Micron89(2016)60–76
ContentslistsavailableatScienceDirect
Micron
jo u rn al h om ep a g e :w w w . e l s e v i e r . c o m / l o c a t e / m i c r o n
Review
Atomic
force
microscopy
for
the
investigation
of
molecular
and
cellular
behavior
Alper
D.
Ozkan,
Ahmet
E.
Topal,
Aykutlu
Dana,
Mustafa
O.
Guler,
Ayse
B.
Tekinay
∗BilkentUniversity,UNAM-InstituteofMaterialsScienceandNanotechnology,Ankara,Turkey
a
r
t
i
c
l
e
i
n
f
o
Articlehistory: Received4May2016 Accepted27July2016 Availableonline29July2016 Keywords:
Atomicforcemicroscopy Biomacromolecules Mechanicalcharacterization Cells
a
b
s
t
r
a
c
t
Thepresentreviewdetailsthemethodsusedforthemeasurementofcellsandtheirexudatesusingatomic forcemicroscopy(AFM)andoutlinesthegeneralconclusionsdrawnbythemechanicalcharacterizationof biologicalmaterialsthroughthismethod.AFMisamaterialcharacterizationtechniquethatcanbe oper-atedinliquidconditions,allowingitsusefortheinvestigationofthemechanicalpropertiesofbiological materialsintheirnativeenvironments.AFMhasbeenusedforthemechanicalinvestigationofproteins, nucleicacids,biofilms,secretions,membranebilayers,tissuesandbacterialoreukaryoticcells;however, comparisonbetweenstudiesisdifficultduetovariancesbetweentipsizesandmorphologies,sample fixationandimmobilizationstrategies,conditionsofmeasurementandthemechanicalparametersused forthequantificationofbiomaterialresponse.AlthoughstandardprotocolsfortheAFMinvestigation ofbiologicalmaterialsarelimitedandminordifferencesinmeasurementconditionsmaycreatelarge discrepancies,themethodisnonethelesshighlyeffectiveforcomparativelyevaluatingthemechanical integrityofbiomaterialsandcanbeusedforthereal-timeacquisitionofelasticitydatafollowingthe introductionofachemicalormechanicalstimulus.Whileitiscurrentlyoflimiteddiagnosticvalue,the techniqueisalsousefulforbasicresearchincancerbiologyandthecharacterizationofdiseaseprogression andwoundhealingprocesses.
©2016ElsevierLtd.Allrightsreserved.
Contents
1. Introduction...60
2. Effectofprobemorphology,compositionandsurfacechemistry...61
3. Atomicforcemicroscopyofunicellularorganisms...62
3.1. Bacterialcellsurfaces...62
3.2. Bacterialsecretions,exudatesandbiofilms ... 64
4. Atomicforcemicroscopyofmammaliancellsandtissues...64
4.1. Cancerdiagnosisandcharacterization ... 67
4.2. Diagnosisofotherdiseases ... 69
4.3. Stemcelldifferentiation...70
4.4. Extracellularsecretionsandtissuemicroenvironments ... 71
5. Futuredirections...71
References...73
1. Introduction
Bothuni-andmulticellularorganismscoordinatetheirbehavior usinganetworkofchemical,electricalandmechanicalsignals,and employavarietyofsensorymechanismstoperceiveandrespond
∗ Correspondingauthor.
E-mailaddress:atekinay@bilkent.edu.tr(A.B.Tekinay).
