR), ascorbate (AsA) and glutathione (GSH) (Asada, 1999).
The current study is designed to answer the following to some questions: (i) Can H2S and/or NO alone and in combination prevent cobalt stress? (ii) Can H2S and NO eliminate the negative effects of cobalt-induced oxidative stress on chloroplasts? (iii) If they have posi-tive effects on chloroplast system, could H2S and/or NO act a regulator on photosynthetic capacity, antioxidant mechanisms and ROS content in the chloroplasts of wheat leaves with excess Co? To evaluate the impact of H2S and NO on tolerance mechanisms under stress conditions, one of the methods applied is water-soluble donors of H2S and NO such as sodium hydrosulphide (NaHS) and sodium nitroprusside (SNP), re-spectively. In the present study, to identify the potential roles of NaHS and SNP application under Co toxicity, several factors were analyzed in the chloroplasts of wheat leaves namely growth, water content (RWC), leaf osmotic potential (ΨΠ), photosynthetic parameters, ROS content (H2O2), expression levels of rubisco, antioxidant enzyme activities (SOD, POX, APX, GR, MDHAR and DHAR) and lipid peroxidation.
2. Material and methods
2.1. Plant material and experimental design
Wheat seeds (Triticum aestivum L. cv. Ekiz) were obtained from the Bahri Dagdas International Agricultural Research Institute Konya, Turkey. Seeds were surface-sterilized in 5 % sodium hypochlorite for 10 min, rinsedfive times with sterile distilled water and then allowed to germinate on double-layer filter paper wetted with distilled water. Germinated wheat seedlings were transferred to half strength Hoagland solution and were grown under the growth chamber conditions (16/8 h light/dark regime at 24 °C, 70 % relative humidity and 350μmol m−2 s−1photosynthetic photonflux density). The seedlings were grown in hydroponic culture containing this solution for 21 d and the solutions were replaced by fresh half-strength Hoagland solution, twice a week. For determination of ideal experimental donors of NO and H2S and stress concentrations, 100 μM SNP (NO donor), 600 μM NaHS (H2S donor) and 150 and 300μM cobalt (Co) were selected based on pre-vious experiments and concentrations found in the literature (Wang et al., 2010;Li et al., 2013; 2015). To further assess whether NO ac-cumulation was involved in responses induced by NaHS, the specific NO scavenger rutin hydrate (RTN) was applied. The wheats were treated with 50μM RTN (a NO scavenger) either singly or in combi-nation with or without SNP. The concentration of RTN was chosen based on the studies ofSingh et al. (2017). This treatment groups were
(1) SNP + RTN, 100 μM SNP together with 50 μM RTN treatments without Co treatments, (2) SNP + NaHS + RTN, 100μM SNP, 600 μM NaHS treatments together with 50μM RTN without Co treatments, (3) Co1+SNP + RTN, 100 μM SNP treatment together with 50 μM RTN application under 150μM Co treatment, (4) Co1+SNP + NaHS + RTN, 100μM SNP treatment, 600 μM NaHS together with 50 μM RTN ap-plication under 150μM Co treatment, (5) Co2+SNP + RTN, 100 μM SNP treatment together with 50μM RTN application under 300 μM Co treatment, (6) Co2+SNP + NaHS + RTN, 100μM SNP treatment, 600 μM NaHS together with 50 μM RTN application under 300 μM Co treatment. Plants were harvested after 72 h of treatment and the leaves were stored at −86 °C until further analyses. The photographs har-vested plants were given as Supplementary data. The experimental design included the twelve treatment groups: (1) Control, Normal conditions without Co, NaHS and SNP applications (2) SNP, 100μM SNP application alone without NaHS and Co treatments (3) NaHS, 600 μM NaHS treatment alone without SNP and Co treatments (4) SNP + NaHS, 100 μM SNP together with 600 μM NaHS treatments without Co treatments (5) Co1, 150μM Co treatment alone without 100 μM SNP and 600 μM NaHS application (6) Co1 + SNP, 150 μM Co treatment together with 100μM SNP application (7) Co1 + NaHS, 150 μM Co treatment together with 600 μM NaHS application (8) Co1 + SNP + NaHS, 100μM SNP treatment together with 600 μM NaHS ap-plication under 150 μM Co treatment (9) Co2, 300 μM Co treatment alone without 100μM SNP and 600 μM NaHS application (10) Co2 + SNP, 300μM Co treatment together with 100 μM SNP application (11) Co2 + NaHS, 300μM Co treatment together with 600 μM NaHS ap-plication (12) Co2 + SNP + NaHS, 100μM SNP treatment together with 600μM NaHS application under 300 μM Co treatment.
2.2. Determination of growth, water content and osmotic potential
Six plants were used for the control group and for each treatment group. The length of seedlings was measured with a ruler. Fresh weights (FW) were measured. After the samples were dried, dry weights (DW) were measured. RGR values were calculated according to the following formula byHunt et al. (2002):
RGR = [ln (DW2)– ln (DW1)] / (t2– t1),
where DW1= dry weight (g) at t1; DW2= dry weight (g) at t2, t1; initial harvest and t2;final harvest.
After 72 h, six leaves were harvested and their fresh weight (FW) were determined. The leaves werefloated on de-ionised water for 6 h and the turgid tissue was blotted dry prior to determining turgid weight (TW). Dry weight (DW) was determined after oven drying at 70 °C. The leaf relative water content (RWC) was calculated by the following formula (Smart and Bingham, 1974):
RWC (%) = [(FW-DW) / (TW-DW)] x 100
Leaves were extracted by crushing the material with a glass rod. Leaf osmotic potential (ΨΠ) was measured by Vapro Vapor pressure Osmometer 5600.ΨΠwas converted to MPa according toSanta-Cruz et al. (2002)by multiplying by a coefficient of 2.408 × 10−3. 2.3. Determination of photosynthetic parameters
A portable fluorometer (FMS-2, Hansatech, King's Lynn, UK) was used to determine the maximal quantum yield of PSII photochemistry (Fv/Fm). Carbon assimilation rate (A), stomatal conductance (gs), in-tercellular CO2 concentration (Ci) and transpiration rate (E) were measured with a portable gas exchange system (LCpro+; ADC, Hoddesdon, UK). The stomatal limitation value (Ls) was measured as 1 – Ci/Ca(Ma et al., 2011).
2.4. Determination of endogenous ion contents in leaves
The Co2+, K+, Ca2+, Mg2+ and Fe2+ contents in leaves were analyzed by Varian Vista-MPX simultaneous inductively coupled plasma optical emission spectrometer (ICP-OES) (Nyomora et al., 1997).
2.5. Determination of endogenous contents of NO and H2S in leaves
Nitric oxide content was measured as detected by Zhou et al. (2005). Absorbance was determined at 540 nm. NO content was cal-culated by comparison to a standard curve of NaNO2.
H2S content was performed as described byNashef et al. (1977). H2S was quantified based on a standard curve of known concentrations of NaHS.
2.6. Isolation of chloroplasts
Intactness of the chloroplasts was detected by using a ferricyanide reduction test (Lilley et al., 1975). The total soluble protein content was completed as suggested byBradford (1976).
2.7. Gene expression analysis
RNA isolation was performed using Qiagen RNeasy kit. Total RNA was treated with DNase I (Fermentas) to remove residual genomic DNA. Then, reverse transcription was detected using RevertAid First Strand cDNA Synthesis Kit (Thermo K1622). These cDNAs were used as tem-plates for quantitative reverse-transcription-PCR (qRT-PCR) using SYBR Select Master Mix (Applied Biosystems). The amount of RNA in each reaction was normalized to the T. aestivum GAPDH gene. Three in-dependent experiments were identified for qRT-PCR assays with the PikoReal 24 Real‐Time PCR System (Thermo Scientific) and the data was analyzed with PikoReal Software version 2.2 (Thermo Scientific). qRT-PCR conditions were as follows: 95 °C for 30 s and 40 cycles of 95 °C for 5 s and 60 °C for 30 s. The amplicons specificities were de-tected by melting curves analysis (60–95 °C) after 40 PCR cycles. T. aestivum grown under controlled conditions was used as a reference point and relative expression levels were calculated with respect to this reference value (set to 1) for the rubisco large subunit (rbcL) gene that was studied. Expression profile of rbcL gene was determined by aver-aging of Ct value of three technical replicates from three biological replications. The primer specificity was confirmed using melting curve analysis. The relative expression of rbcL gene was calculated by the 2− DDCT method using Ct value (Livak and Schmittgen, 2001).