tointernalorexternalregulatorycues(Riccaetal.,2013;Johnson, 2013).Inunicellularorganisms,suchsignalsmayassistin feed-ing,attractingconspecifics,synchronizingreproductivecyclesor initiatingdefensemechanismsinahostileenvironment(Dufour andLevesque,2013);whilemulticellularlifeutilizescell signal-ingnetworkstoregulatecellrecruitment,adhesion,differentiation, proliferationanddeath(WattandHuck,2013;Ravichandran,2003; Zoranovicetal.,2013;JaenischandBird,2003;OwensandWise, 1997).Asthelattercategoryofprocessesareintegraltosustain complexlife,thecharacterizationofregulatorysignalsisofgreat http://dx.doi.org/10.1016/j.micron.2016.07.011
A.D.Ozkanetal./Micron89(2016)60–76 61 importancetothemedicalandbiologicalsciences,andmuchwork
hasbeenperformedtoelucidatethelinksbetween environmen-talcuesandcellularprocesses(Andoetal.,2013;Carvalhoetal., 2013;Dorobantuetal.,2012).However,whilethechemicaland biological environment of cells are relatively well-defined, the mechanicalpropertiesofcellsandtheirimmediateenvironment areinvestigatedonlytoalesserdegree;partlybecauseofthehigh complexityandvariabilityofthemechanicalinteractionsexhibited bycells andpartly duetolimitationsassociated withthe high-resolutionmechanicalprobingofcellsurfacesandinteriors(Cohen andKalfon-Cohen,2013).Nonetheless,considerableefforthasbeen spenttoestablishhowcellsperceiveandactuponthephysical char-acteristicsofnearbysubstrates(Shaoetal.,1996),andtodetermine howthemechanicalpropertiesofcellsandtissuesarealteredin responsetodiseasestateorenvironmentalfactors,usingmaterial characterizationtoolssuchasmagnetictwistingcytometry,optical tweezers,microneedleprobes,scanningacousticmicroscopyand atomicforcemicroscopy(NeumanandNagy,2008).
Atomicforcemicroscopy(AFM)isacharacterizationtoolthat measures the topology and material properties of surfaces by recordingthedeflectionofametallicprobe(or“tip”)asitmoves overthetargetsurface.AFMcanbeoperatedunderthree princi-palmodes:Incontactmode,thetipisdraggeddirectlyoverthe surfaceanddeflectsawayduetoarepulsiveCoulombic interac-tion,while in non-contact modeit is held at a short(typically <100nm)distanceoverthesampleandoscillatesatafrequency thatdependsontheattractivevanderWaalsforcesactingupon it.Intappingorintermittentcontactmode,thetipiskept oscil-latingabovethesample,andtheoscillationfrequencychangesas thetipapproachesthesurfaceatregularintervals(Giessibl,2003). Contactand intermittentmodesareparticularlysuitableforthe probingofbiologicalsamples,duetotheirapplicabilityinliquid media(Danino,2008).Despiteconsiderablelossesinresolution,a liquidsampleenvironmentallowscellularimaginginanative(or pseudo-native)environmentand,moreimportantly,permitsthe directinvestigationofmechanicalchangesonalivecellsurfacein responsetoanintroducedstimulus(Liuetal.,2005).Time-lapse elastographstakeninthisfashionhavebeenutilizedforadiverse arrayofapplications,includingtovisualizetheformationof amy-loid(Harperetal.,1997)orcollagen(Revenkoetal.,1994)fibers underdifferentenvironmentalconditions,determinehow mem-braneintegrityisalteredin thepresenceofantibiotics(Fantner etal.,2010a),orrecordtheproductionanddissolutionof cytoskele-talelementsduringcellmovement(RotschandRadmacher,2000). Inaddition,itispossibletoutilizetheAFMtipasastimulusto elicitaresponsefromthetargetcell,andtheprobeitselfcanbe functionalizedwithligandmoleculestodeterminetheaffinityof thecellmembranetoaparticularbiologicalmoiety.
DuetotheversatilityandpotentialapplicationareasofAFM, thetechniquehasattractedsubstantialinterestinbiomechanical research,andhasbeenusedinthecharacterizationofagreat vari-etyoftissues,cellsandsub-cellularstructuresinbothlivecondition andfollowingfixinganddrying.Thepresentreviewaimstocover thosestudiesthatfocusonthedifferencesinmechanicalproperties associatedwithpathologicalconditionsorchangesin environmen-talcues,andemphasizestheimportanceofthemechanicalUmwelt inmodulatingthebehaviorofbothsingle-celledandmulticellular systems.
2. Effectofprobemorphology,compositionandsurface chemistry
BeforediscussingtheAFMimagingofbiologicalmaterials,the importanceofAFMtipchoiceshouldbeunderlined.The diame-ters,materials,morphologiesandcantileverlengthsofcommercial
AFMprobesshowconsiderablevariance,andoptimalperformance requirestheuseofaprobeconductivetothetaskathand.The com-positionofthesamplematerialshouldbetakenintoconsideration tochoosethespringconstantoftheAFMprobe,assoftermaterials, suchascells,maybedamagedoverrepeatedcontactwiththeAFM tip(Costa,2003).Inaddition,dependingontheareatobescanned, itmaybedesirabletoincreaseordecreasethetipdiameter.Larger tipsareassociatedwithlowerresolution,butcanbeutilizedtoscan largersampleareaswithoutcompromisingtipintegrity,assharper tipsmayexperiencesignificantwearoverlongscanningdistances, suchaswhenscanningcells.Ontheotherhand,sharpertipsare capableofresolvingsmallerfeaturestoagreaterextent,whichis invaluablewhenmeasuringproteinsandothernanoscale biolog-icalmaterials.Consequently,differencesinmaterialstiffnessthat areevidentundernanoscaleinvestigationmaybeunmeasurable usingmicroscaletips(Stolzetal.,2009a).Ifadhesiondataistobe collected,thematerialandmorphologyoftheAFMtip(alongside substrateproperties)alsodeterminesthesuitablemodelforusein elasticitycalculations(Fig.1).
AFMprobescanalsobefunctionalizedinordertocharacterize theinteractionbetweentwospecifictypesofbiologicalmoieties, suchasbetweenareceptoranditsligand.Thistypeofinteraction isbestexemplifiedbybiotinandavidin,usedbyColtonetal.in theirhallmarkpapertoillustratethepossibilityofusingAFMto directlyevaluatethestrengthofmolecularinteractions(Leeetal., 1994).Mechanicalpropertiesofawidevarietyofproteinshavenow beenelucidated,includingtheinteractionsbetweenantibodiesand theircorrespondingantigens(Allenetal.,1997),actinandmyosin (Koderaetal.,2010),osteopontinandintegrin(Leeetal.,2007),and variouscelladhesionproteoglycans(Dammeretal.,1995).Such proteinscaneitherbecovalentlytetheredtothetargetmaterial (Ebneretal.,2007;Kamruzzahanetal.,2006)orattachedbydrying theproteinsampleonthesurface(Florinetal.,1995).Inaddition, themechanicalstrengthoftheconstituentdomainsofasingle pro-teincanbeevaluatedbyattachingthatproteintoasurfaceand usingtheAFMtiptostretchit(Lietal.,2003).Thisresultsinthe gradualunfoldingoftheprotein,andtheunwindingofeachdomain isassociatedwithamomentarydropinforce.Tensile characteris-ticsoftheimmunoglobulinandfibronectinIIIdomainsoftitinwere investigatedusingthismethod(Riefetal.,1998),andtheabilityof thebacterialribonucleasebarnasetowithstandforcewaslikewise evaluatedbyincorporatingthisproteinintoachimericconstruct consistingoffourTII27and threebarnasesubunits(Bestetal., 2001).