Primers were designed using the IDT-PrimerQuest tool (http://eu. idtdna.com/primerquest/ home/index) and validated using IDT-OligoAnalyzer 3.1 (https://eu.idtdna.com/calc/analyzer) for homo-logue cDNA sequences. Based on sequences in the GenBank database (Accession number:LT576864.1), the following gene-specific primers were designed with Primer Premier 5.0 and used for amplification: Rubisco large subunit (rbcL): forward primer, 5′-GGTCGTCCTTTATTG GGATGT-3′ and reverse primer, 5′−CCACCACGTAGACACTCAT AAC-3′; GAPDH: forward primer, 5′-AAGGAGGAGTCTGAGGGAAA-3′ and reverse primer, 5′-GAAGATGCTGGACCTGTTGT-3′. The primers were synthesized by Macrogen (Rockville, USA).
2.8. Determination of H2O2content and lipid peroxidation levels in the chloroplasts
Determination of H2O2content was measured according toLiu et al. (2010). The level of lipid peroxidation was measured according toRao and Sresty (2000). TBARS concentration was calculated from the ab-sorbance at 532 nm, and measurements were corrected for nonspecific turbidity by subtracting the absorbance at 600 nm. The concentration of TBARS was calculated using an extinction coefficient of 155
mM−1cm−1.
2.9. Identification of isozyme and/or enzyme compositions in chloroplasts For SOD (EC 1.15.1.1) isozyme activity, samples were subjected to non-denaturing polyacrylamide gel electrophoresis (PAGE) as described byLaemmli (1970). Chloroplastic SOD activity assay was based on the method ofBeauchamp and Fridovich (1971). The isozymes and enzyme activity of POX (EC 1.11.1.7) were based on the method described by Seevers et al. (1971) and Herzog and Fahimi (1973), respectively. Electrophoretic APX separation was performed according toMittler and Zilinskas (1993). APX (EC 1.11.1.11) enzyme activity was measured according toNakano and Asada (1981). GR (EC 1.6.4.2) activity was measured according to Foyer and Halliwell (1976). NADPH oxidase (NOX) isozymes were identified as described bySagi and Fluhr (2001). NOX (EC 1.6.3.1) activity was measured according toJiang and Zhang (2002).
Gels stained for SOD, POX, APX and NOX activities were photo-graphed with the Gel Doc XR + System and then analyzed with Image Lab software v4.0.1 (Bio-Rad, California, USA). Known standard amounts of enzymes (0.5 units of SOD and 0.2 units of POX) were loaded onto gels. For each isozyme set/group, the average values were significantly different at p < 0.05 using Tukey’s post-test.
2.10. Determination of the contents of ascorbate and dehydroascorbate in chloroplasts
Total and reduced ascorbate (AsA) contents were done according to the method ofDutilleul et al. (2003)with modifications. The oxidized form of ascorbate (DHA, dehydroascorbate) was measured using the formula DHA = Total AsA− Reduced AsA.
2.11. Determination of the activity of monodehydroascorbate reductase and dehydroascorbate reductase in chloroplasts
Monodehydroascorbate reductase (MDHAR; EC 1.6.5.4) activity
was assayed by the method of Miyake and Asada (1992). Dehy-droascorbate reductase (DHAR; EC 1.8.5.1) activity was measured ac-cording toDalton et al. (1986).
2.12. Determination of the contents of glutathione and oxidized glutathione in the chloroplasts
The glutathione (GSH) was assayed according toParadiso et al. (2008). Oxidized glutathione (GSSG) was determined after removal of GSH by 2-vinylpyridine derivatization. GSH redox state (%) was de-termined by calculating the ratio of GSH to total glutathione (GSH + GSSG) according toShi et al. (2013).
2.13. Statistical analysis
The experiments were carried out in triplicates and each data was expressed as the means of six replicates. All data obtained were sub-jected to a one-way analysis of variance (ANOVA). Statistical analysis of the values was performed by using SPSS 20.0. Comparisons with p < 0.05 were considered significantly different.
3. Results
RGR in wheat exposed to different cobalt concentrations are shown inFig. 1A. Depending on Co concentrations, the marked reduction was observed in RGR of wheat. The reduction rate of RGR reached the maximum levels in wheat with 300μM Co (by 2.1-fold). However, SNP and/or NaHS under stress or non-stress had a significant induction in RGR. As shown inFig. 1B, the presence of Co stress in the growth media caused a considerable decrease in RWC as compared to the control group. The maximum decline was measured at 300μM Co by 10 % for 72 h. While, under non-stress conditions, the application of SNP and/or NaHS did not show any change in RWC, the alone or combined form of SNP and NaHS treatments in response to Co stress resulted in en-hancement in RWC. Both Co concentrations caused a decrease inΨΠ (Fig. 1C). For example, under 300 μM Co toxicity, ΨΠ dropped to
Fig. 1. Effects of exogenous 100 μM SNP and 600 μM NaHS treatment on growth (RGR, g g−1day−1, A), relative water content (RWC, %, B) and osmotic potential
-1.64 MPa from-1.34 MPa compared with control group. The combined or alone treatments of SNP and NaHS did not affect ΨΠ, whereas in plants with SNP and/or NaHS under Co stressΨΠincreased compared with stress alone.
Compared with control group, Co treatments caused a reduction in Fv/Fm(Fig. 2A). Besides, the enhancement in Fv/Fmwas triggered in wheat leaves with SNP or NaHS plus Co stress as compared to the stress treatments alone. Similarly, a remarkable increase in Fv/Fmwas de-tected only in wheat plants with SNP plus NaHS. As given inFig. 2B, Co stress significantly inhibited carbon assimilation rate (A) of wheat leaves and the highest inhibition level of A was at 300μM Co (50.1 %). The plants treated with SNP and NaHS under excess Co had higher A values than Co treatments alone. A similar effect was noticed in re-sponse to the applications of SNP or NaHS under control conditions. Also, there was a noticeable rise at SNP + NaHS (30.8 % increase). Co stress led to a considerable decrease in gsand Ciof wheat leaves (Fig. 2C and 2D). On the other hand, the elevated levels of gs and Ciwere maintained by both alone and in combination of SNP and NaHS under stress. When excess Co was treated to the wheat plants, the transpira-tion rate (E) decreased (Fig. 2E) and the decline in E was more sig-nificant in 300 μM Co-treated wheat (2.1- fold). A remarkable effect was created on E levels by SNP and/or NaHS alone and together with Co stress. These changes on Fv/Fm, gs, Ciand E were reversed by RTN
application to SNP or SNP + NaHS plants. As shown inFig. 2F, after exposure to 150μM and 300 μM Co applications, 33.3 % and 59.5 % increases in stomatal limitation value (Ls) were detected in wheat leaves, respectively. However, Co stress-induced increment in Lswas prevented by SNP or NaHS applications. However, no change in Lswas observed with alone or in combination of SNP and/or NaHS. RTN ap-plication in plants with SNP and NaHS under stress caused an increase in Ls.
As presented data inFig. 3A, Co stress increased endogenous con-tent of Co2+in wheat leaves. The greatest concentration (1.42μmol g−1DW) was recorded in 300μM Co-treated wheat. On the other hand, the addition of SNP and NaHS to stress-treated plants decreased the contents of Co2+ for 72 h. Also, under control conditions, SNP and NaHS treatments alone did not cause a significant effect on the content of Co2+. While, after stress treatments, the concentrations of K+, Ca2+, Fe2+presented a decline in leaves (Fig. 3B, C and E), the endogenous content of Mg2+was similar to the control groups (Fig. 3D). In response to low Co treatments, SNP and SNP + NaHS significantly increased K+ content (Fig. 3B), in high Co treatment-treated leaves, the enhancement in K+content was detected by NaHS application. All the treatment groups had the same K+content in the leaves with the exception of Co1+SNP + NaHS treatments, which had a much high level (Fig. 3B). Regarding the contents of Ca2+and Mg2+, the maximum concentration
Fig. 2. Effects of exogenous 100 μM SNP, 600 μM NaHS treatment and rutin hydrate (RTN, 50 μM) on chlorophyll efficiency (Fv/Fm, A), carbon assimilation rate (A,
μmol m−2s-1, B), stomatal conductance (g
s, mmol m−2s-1, C), intercellular CO2concentrations (Ci,μmol mol-1, D), transpiration rate (E, mmol m−2s-1, E) and
in leaves was 177.9 and 99.4μmol g−1DW in leaves of the combination treatments at 300μM Co stress, respectively (Fig. 3C). Besides, it was found that the endogenous content of Fe2+was induced by SNP and/or NaHS application under stress except for Co2 plus NaHS (Fig. 3E).