DNAandRNAcanalsobeimmobilizedandcharacterizedina similarmanner,andthemechanicalinvestigationofDNAmolecules ofvaryinglengthsandconfigurationshasbeenperformedusing AFM(Maoetal.,1999; Hansmaetal.,1995).In additiontothe determinationofcovalentbondstrengthinssDNAordsDNA,itis alsopossibletoevaluatethestrengthofthebondsbetween com-plementarystrandsinashortdsDNApiece,ortodeterminethe forcesnecessarytostretchanintactDNAmolecule(Hansmaetal., 1996).High-resolutionAFMimagingcanalsobeusedto character-izethephysicalstructureofaDNAhelix(Fig.2),andmorecomplex DNAarchitecturesandDNA–proteininteractionscanbevisualized andcharacterizedusingatomicforcemicroscopy.Yanevaetal.,for example,confirmedthatDNA-dependentproteinkinase(DNA-PK) canbindtoDNAwithouttheassistanceofKuproteins,andthatthe lattershowsatime-dependentpreferenceforstrandends,by visu-alizingDNA-KuandDNA-DNA-PKinteractionsusingAFM(Yaneva etal.,1997).Theaffinitybetweencellsandspecificproteinscanalso beassessedbyindentingthecellofinterestwithanAFMtip func-tionalizedwiththeproteinofinterest(Hanetal.,1995).Gaubetal. reportedamethodtodistinguishbetweenindividualredbloodcell originsinamixtureofA-andO-grouperythrocytes,usinganAFM tipfunctionalizedwithHelixpomatialectin(Grandboisetal.,2000).
62 A.D.Ozkanetal./Micron89(2016)60–76
Fig.1. Comparisonofmicro-andnanoindentationfortheidentificationofchangesassociatedwithaginginthecartilageofC57BL/6mice.Microindentationresultscould detectnodifferencebetween1,10and19-montholdindividuals,whilenanoindentationwasabletodeterminethatthecartilageelasticityofnon-arthriticmicechanges withage.ReplicatedwithpermissionfromStolzetal.(2009b).
Fig.2. AFMtopographyofaplasmid,showingthegeneralappearanceoftheDNA helixundervaryingpeakforces(a–d).Minorandmajorgroovescanbeobserved inAFMimages(a–d,insets),andtheDNAstructurecanbecompressedunderhigh peakforces(e,f).Whitearrowdenotesadislocationinaplasmidloopcreatedby highloadforces.ReplicatedwithpermissionfromPyneetal.(2014).
Thislectindisplaysstrongaffinitytoglycolipidsthatarepresentin themembranesofA-groupbutnotO-grouperythrocytes,resulting inhigherruptureforcesassociatedwiththeformer.Thedifferences
inadhesiveforcesarethenutilizedtocreateamapwhereindividual A-andO-groupcellscanbeidentified.
3. Atomicforcemicroscopyofunicellularorganisms
ArepresentativeselectionofAFMstudiesonthestiffness char-acterizationofbacteria,yeastsandcellularsecretionsisprovided inTable1.Itisreadilyevidentthatseveralmeansofsample prepa-ration are available for measurement, and that values suchas tip-sampleadhesion,F-dcurveslopesandcellularspringconstants canallbeusedtocomparethemechanicalintegritiesofbiological samples;consequently,onlythesestudiesdetailingthefullrange ofmeasurementconditionswereincludedintothetable.Bothair andliquidimaginghavebeenperformedformechanical investi-gations;however,biologicalmaterialsareoftenviscoelasticand maydisplaylargechangesinelasticbehaviordependingon envi-ronmentalhumidity.Assuch,samplesinairtendtohavemuch largerYoung’smodulicomparedtosamplesimagedinliquids(e.g. a10-folddifferencewasobservedinbetweentheelasticmoduliof air-driedandrehydratedmurinesacculifromEscherichiacoli(Yao etal.,1999)).Given thedifferences inmeasurementtechniques andsamplepreparationmethods,aswellasthenaturalvariance inthematerialpropertiesofbacterialcellsandtheirsecretions,it isusuallypreferabletocompareresultswithinstudiesratherthan assumingagivenstiffnessvaluewillapplyunderother experimen-talconditions.
3.1. Bacterialcellsurfaces
Unlikemanyvertebratecelllines,bacterialcellsarenot depen-dentonahighlyspecificsetofenvironmentalconditionstosurvive, andcantolerateextendedAFMimagingsessionswithout detrimen-taleffects(Ramanetal.,2011;Franciusetal.,2008).Theireaseof procurement,non-demandinggrowthconditionsandthefactthat manylaboratoryspecieseitherare,orserveasmodelsfor,common pathogensmakebacteriapopulartargetsforAFMimaging. Bacte-riamustbeimmobilizedpriortoimaginginliquidmedia,astheir mobilityotherwisemakesitimpossibletoimage,andeven ses-silebacteriacanbelaterallypushedbytheAFMtip(Doktyczetal., 2003).Immobilizationcanbeperformedbydryingandrehydrating, electrostaticbindingtoapositivelychargedsurface(e.g.gelatinor
A.D. Ozkan et al. / Micron 89 (2016) 60–76 63 Table1
Mechanicalcharacterizationofmembranes,secretionsandsingle-celledorganismsbyAFM.