Under control conditions, SNP or NaHS alone did not cause an increase in the concentrations of K+, Ca2+, Fe2+ and Mg2+, however, a sig-nificant increment was observed in the contents of Mg2+
and Fe2+after 72 h of the combination form.
Fig. 3. Effects of exogenous 100 μM SNP and 600 μM NaHS treatment on ion contents (Co2+(A), K+(B), Ca2+(C), Mg2+(D) and Fe2+(E) contents (μmol g−1DW))
in wheat leaves exposed to 150μM (Co1) and 300 μM (Co2) Co stress for 72 h.
Fig. 4. Effects of exogenous 100 μM SNP, 600 μM NaHS treatment and rutin hydrate (RTN, 50 μM) on the contents of NO (A, nmol g−1FW) and H
2S (B,μmol g−1
The results of the contents of NO and H2S are depicted inFig. 4. In wheat plants under Co stress, the endogenous contents of NO improved significantly (Fig. 4A). Both SNP and/or NaHS caused a further increase of NO content under control and stress conditions. The treatments of RTN along with SNP or SNP under stress decreased the endogenous NO levels as compared to plants with SNP alone or Co + SNP, respectively. The same variation in NO content was observed in the combination SNP and NaHS under stress. When compared to the control group, H2S content was induced in plants exposed to the stress treatments (Fig. 4B). The addition of SNP or NaHS together with Co stress induced the en-dogenous H2S content. However, after a scavenger of NO (RTN) ap-plication to SNP or SNP + NaHS treated plants under stress, the in-duction in the endogenous levels of H2S content was reversed.
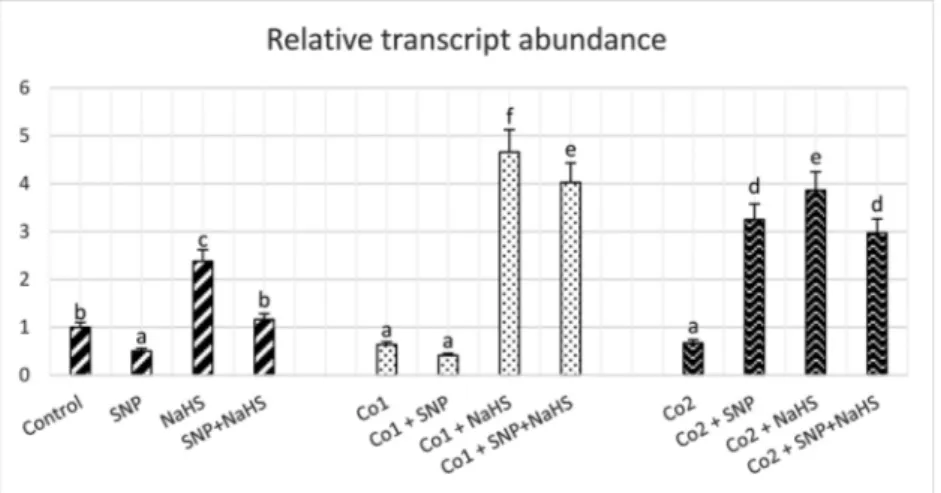
As shown inFig. 5, during the experimental period, the enhanced concentrations of Co treatments decreased the transcription levels of rbcl gene in wheat leaves. Except for 150μM Co plus SNP, the higher transcription of rbcl was observed in the chloroplasts of wheat plants applied to SNP and/or NaHS under Co stress. While, SNP treatment under control conditions had a decrease in the rubisco transcript abundance, NaHS applications alone enhanced the expression of this gene by 2.3-fold. Also, under the combination of SNP and NaHS, there was no effect on the expression of rbcl as compared to the control group.
Fig. 6A reveals that depending on Co treatments, a notable increase in H2O2content was observed. While under SNP and/or NaHS alone there was no effect on H2O2content, the wheat plants with SNP and/or NaHS in response to stress treatments had a decrease in H2O2content. SNP and/or NaHS-induced TBARS content was similar to the control group. However, after Co treatments to wheat plants, the levels of lipid peroxidation (TBARS content) were induced (Fig. 6B) and reached the maximum levels at 300μM Co by 1.2-fold. On the other hand, SNP and/ or NaHS alleviated the increament in TBARS content of both Co con-centrations-treated wheat. After RTN was added to SNP or SNP + NaHS under stress, the levels of lipid peroxidation were elevated in compar-ison with those of the SNP- and SNP plus NaHS-treated plants, re-spectively.
As illustrated byFig. 7A, three chloroplastic SOD isozymes (Cu/Zn-SOD1-3) were observed in the evaluation of SOD isozyme profiles for 72 h. During the experimental period, only one isozyme, Cu/Zn-SOD, was detected, but Fe-SOD and Mn-SOD could not be identified. The activity of SOD in chloroplasts of wheat was either unaffected or low-ered by stress (Fig. 7B) when compared to the control group. SOD en-zyme activity exhibited a similar activity with the isoen-zyme staining pattern and the decrease in SOD enzyme activity in plants with 300μM Co stress was depending on the declined intensities of Cu/Zn-SOD1-2. On the other hand, a significant enhancement was observed in SOD
activity of SNP and/or NaHS-treated leaves under stress conditions, especially for Cu/Zn-SOD3 isozyme. Whereas, the alone and the com-bined treatments of SNP or NaHS decreased SOD activity, which showed lower intensities of Cu/Zn-SOD1-2.
In our study, three POX isozymes were detected by native page analysis (POX1-2-3) (Fig. 8A). The plants treated with 150 and 300μM Co had lower total activity of POX enzyme than the control groups by 41.8 % and 57 % decrease (Fig. 8B) in terms of the all POX isozymes (Fig. 8A). This effect was reversed by SNP and NaHS treatments under stress and non-stress conditions.
Gel analysis revealed thatfive APX isozymes (APX1-5) in treatment groups (Fig. 9A). Despite of the disappearance in APX5 under 300μM Co treatments (Fig. 9A), the enzyme activity and the isoforms of APX increased under Co stress (Fig. 9B). At both Co treatments, especially APX3 isozyme, which was the most intense, was responsible for this change. Also, the increased APX activity under stress was maintained with the applications of SNP and/or NaHS. Similarly, SNP and/or NaHS applications under control conditions caused an increase in APX en-zyme/isozyme activity.
Chloroplastic GR enzyme activity decreased in wheat under Co stress (Fig. 10A). The maximum reduction in GR activity was at 150μM Co by 30.1 %. GR activity increased under SNP and/or NaHS plus Co stress compared to the stress alone. Similarly, after SNP and/or NaHS applications under non-stress conditions, GR activity was higher than the control group. As suggested inFig. 10B, gel assays for detecting NOX activity revealed that there were eight NOX bands identified in the chloroplasts of wheat leaves (NOX1-8). Similar to the enzyme activity (Fig. 10C), under Co stress, the intensity of NOX isoform was stronger than the control group. This change depends on the induced intensity of NOX8 isozyme and the new identified isozyme, NOX5, under Co stress. The increased NOX activity under stress did not protect with SNP and/ or NaHS treatments and the activity decreased as compared to the stress treatments. On the other hand, the wheat leaves exposed to SNP and/or NaHS alone demonstrated an increase in NOX activity.
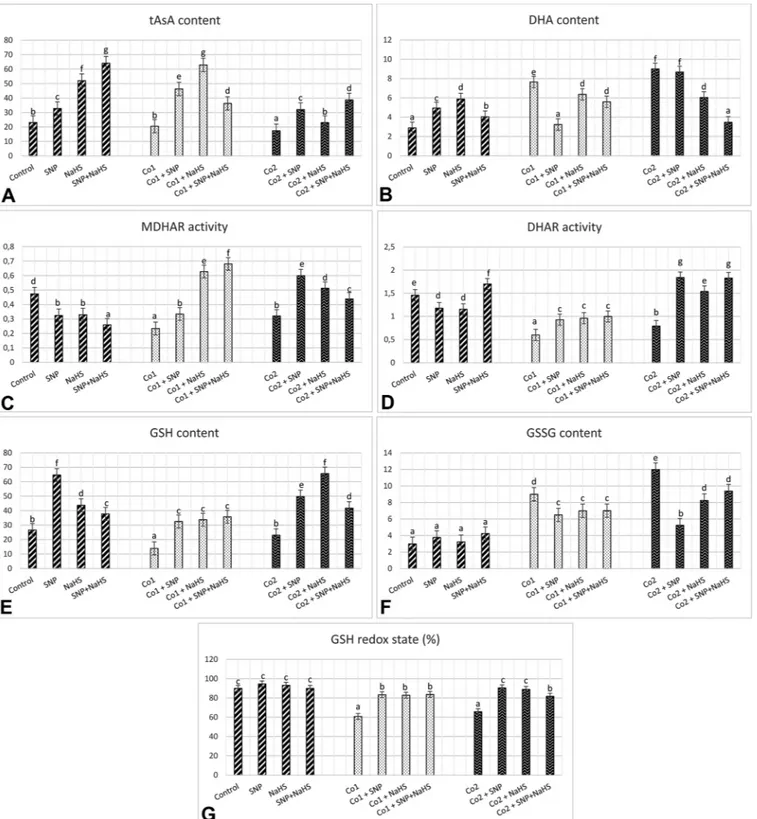
tAsA content under 150 and 300μM Co was similar to the control group or decreased, respectively (Fig. 11A). However, tAsA content of stress + SNP or NaHS treatments was higher than that of Co stress, as parallel to the group of SNP or NaHS alone. On the other hand, there was an opposite observation between tAsA and DHA content. While, stress caused an increase in DHA content, SNP or NaHS treatments could not maintain the increase in this content (Fig. 11B). The levels of tAsA/DHA decreased significantly under Co stress but, were clearly mitigated by SNP and/or NaHS (Fig. 11A-B). The changes between MDHAR and DHAR were similar in wheat (Fig. 11C-D). After 72 h from the beginning of treatments, both Co treatments led to a remarkable reduction in the activities of MDHAR and DHAR (Fig. 11C and D).
Fig. 5. Effects of exogenous 100 μM SNP and 600 μM NaHS treatment on qRT-PCR analysis of expression of rubisco large subunit in wheat leaves exposed to 150 μM (Co1) and 300μM (Co2) Co stress for 72 h.
Interestingly, the maximum reduction in MDHAR and DHAR was at 150 μM Co by 2-fold and 2.4-fold, respectively. However, the wheat leaves with stress plus SNP and NaHS had high activities of MDHAR and DHAR as compared to the stress treatments. On the other hand, a sig-nificant decline in MDHAR activity was determined by SNP or NaHS alone and their combined treatments in wheat leaves (Fig. 11C). Be-sides, when SNP or NaHS were applied alone, a reduction in DHAR was observed but a combination of both caused an increase in DHAR ac-tivity (Fig. 11D). After exposure to Co stress for 72 h, no increase was observed in GSH content, however, a dramatic increase was observed under the combination of SNP and NaHS with/without stress. (Fig. 11E). The contents of GSSG in the wheat leaves increased sub-stantially for the experimental period when under stress, compared to the content level of the control group (Fig. 11F). However, this effect did not maintain in wheat with stress plus SNP or NaHS. The levels of GSH/GSSG followed a similar pattern with ratio of tAsA/DHA (Fig. 11E-F). Additionally, SNP and NaHS treatments minimized the risk of reducing GSH redox state (the ratio of GSH content to total glutathione (GSH + GSSG)) in response to Co stress (Fig. 11G).
4. Discussion
Negative impacts of Co on the growth of plants have already been reported (Begović et al., 2016). In the current study, this result is in concordance with measured RGR in stress-treated plants. The inhibition of RGR by Co stress could be associated with damaged photosynthesis system. The reduction in RGR induced by Co might be possible for other reasons for the declined mitotic activity in cells or impairment of the elongation zone of the root tips (Yuan and Huang, 2016) and Co-mediated disruption of uptake of beneficial elements required for plant regulation (Ali et al., 2018b). In the current study, Co treatments caused the high accumulation of Co2+content in wheat leaves. However, in wheat plants under stress or non-stress conditions, NaHS application improved the growth of plants. This result correlated with the study conducted byShi et al. (2014)where NaHS significantly alleviated Cd-induced growth inhibition in bermudagrass plants. Similar to this group, NO-stimulated increment in RGR of wheat plants was observed. In line with our data,Vaishnav et al. (2016)described that 100μM SNP application promoted the growth of soybean seedlings under NaCl. The
Fig. 6. Effects of exogenous 100 μM SNP, 600 μM NaHS treatment and rutin hydrate (RTN, 50 μM) on hydrogen peroxide (H2O2,μmol g−1FW, A) and lipid
peroxidation (TBARS, nmol g‒1FW, B) in wheat leaves exposed to 150μM (Co1) and 300 μM (Co2) Co stress for 72 h.
Fig. 7. Effects of exogenous 100 μM SNP and 600 μM NaHS treatment on relative band intensity of different types of chloroplastic superoxide dismutase isoenzymes (SOD, A) and SOD activity (B, units mg−1protein) in wheat leaves exposed to 150μM (Co1) and 300 μM (Co2) Co stress for 72 h.
Fig. 8. Effects of exogenous 100 μM SNP and 600 μM NaHS treatment on relative band intensity of different types of chloroplastic peroxidase isoenzymes (POX, A) and POX activity (B, units mg−1protein) in wheat leaves exposed to 150μM (Co1) and 300 μM (Co2) Co stress for 72 h.
Fig. 9. Effects of exogenous 100 μM SNP and 600 μM NaHS treatment on relative band intensity of different types of chloroplastic ascorbate peroxidase isoenzymes (APX, A) and APX activity (B, units mg−1protein) in wheat leaves exposed to 150μM (Co1) and 300 μM (Co2) Co stress for 72 h.
reason behind the improvement in SNP-induced RGR might be the decline in the endogenous contents of Co2+(as observed in our study), the alteration of Co redox state and the inactivation of metals by the modification of phytochelatins as reported by Amooaghaie and Enteshari (2017).
RWC reflects a measure of plant water status connecting with the metabolic activity in plants (Lawlor, 2002). Therefore, it can be ex-trapolated that there is a relationship between RWC and biomass. As expected, our study showed that both levels of RWC and RGR decreased under Co stress. According toSrivastava et al. (2015), Cd-treated plants also showed a significant decline in RWC. Furthermore, the addition of H2S donor to growth media increased plant available water (RWC) in wheat leaves under both Co treatments. This is in agreement with present results in which the enhancement in RWC in NaHS-treated Se-samum indicum was observed under lead stress (Amooaghaie and Enteshari, 2017). Also,Tan et al. (2008)reported that NO donor ex-posure under osmotic stress maintained the high-water content in wheat. The possible reason behind NaHS-stimulated increased RWC in plants might be the induction of water uptake by roots, the induced stomatal conductance (gs) (as detected in our study) or the increased proline accumulation, as suggested byAli et al. (2014). Another reason for the improved RWC, RGR and photosynthesis in wheat leaves under Co plus NaHS and/or SNP might be due to their impacts on improving uptake of K, Ca, P, S, Mg and Fe (especially K+content, in osmor-egulation), as observed in plants with SNP and NaHS treatments in response to cadmium and aluminum stress by Xu et al. (2014) and Dawood et al. (2012), respectively. Also, the increased uptake of these nutrients observed that the increment in photosynthesis (especially in Fv/Fm) might be connection with the endogenous levels of NO and H2S in wheat leaves. This was accordance with the data reported byZiogas et al. (2015) andKaya et al. (2019). H2S plays a role in the ionic homeostasis, the efflux and influx of ions (K+ and Na+) to vacuole under stress conditions (Zhao et al., 2018) and modifies the transloca-tion or uptake of metals (Li et al., 2013a, Sun et al. 2013). Thus, the improvedΨΠof wheat leaves might be connected with the elevated K+
content at SNP or NaHS plus stress.