Sample Tipproperties Imagingconditions Elasticproperties Reference
Sulfate-reducingbacteria Siliconnitride,k=0.06N/m(nominal) Contactmode,air,sampleonmica (Adhesion)−3.9to−4.3nNatcellsurface,−5.1 to−5.9nNatcell-substrateboundary,−6.5to −6.8nNatcell–cellboundary
Fangetal.(2000)
EnteroaggregativeEscherichiacoli, wild-typeanddispersinmutant
Silicon,k=2.8N/m(nominal) Contactmode,liquid(distilledwater)andair, sampleongelatin-treatedmica
(F-dslope)0.133forwild-typestrainonagar, 0.069forwild-typestraininbroth,0.81for dispersinmutantonagar,0.78fordispersin mutantinbroth
Beckmannetal.(2006)
Bacillussubtilis,Micrococcusluteus, Pseudomonasputida,twostrainsof Escherichiacoli
Siliconnitride,k=0.32N/m(nominal) Contactmode,liquid(HMbuffer),sampleon APTEScoverslip
(Springconstant)variesbetween0.16±0.01to 0.41±0.01,higherinGram-positivecells
Volleetal.(2008)
Pseudomonasaeruginosa Siliconnitrideandsiliconnitridewithsilicon oxidetips,k=0.07±0.01(calibratedbythe thermalmethod)
Contactmode,liquid(milliQwater),sampleon poly-l-lysine-coatedglass
(Springconstant)0.044±0.002N/mfor unfixed,0.11±0.03N/mfor
glutaraldehyde-fixedcells;creepdeformation behavioralsoinvestigated
Vadillo-Rodriguezetal.(2008)
Klebsiellaterrigena Siliconnitride,k=0.06N/m(nominal) Contactmode,liquid(potassiumphosphate bufferatpH6.8),sampleonpolycarbonate membranefilter/poly-l-lysine-coated glass/immobilizedontipbygluteraldehyde fixation
(Adhesion)−0.26±0.05nNformembrane filter,−0.5±0.2forpoly-l-lysine,−35±2nN forgluteraldehydefixation,otheradhesion parametersalsomeasured
Vadillo-Rodriguesetal.(2004)
TwoStreptococcussalivariusstrains Siliconnitride,k=0.03N/m(nominal) Contactmode,liquid(deionizedwateror0.1M KClsolution),sampleonpolycarbonate membranefilter
(Adhesionandrepulsion)Fibrillatedstrain showsalargerrepulsionrange,interpretedto reflectthelayeroffibrils;retractionresultsin threeadhesionforcespotentially
correspondingtothreedifferentlengthsof fibrilsobservedbyelectronmicroscopy
vanderMeietal.(2000)
Desulfovibriodesulfuricans, Pseudomonassp.andan unidentifiedlocalmarineisolate
Siliconnitride,k=0.12±0.02N/m(calibrated bythethermalmethod)
Contactmode,liquid(artificialseawater), samplecoatedontipandbroughtinto interactionwithmetals
(Adhesion)Allthreeisolatesadhereto aluminumbetterthanmildsteel,stainless steel316andcopper;Desulfovibrioand Pseudomonasadherebetterthanthemarine isolate.