Co-induced oxidative stress altered the pigments, stomatal func-tioning, electron transport chain and thylakoid membrane related to photosynthetic capacity (Ali et al., 2010). As excepted, excess Co con-centrations distinctly caused a significant decline of chlorophyll fluor-escence (Fv/Fm) representing the maximum quantum efficiency of PSII and degradation of photosynthetic pigments, as suggested by Singh et al. (2015). The decline of K+and/or Mg2+is related to the decrease in photosynthetic rate and regulation of carbonfixation (Mengel and Kirby, 2001). The reason behind the decrease in Fv/Fmunder stress in the present study might be attributed to the decreased in K+and un-changed Mg2+contents. Also, according toFlexas et al. (2002), sto-matal conductance (gs) could be a good indicator of stress intensity in relation to photosynthesis. In the present study, the decline in gswas accompanied by dramatic decreases in A and E under Co treatments. Also, the reduction in photosynthesis in Co-treated wheat leaves was accompanied by a lower gsbecause of stomatal closure and this situa-tion resulted in less promoted of biomass (RGR). When in response to stress the carbon assimilation rate (A) are suppressed, the photo-chemical energy could not be dissipated through CO2 assimilation (Wada et al., 2019). In plants exposed to stress, one reason for the re-duction in the photosynthesis rate is the stomatal closure and thus in-tercellular CO2 concentration (Ci) is lower than control conditions (Engineer et al., 2016). In the current study, both Ciand Ci/Ca(data not shown) levels decreased after Co stress treatments to wheat plants. This result is in agreement with the data ofAshraf et al. (2002)which also showed that there was a slight reduction in Ci/Ca(intercellular CO2/ ambient CO2) under stress. Under the different stress conditions, there are some restriction called stomatal and mesophyll limitations on carbon assimilation rates due to the changes of CO2diffusion to the intracellular leaf space in plants (Acosta-Motos et al., 2017). If there is a reduction in A and then a decrease in Ciin treated plants, the stomatal limitation is reported. On the other hand, in the mesophyll limitation, a reduction in A and an increase in Ciare observed (Cano et al., 2013). In our study, when these parameters were evaluated, it was observed that
Fig. 10. Effects of exogenous 100 μM SNP and 600 μM NaHS treatment on chloroplastic GR activity (A, units mg−1protein), relative band intensity of different types
there was a stomatal limitation in the wheat leaves exposed to Co stress. Both NaHS and SNP applications improved the photosynthetic perfor-mance (Fv/Fm) under Co stress, confirming earlier findings on sesame and cotton under lead and salt stress, respectively (Amooaghaie and Roohollahi, 2017;Liu et al., 2014). Besides,Hawkins and Lewis (1993) reported no clear relationship between growth and photosynthetic ca-pacity under salt stress. However, because of increasing dry matter production (RGR) and plant height (data not shown) in wheat leaves
with SNP and NaHS treatments the positive correlation was observed among RGR, Fv/Fmand A. The reason behind the promoted levels of Fv/ Fmand A induced by SNP or NaHS might be the improved biogenesis of chloroplast through increasing number of grana lamellae, the bio-synthesis of chlorophyll, the modifying photochemical reaction of PSII against stress, thiol redox modification and regulation of rubisco ac-tivity, as suggested byMustafa et al. (2015)andKong et al. (2016). Also, except for Co2+NaHS, the induced endogenous content of Fe2+
Fig. 11. Effects of exogenous 100 μM SNP and 600 μM NaHS treatment on chloroplastic total ascorbate content (tAsA, nmol g−1FW, A), dehydroascorbate content
(DHA, nmol g−1FW, B), monodehydroascorbate reductase activity (MDHAR,μmol mg−1protein, C), dehydroascorbate reductase activity (DHAR,μmol mg−1 protein, D), glutathione content (GSH, nmol g−1FW, E), oxidized glutathione (GSSG, nmol g−1FW, F) and GSH redox state (%, G) in wheat leaves exposed to 150μM (Co1) and 300μM (Co2) Co stress for 72 h.
was related to the improved photosynthetic capacity after the combi-nation of SNP and NaHS under stress which was important element for chlorophyll synthesis as suggested by Sarafi et al. (2018). SNP and NaHS triggered the considerable increase in A and gswith increasing CO2concentrations (Ci) through higher CO2uptake. Similarly, NaHS in strawberry expose to NaCl increased the photosynthetic rate, gsand RWC (Christou et al., 2013). The same researchers have demonstrated that H2S is connected with guard cell signaling through abscisic acid-dependent signaling network. Similarly, in the current study, the im-provement in gsof plants with NaHS plus Co stress was supported the role of H2S on stoma regulation. As expected, in the SNP and NaHS applications exposed to stress-treated wheat leaves, the increased levels of gswere parallel to the enhancement in Ciand E. On the other hand, the levels of Ci/Caare connected with the stomatal limitation (Ls, 1- Ci/ Ca) in plants. Previous study also reported increases in Lswith the se-verity of stress (Gulías et al., 2002), as observed in our study. Besides, Farquhar et al. (1980)identified a steady-state mechanistic model of C3 leaf photosynthetic carbon assimilation (A) that A is limited by the slower of the levels of rubisco-catalyzed carboxylation (rubisco-limited) under stress conditions. Therefore, the limitations in photosynthesis induced by stress are associated with the lower rubisco activity (Hu et al., 2009). In the present study, the expression of rubisco was down-regulated in the chloroplasts of Co stress-treated wheats. On the other hand, NaHS and/or SNP applications alleviated the rubisco activity reduced by stress at the transcriptional levels, which is in accordance with thefindings ofChen et al. (2013)that Spinacia oleracea treated with NaHS increased the gene expression encoding rubisco large sub-unit in chloroplast. In NaHS and SNP treated-wheat plants, the induced transcript levels of rubisco large subunit were in accordance with the induced rate of CO2fixation in photosynthesis, RGR and Fv/Fm. So, SNP and NaHS might play a role in the recovery of both components sto-matal (Ls, gsand E) and non-stomatal (Fv/Fmand the rubisco expres-sion).
In chloroplasts, ROS production occurs by the direct transfer of the excitation energy from chlorophyll molecules or oxygen reduction in the Mehler reaction under stress conditions (Gill and Tuteja, 2010). Plants have an antioxidant defence system to combat with metal-in-duced excess ROS production (Aziz et al., 2013) and the combined action of SOD, POX and AsA-GSH cycle is essential for scavenging of ROS levels in chloroplasts (Diao et al., 2014). In the present study, Co stress did not trigger SOD activity in chloroplast which is thefirst step for tolerance against the radicals. The reason behind might be due to disturbing of SOD enzyme subunits or binding/absence of cofactors to SOD such as Fe2+, Cu2+and Zn2+by Co treatments. The impaired SOD activity was correlated with the reduction of Fe2+content under stress
and as observed bySree et al. (2015)the transport of Fe2+from roots to shoots was inhibited by Co stress. However, H2O2accumulation was induced in the chloroplast of wheat under Co stress. Furthermore, NADPH oxidase (NOX) activity is considered an important source of superoxide radicals and H2O2 in plants (Miller et al., 2009). In the current study, in wheat leaves, the reasons for accumulation of these radicals under Co stress might be a decline in scavenging capacity of SOD enzyme and an increase in NOX activity. This situation could contribute to the increase in TBARS and H2O2content. This result is in agreement withAli et al. (2018a)in which the exposure of excessive Co levels led to enhanced H2O2content in Brassica napus. Another possible cause of H2O2accumulation under Co stress in chloroplasts of wheat leaves is the diffusion to the chloroplast from other cellular compart-ments or cytoplasm. On the other hand, in the presence of NaHS, the activated total SOD eliminated the toxic levels of radicals induced by osmotic and cadmium stress in sweet potato and Salix matsudana, re-spectively (Yang et al., 2018;Zhang et al., 2009). Also, an increase in SOD activity was found under NO donor plus Co stress (Song et al., 2006). These reports were in accordance with our results that SOD eliminated the toxic levels of radicals induced by Co stress in wheat chloroplasts. Also, the mentioned studies about the relationship be-tween SOD activity and NaHS or SNP applications under stress condi-tions, generally were on the total activity of this enzyme. This work proved that NaHS and/or SNP could also induce SOD activity in chloroplasts against Co stress. NO is regulated by negatively modulating the Cu/Zn-SOD expression (da-Silva et al., 2018). In the present study, although, in stress-treated wheat, the intensities of SOD isozyme (especially for Cu/Zn-SOD1-2) was lower than control group or did not change, the total levels of all SOD isozymes including Cu/Zn-SOD1-3 were induced by NaHS and/or SNP applications plus stress.