Shengetal.(2007)
B.mycoides Silicon,k=0.064N/mand0.4N/m Contactmode(constantheight),liquid (0.145MNaCl),samplecoatedontipand broughtintointeractionwithglass
(Adhesion)7.4±3.7nNofadhesionto hydrophilicglasssurface,49.5±14.42nNof adhesiontohydrophobic-coatedglasssurface
Bowenetal.(2002)
Marinebacterialdepositions Silicon,k=45.7N/m(calibratedbytheadded massmethod)
Tappingmode,air,sampledepositedon fluoridatedandnon-fluoridatedpolyurethane
(Young’smodulus)between1.5and2.2GPa Bakkeretal.(2003)
Bacterialdepositions,suspectedto beextracellularpolymeric substances
Siliconnitride,kvariesfrom0.03to0.5N/m (nominal)
Contactmode,liquid(MilliQwater),sample depositedonpolystrene
(Adhesion)Forcesof0.8±0.2nNobservedover barepolystyrene,asopposedto0.2±0.2nN aftercellattachment
vanderAaandDufrene(2002)
P.aeruginosapili Siliconnitride,0.008±0.004N/m Contactmode,liquid(water),sampleattached topoly-l-lysine-coatedtipsandbroughtinto interactionwithmicasurface
(Adhesion)Ruptureforcesof95pNduring retraction.
Touhamietal.(2006)
E.colibiofilms Siliconnitride,k=0.07-0.4N/m Contactmode,air,sampledepositedonglass (Adhesion)Pull-offforcesof122.65pNfor cell-tipinteractionand51.79pNforglass-tip interaction
Ohetal.(2007)
Bacterialcellulosefibers Siliconnitridek=1.03±0.05N/m(calibrated bythethermalmethod,nominalkof0.5N/m notusedduetolargediscrepancy)
Contactmode,air,sampleonsilicon nitride-coatedsilicongrating
(Young’smodulus)78±17GPa Guhadosetal.(2005)
E.coliandE.colispheroplasts Siliconnitride,k=0.1and0.01N/m(nominal, actualspringconstantscalibratedbythe thermalmethod)
Contactmode,liquid(TBS2buffer),sampleon APTES/Glutmica
(Springconstant)0.194N/mforintactcells, 0.571N/mforfixedspheroplasts
Sullivanetal.(2007)
Saccharomycescerevisiae Siliconnitride,k=0.008±0.4N/m(calibrated bythethermalmethod)
Contactmode,liquid(milliQwater),sampleon polycarbonatemembranefilter
(Young’smodulus)6.1±2.4MPaonbudscar, 0.6±0.4MPaonsurroundingcellwall
Touhamietal.(2003)
Aspergillusnidulans Siliconnitride,k=0.47±0.06N/m(calibrated bythethermalmethod)
Contactmode,liquid(PBS),sampleon poly-l-lysine-coatedglass
(SpringconstantandYoung’smodulus) 0.29±0.02N/mand110±10MPafor wild-typehyphaeincompletemedium, decreasesformutantstrainlackingachitin synthesisgene,aswellasinthepresenceof 0.6MKCl.
64 A.D.Ozkanetal./Micron89(2016)60–76 poly-l-lysine),entrapmentinadhesiveproteins,covalentbinding
toamino-orcarboxyl-functionalizedsurfacesorbyphysical con-finementinmicrowellsorporousmembranes(Doktyczetal.,2003; Suoetal.,2008).Itshouldbekeptinmindthattheimmobilization methodmayalterthesurfacepropertiesoftheentrappedcells,e.g. bydirectlyalteringthebacterialsurfacechemistryortriggeringa defensemechanismagainsttheenvironmentalstressesassociated withtheimmobilizationtechnique.Duetothesmallsizesof bacte-ria,itisalsopossibletofunctionalizeAFMtipswithbacterialcells, whichcanthenbeusedtotesttheinteractionbetweenthe bac-teriumandmaterialsurfaces.Tingetal.,forexample,usedsuchtips toshowthattheGram-negativebacteriaMassiliatimonaeand Pseu-domonasaeruginosaadherebettertostainlesssteelsurfacesthan doestheGram-positiveBacillussubtilis(Harimawanetal.,2011).