To mitigate H2O2and modulating redox state, AsA-GSH cycle per-forms in chloroplasts of plants, which contains four enzymes (APX, GR, MDHAR and DHAR) and two non-enzymatic antioxidants (AsA and GSH) (Foyer and Noctor, 2011). Another enzyme for detoxifying the toxic H2O2levels is POX (Miller et al., 2009). Our results showed that the higher activity of APX under stress also cannot eliminate the excess H2O2due to insufficient substrates provided by low contents/activities of tAsA, GR, MDHAR and DHAR. Also, due to the inhibition of SOD and POX, the increasing Co treatments coincided with the enhanced levels of H2O2and TBARS in the wheat chloroplasts. These findings were clearly matched with the previous studies that the higher TBARS con-tent accumulated in spinach leaves exposed to Co stress (Pandey et al., 2009). To eliminate H2O2 toxic levels, AsA is converted to mono-dehydroascorbate (MDHA), which is degraded to mono-dehydroascorbate (DHA). MDHA and DHA are reduces to AsA by MDHAR and DHAR
Fig. 12. The proposed action sites and pathways of exogenous SNP and NaHS on the antioxidant system in the chloroplasts of wheat under Co stress. Hydrogen peroxide (H2O2) generated with Co stress was scavenged AsA-GSH cycle (Asada-Halliwell pathway). The applications of SNP and NaHS cause an increase in the AsA/
using GSH as the reducing substrate, respectively. In the chloroplast, an NADH-dependent MDHAR activity plays a role in the regeneration of AsA thus maintains the redox state of AsA and GSH. (Foyer and Noctor, 2011).Mittova et al. (2003)reported that some plants had the higher activity of MDHAR than DHAR in mitochondria and chloroplasts. As well as MDHAR activity, DHAR in the AsA-GSH pathway is mediated in reduction of DHA. However, in the present study, in the chloroplast of Co stress treated-wheat leaves, the regeneration of AsA could not be achieved sufficiently because of a decline in MDHAR and DHAR ac-tivity. Also, Co stress led to decline in AsA/DHA and a lower percentage of AsA was transformed to the oxidized state in response to Co stress. In plants, GSH, another antioxidant, involves in the detoxification of peroxidation products of lipids and proteins, the mitigation of ROS and the modulation of the GSH/GSSG ratio to maintain a higher reducing power and delivering information of ROS to the redox signal network (Gajewska and SkŁodowska, 2010). On the other hand, GSH-dependent DHAR and GR play a role in the GSH regeneration cycle. However, in the present study, because of a decrease in both activities, the re-generation of GSH was not maintained under Co stress. The opposite result was noted byYang et al. (2018)in Salix matsudana. In the present study, in the presence of H2S and NO donors, the activities of POX, APX, GR, MDHAR and DHAR, and GSH content were positively affected in Co-treated wheat leaves. Also, these results correlated with Yu et al. (2013) who detected enhanced APX and GR in cucumber seedlings under NaHS plus stress. Likewise, when compared to the stress alone treated wheat plants, exogenous addition of SNP together with NaCl stress significantly increased the activities of MDHAR, DHAR and GR as suggested by Hasanuzzaman et al. (2011). In this study, with their ability to induce the enzymes of AsA-GSH cycle under excess Co, the applications of NaHS and SNP had direct capability to regenerate AsA and GSH and maintain reduced pool of AsA in wheat leaves. Therefore, SNP and NaHS were also helpful in protecting a high level of AsA/DHA. Similar data observed in GSH/GSSG with increased GSH content and reduced GSSG, as reported byShi et al. (2013)in Cynodon dactylon. The high ratio in GSH/GSSG and the elevated GSH content/redox state, which was detected in our study, showed that it could be role in re-cycling of AsA and GSH by providing a redox state and reducing oxi-dative stress. As accompanied by all these observations mentioned above, NaHS and SNP treatments provided the low levels of H2O2and TBARS under Co toxicity. This result provided that NaHS or SNP might play a role in the H2O2-mediated signaling network in the chloroplasts of wheat. Besides, Dong et al. (2014) reported that these reduced contents were in parallel with the increment of NO accumulation in plants with copper stress and NO applications. In the current study, the prevention of TBARS reduction and decline in NO content in SNP plus RTN (NO scavenger) confirmed that NO accumulation induced by exogenous application or stress might play a role in Co stress tolerance. As well as the accumulation of NO, one possible explanation for de-crease in the amount of H2S with RTN under stress is connection be-tween NO signaling and H2S. As observed by the results ofSingh et al. (2015)the application of NaHS increased NO by regulating nitrate re-ductase. Therefore, both exogenous applications of NaHS and SNP in-creased NO production. In the present study, an increase in activities of APX and GR might be associated with the exposure of H2S and H2 S-mediated NO increment, as reported byWang et al. (2012).
5. Conclusion
Based on the observations, a model for SNP and NaHS-mediated responses on the antioxidant system in the chloroplasts of wheat under high Co toxicity was proposed in the current study (Fig. 12). Our findings indicated that excess Co inhibited RGR, RWC and ΨΠin wheat leaves. Also, the wheat exposed to Co stress lowered the photosynthetic capacity as observed by a decrease in A, gs, E and Ciand an increase in Ls. In parallel to the declination in Fv/Fm, Co stress impaired the ex-pression of rubisco in the chloroplasts of wheat. Although, under stress
conditions, only APX activity was activated, H2O2 content and lipid peroxidation (TBARS content) were triggered in wheat leaves because of its insufficient activation. On the other hand, the donor applications of NO and H2S (SNP and NaHS, respectively) successfully eliminated negative effects of stress on these parameters (RWC, ΨΠand photo-synthetic parameters). SNP and NaHS might play roles in both the re-covery in different stomatal components (Ls, gsand E) and non-stomatal components (Fv/Fmand the rubisco expression) under Co stress. Pho-tosynthetic efficiency conferred by SNP and NaHS was reversed by treatment together with RTN, and the contents of NO and H2S were reduced. Also, SNP induced H2S accumulation, suggesting that NO as an upstream signal regulates the H2S production. The activities of SOD, APX, GR, MDHAR and DHAR and the contents of AsA and GSH in the Co-stressed chloroplasts were enhanced by SNP and NaHS applications, which were consistent with the ability of SNP and NaHS to reduce the content of H2O2and TBARS in this organelle. On the other hand, the antioxidant role of SNP and NaHS against Co stress was due to the modulation enzymatic-non-enzymatic antioxidants including AsA-GSH cycle. As well as the induced this cycle, the toxic accumulation of H2O2 was eliminated by the increment in POX activity. Exogenous of NaHS and SNP together with stress significantly ameliorated Co-induced toxic effects on chloroplast system in wheat, which coincided with the de-creased levels of H2O2and TBARS, through the increased POX activity and the enhanced enzymes of the ascorbate-glutathione cycle resulting in the maintaining of the redox state in the chloroplasts.
Declaration of Competing Interest
The authors declare that they have no known competingfinancial interests or personal relationships that could have appeared to influ-ence the work reported in this paper.
Acknowledgment
We are thankful to Bahri Dagdas International Agricultural Research Institute for providing the wheat seeds. For this study, NaHS was ob-tained from Yasemin Teksen, Assist. Prof. Dr. and we thank her for chemical support. The authors are also grateful to Assist. Prof. Dr. Nabeelah Bibi Sadeer from University of Mauritius for helpful com-ments to improve the manuscript. This work was supported by Selcuk University Scientific Research Projects Coordinating Office (Grant Number 17401061).
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jhazmat.2020.122061.
References
Acosta-Motos, J.R., Ortuna, M., Bernal-Vicente, A., Diaz-Vivancos, P., Sanchez-Blanco, M., Hernandez, J.A., 2017. Plant responses to salt stress: adaptive mechanisms. Agronomy 7, 18–56.
Ali, B., Hayat, S., Hayat, Q., Ahmad, A., 2010. Cobalt stress affects nitrogen metabolism, photosynthesis and antioxidant system in chickpea (Cicer arietinum L.). J. Plant Interact. 5 (3), 223–231.
Ali, B., Song, W., Hu, W., Luo, X., Gill, R., Wang, J., Zhou, W., 2014. Hydrogen sulfide alleviates lead-induced photosynthetic and ultrastructural changes in oilseed rape. Ecotoxicol. Environ. Saf. 102, 25–33.
Ali, E., Hussain, N., Shamsi, I.H., Jabeen, Z., Siddiqui, M.H., Jiang, L.X., 2018a. Role of jasmonic acid in improving tolerance of rapeseed (Brassica napus L.) to Cd toxicity. J. Zhejiang University-SCIENCE B. 19 (2), 130–146.
Ali, N., Schwarzenberg, A., Yvin, J.-C., Hosseini, S.A., 2018b. Regulatory role of silicon in mediating differential stress tolerance responses in two contrasting tomato genotypes under osmotic stress. Front. Plant Sci. 9, 1475.
Amooaghaie, R., Enteshari, S., 2017. Role of two-sided crosstalk between NO and H2S on
improvement of mineral homeostasis and antioxidative defense in Sesamum indicum under lead stress. Ecotoxicol. Environ. Saf. 139, 210–218.
Amooaghaie, R., Roohollahi, S., 2017. Effect of sodium nitroprusside on responses of Melissa officinalis to bicarbonate exposure and direct Fe deficiency stress.
Photosynthetica 55 (1), 153–163.
Asada, K., 1999. The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 601–639. Ashraf, M., Karim, F., Rasul, E., 2002AsaAAsaa. Interactive effects of gibberellic acid
(GA3) and salt stress on growth, ion accumulation and photosynthetic capacity of two
spring wheat (Triticum aestivum L.) cultivars differing in salt tolerance. Plant Growth Regul. 36 (1), 49–59.
Aziz, R., Shahbaz, M., Ashraf, M., 2013. Influence of foliar application of triacontanol on growth attributes, gas exchange and chlorophyllfluorescence in sunflower (Helianthus annuus L.) under saline stress. Pak. J. Bot. 45 (6), 1913–1918. Beauchamp, C., Fridovich, I., 1971. Superoxide dismutase: improved assays and an assay
applicable to acrylamide gels. Anal. Biochem. 44 (1), 276–287.
Begović, L., Mlinarić, S., Dunić, J.A., Katanić, Z., Lončarić, Z., Lepeduš, H., Cesar, V., 2016. Response of Lemna minor L. To short-term cobalt exposure: the effect on photosynthetic electron transport chain and induction of oxidative damage. Aquat. Toxicol. 175, 117–126.
Bradford, M.M., 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72 (1-2), 248–254.
Cano, F., Sanchez-Gomez, D., Rodriguez-Calcerrada, J., Warren, C., Gill, L., Aranda, I., 2013. Effects of drought on mesophyll conductance and photosynthetic limitations at different tree canopy layers. Plant Cell Environ. 36 (11), 1961–1980.
Chen, J., Wang, W.H., Wu, F.H., You, C.Y., Liu, T.W., Dong, X.J., He, J.X., Zheng, H.L., 2013. Hydrogen sulfide alleviates aluminum toxicity in barley seedlings. Plant Soil 362 (1-2), 301–318.
Chen, J., Wu, F.H., Wang, W.H., Zheng, C.J., Lin, G.H., Dong, X.J., He, J.X., Pei, Z.M., Zheng, H.L., 2011. Hydrogen sulphide enhances photosynthesis through promoting chloroplast biogenesis, photosynthetic enzyme expression, and thiol redox mod-ification in Spinacia oleracea seedlings. J. Exp. Bot. 62 (13), 4481–4493. Cheng, W., Zhang, L., Jiao, C., Su, M., Yang, T., Zhou, L., Peng, R., Wang, R., Wang, C.,
2013. Hydrogen sulfide alleviates hypoxia-induced root tip death in Pisum sativum. Plant Physiol. Biochem. 70, 278–286.
Christou, A., Manganaris, G.A., Papadopoulos, I., Fotopoulos, V., 2013. Hydrogen sulfide induces systemic tolerance to salinity and non-ionic osmotic stress in strawberry plants through modification of reactive species biosynthesis and transcriptional regulation of multiple defence pathways. J. Exp. Bot. 64 (7), 1953–1966. Corpas, F.J., Gonzalez-Gordo, S., Canas, A., Palma, J.M., 2019. Nitric oxide and hydrogen
sulfide in plants: which comes first? J. Exp. Bot. 70 (17), 4391–4404.
da-Silva, C.J., Mollica, D.C., Vicente, M.H., Peres, L.E., Modolo, L.V., 2018. NO, hydrogen sulfide does not come first during tomato response to high salinity. Nitric Oxide 76, 164–173.
Dalton, D.A., Russell, S.A., Hanus, F.J., Pascoe, G.A., Evans, H.J., 1986. Enzymatic-re-actions of ascorbate and glutathione that prevent peroxide damage in soybean root-nodules. Proc. Natl. Acad. Sci. U.S.A. 83 (11), 3811–3815.
Dawood, M., Cao, F., Jahangir, M.M., Zhang, G., Wu, F., 2012. Alleviation of aluminum toxicity by hydrogen sulfide is related to elevated ATPase, and suppressed aluminum uptake and oxidative stress in barley. J. Hazard. Mater. 209, 121–128.
Diao, Y., Xu, H., Li, G., Yu, A., Yu, X., Hu, W., Zheng, X., Li, S., Wang, Y., Hu, Z., 2014. Cloning a glutathione peroxidase gene from Nelumbo nucifera and enhanced salt tolerance by overexpressing in rice. Mol. Biol. Rep. 41 (8), 4919–4927. Dong, Y., Jinc, S., Liu, S., Xu, L., Kong, J., 2014. Effects of exogenous nitric oxide on
growth of cotton seedlings under NaCl stress. J. Soil Sci. Plant Nutr. 14 (1), 1–13. Dooley, F.D., Nair, S.P., Ward, P.D., 2013. Increased growth and germination success in
plants following hydrogen sulfide administration. PLoS One 8 (4), e62048. Dutilleul, C., Garmier, C., Noctor, G., Mathieu, C., Chétrit, P., Foyer, C.H., De Paepe, R.,
2003. Leaf mitochondria modulate whole cell redox homeostasis, set antioxidant capacity, and determine stress resistance through altered signaling and diurnal reg-ulation. Plant Cell 15, 1212–1226.
Engineer, C.B., Hashimoto-Sugimoto, M., Negi, J., Israelsson-Nordström, M., Azoulay-Shemer, T., Rappel, W.-J., Iba, K., Schroeder, J.I., 2016. CO2sensing and CO2
reg-ulation of stomatal conductance: advances and open questions. Trends Plant Sci. 21 (1), 16–30.
Fan, H.F., Du, C.X., Guo, S.R., 2013. Nitric oxide enhances salt tolerance in Cucumber seedlings by regulating free polyamine content. Environ. Exp. Bot. 86, 52–59. Fang, H., Jing, T., Liu, Z., Zhang, L., Jin, Z., Pei, Y., 2014. Hydrogen sulfide interacts with
calcium signaling to enhance the chromium tolerance in Setaria italica. Cell Calcium 56 (6), 472–481.
Farquhar, G.D., von Caemmerer, S.V., Berry, J., 1980. A biochemical model of photo-synthetic CO2assimilation in leaves of C3 species. Planta 149 (1), 78–90.
Flexas, J., Bota, J., Escalona, J.M., Sampol, B., Medrano, H., 2002. Effects of drought on photosynthesis in grapevines underfield conditions: an evaluation of stomatal and mesophyll limitations. Funct. Plant Biol. 29 (4), 461–471.
Fotopoulos, V., Christou, A., Antoniou, C., Manganaris, G.A., 2015. Hydrogen sulphide: a versatile tool for the regulation of growth and defence responses in horticultural crops. J. Hortic. Sci. Biotechnol. 90 (3) 227.234.
Foyer, C.H., Halliwell, B., 1976. The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133 (1), 21–25. Foyer, C.H., Lelandais, M., Kunert, K.J., 1994. Photooxidative stress in plants. Physiol.
Plant. 92 (4), 696–717.
Foyer, C.H., Noctor, G., 2011. Ascorbate and glutathione: the heart of the redox hub. Plant Physiol. 155 (1), 2–18.
Gajewska, E., SkŁodowska, M., 2010. Differential effect of equal copper, cadmium and nickel concentration on biochemical reactions in wheat seedlings. Ecotoxicol. Environ. Saf. 73 (5), 996–1003.
García-Mata, C., Lamattina, L., 2013. Gasotransmitters are emerging as new guard cell signaling molecules and regulators of leaf gas exchange. Plant Sci. 201, 66–73.
Gill, S.S., Tuteja, N., 2010. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 48 (12), 909–930. Gulías, J., Flexas, J., Abadía, A., Madrano, H., 2002. Photosynthetic responses to water
deficit in six Mediterranean sclerophyll species: possible factors explaining the de-clining distribution of Rhamnus ludovici-salvatoris, an endemic Balearic species. Tree Physiol. 22 (10), 687–697.
Hancock, J.T., Whiteman, M., 2014. Hydrogen sulfide and cell signaling: team player or referee? Plant Physiol. Biochem. 78, 37–42.
Hasanuzzaman, M., Hossain, M.A., Fujita, M., 2011. Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol. Rep. 5 (4), 353.
Hawkins, H.J., Lewis, O., 1993. Combination effect of NaCl salinity, nitrogen form and calcium concentration on the growth, ionic content and gaseous exchange properties of Triticum aestivum L. cv. Gamtoos. New Phytologist. 124 (1), 161–170. He, H., Li, Y., He, L.F., 2019. Role of nitric oxide and hydrogen sulfide in plant aluminum
tolerance. Biometals 32, 1–9.
Herzog, V., Fahimi, H., 1973. Determination of the activity of peroxidase. Anal. Biochem. 55 (554), e62.
Hou, Z., Wang, L., Liu, J., Hou, L., Liu, X., 2013. Hydrogen sulfide regulates ethyle-ne‐induced stomatal closure in Arabidopsis thaliana. J. Integr. Plant Biol. 55 (3), 277–289.
Hu, H., Shen, W., Li, P., 2014. Effects of hydrogen sulphide on quality and antioxidant capacity of mulberry fruit. Int. J. Food Sci. Technol. 49 (2), 399–409.
Hu, L., Wang, Z., Huang, B., 2009. Photosynthetic responses of bermudagrass to drought stress associated with stomatal and metabolic limitations. Crop Sci. 49 (5), 1902–1909.
Hunt, R., Causton, D.R., Shipley, B., Askew, A.P., 2002. A modern tool for classical plant growth analysis. Ann. Bot. 90 (4), 485–488.
Jiang, M., Zhang, J., 2002. Involvement of plasma-membrane NADPH oxidase in abscisic acid- and water stress-induced antioxidant defense in leaves of maize seedlings. Planta 215 (6), 1022–1030.
Jin, Z., Xue, S., Luo, Y., Tian, B., Fang, H., Li, H., Pei, Y., 2013. Hydrogen sulfide inter-acting with abscisic acid in stomatal regulation responses to drought stress in Arabidopsis. Plant Physiol. Biochem. 62, 41–46.
Kaya, C., Higgs, D., Ashraf, M., Alyemeni, M.N., Ahmad, P., 2019. Integrative roles of nitric oxide and hydrogen sulfide in melatonin‐induced tolerance of pepper (Capsicum annuum L.) plants to iron deficiency and salt stress alone or in combination. Physiol. Plant.https://doi.org/10.1111/ppl.12976.
Kong, D., Hao, Y., Cui, H., 2016. The WUSCHEL related homeobox protein WOX7 reg-ulates the sugar response of lateral root development in Arabidopsis thaliana. Mol. Plant 9 (2), 261–270.
Kushwaha, B.K., Singh, V.P., 2019. Glutathione and hydrogen sulfide are required for sulfur‐mediated mitigation of Cr(VI) toxicity in tomato, pea and brinjal seedlings. Physiol. Plant.https://doi.org/10.1111/ppl.13024.
Laemmli, U.K., 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685.
Lawlor, D.W., 2002. Limitation to photosynthesis in water‐stressed leaves: stomata vs. metabolism and the role of ATP. Ann. Bot. 89 (7), 871–885.
Li, S.W., Li, Y., Yeng, Y., Zeng, X.Y., Ma, Y.H., 2019. Nitric oxide donor improves ad-ventitious rooting in mung bean hypocotyl cuttings exposed to cadmium and osmotic stresses. Environ. Exp. Bot. 164, 114–123.
Li, J., Jia, H., Wang, J., Cao, Q., Wen, Z., 2014. Hydrogen sulfide is involved in main-taining ion homeostasis via regulating plasma membrane Na+/H+antiporter system
in the hydrogen peroxide-dependent manner in salt-stress Arabidopsis thaliana root. Protoplasma 251 (4), 899–912.
Li, Z.G., Ding, X.J., Du, P.F., 2013. Hydrogen sulfide donor sodium hydrosulfide-im-proved heat tolerance in maize and involvement of proline. J. Plant Physiol. 170 (8), 741–747.
Lilley, R.M., Fitzgerald, M., Rienits, K., Walker, D., 1975. Criteria of intactness and the photosynthetic activity of spinach chloroplast preparations. New Phytol. 75 (1), 1–10.
Liu, G., Li, X., Jin, S., Liu, X., Zhu, L., Nie, Y., Zhang, X., 2014. Overexpression of rice NAC gene SNAC1 improves drought and salt tolerance by enhancing root development and reducing transpiration rate in transgenic cotton. PLoS One 9 (1), e86895. Liu, Z.J., Guo, Y.K., Bai, J.G., 2010. Exogenous hydrogen peroxide changes antioxidant
enzyme activity and protects ultrastructure in leaves of two Cucumber ecotypes under osmotic stress. J. Plant Growth Regul. 29 (2), 171–183.
Livak, K.J., Schmittgen, T.D., 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCTmethod. Methods 25 (4), 402–408. Ma, L., Zhang, H., Sun, L., Jiao, Y., Zhang, G., Miao, C., Hao, F., 2011. NADPH oxidase
AtrbohD and AtrbohF function in ROS-dependent regulation of Na+/K+homeostasis
in Arabidopsis under salt stress. J. Exp. Bot. 63 (1), 305–317.
Mancardi, D., Penna, C., Merlino, A., Del Soldato, P., Wink, D.A., Pagliaro, P., 2009. Physiological and pharmacological features of the novel gasotransmitter: hydrogen sulfide. Biochimica et Biophysica Acta (BBA)-Bioenergetics 1787 (7), 864–872. Mengel, K., Kirby, E.A., 2001. Principles of Plant Nutrition, 5th ed. Kluwer Academic
Publishers, Dordrecht, The Netherlands.
Miller, G., Schlauch, K., Tam, R., Cortes, D., Torres, M.A., Shulaev, V., Dangl, J.L., Mittler, R., 2009. The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci. Signal. 2 (84) ra45-ra45.
Mittler, R., Zilinskas, B.A., 1993. Detection of ascorbate peroxidase-activity in native gels by inhibition of the ascorbate-dependent reduction of nitroblue tetrazolium. Anal. Biochem. 212 (2), 540–546.
Mittova, V., Tal, M., Volokita, M., Guy, M., 2003. Up‐regulation of the leaf mitochondrial and peroxisomal antioxidative systems in response to salt‐induced oxidative stress in the wild salt‐tolerant tomato species Lycopersicon pennellii. Plant Cell Environ. 26 (6),