AFMstudiesofbacteriafrequentlyfocusonthemechanismsby whichcertainmoleculesinhibitthegrowthofpathogens. Many antibiotics(e.g.beta-lactamantibiotics,polymyxinsand glycopep-tideantibiotics)actbyinhibitingthesynthesisofbacterialcellwalls ormembranes,andtherebyalterthemembraneintegrityofthe affectedbacteria.Otherantibiotic-mediatedeffects,suchas mem-branethinning(Meckeetal.,2005)orporeformation(Mulleretal., 1999),canalsobeobservedbyAFM,andtheeffectsofless con-ventionalantibiotics,suchasplantextracts(Perryetal.,2009)and peptidesequences(daSilvaandTeschke,2005;Meinckenetal., 2005),canalsobeassessed(Fig.3).TheantimicrobialpeptidePGLa hasbeendemonstrated tolowerthestiffness ofEscherichiacoli membranesandcreatemicelle-likestructuresaroundcell mem-branespriortotheireventualrupture,whilegarlicextractwasalso associatedwithmembranedisruption.Chitosanand chitooligosac-charides(COSs)werealsotestedfortheirantimicrobialeffecton E.coliandStaphylococcusaureus,andthethickpeptidoglycanwallof S.aureuswasfoundtoallowthisbacteriumtobetterretainits over-allmorphology,despiteexperiencingasignificantdecreaseincell rigidity(Fernandesetal.,2009).Whiletheeffectsofantibacterial moleculesmaybeapparentevenunderdryimaging,itisalso possi-bletoquantifytheresultingchangesinatime-dependentmanner bycharacterizingthemechanicalpropertiesofbacterialcellsin liq-uidbeforeandafterintroducingtheantibioticinquestionintothe medium(Fantneretal.,2010a).Thistechniquehastheadvantageof ensuringthatthechangesobservedarefullyduetotheantibiotic,as opposedtoacombinationofitandthedryingprocess,asbacterial cellwallsareviscoelasticandmaygreatlyaltertheirmechanical propertiesinresponsetorelativehumidity(ThwaitesandSurana, 1991).
Avarietyofsubcellularelementscanalsobeidentifiedon mem-branesurfacesusingAFM.Bothbacterialandeukaryoticcellscan beusedintheseefforts,andlipidbilayerscanbesubstitutedas simplifiedmodelsofcellmembranes(Buttetal.,1990;Kuznetsov andMcPherson,2011).Thestructureandfunctionofmembrane proteins(Buttetal.,1990;Fotiadisetal.,2003;Yuanetal.,2002), porecomplexes(Stoffleretal.,1999),gapjunctions(LalandJohn, 1994),amyloidaggregates(Connellyetal.,2012)andavarietyof membrane-componentlipids(Gyorvaryetal.,2003a),aswellas themodeofactionofcholeratoxin(Mouetal.,1995),were inves-tigatedbyatomicforcemicroscopy.Ofparticularinterestarethe recentdevelopmentsinAFM-basedrapid,high-resolutionimaging methods,whichhavegrantedsubstantialinsightintothenature ofsmallsurfaceelements.Processessuchastheself-assemblyof bacterialS-layerproteins(Gyorvaryetal., 2003b), protein fold-ing(Mulleretal.,2002),misfolding(Oberhauseretal.,1999)and crystallization(Reviakineetal.,1998)events,themotionof “walk-ing”proteins(Preineretal.,2014)anddrug-membraneinteractions (Berquandetal.,2004)havebeenthesubjectofreal-timeimaging studies.
3.2. Bacterialsecretions,exudatesandbiofilms
Itiswell-knownthatcellsinmulticellularorganismsenhance theirsurvivalandcoordinationthroughtheproductionofan extra-cellularmatrix;however, this property isnot unique tohigher eukaryotes. Bacteria also secrete proteins, polysaccharides and quorumsensingmoleculesthatrelayinformationbetween con-specificcellsandserveasabufferagainstenvironmentalstresses. Inaddition,thesurfaceattachmentof bacteriaisalsomediated byextracellulardepositions,theadhesioncapacitiesofwhichcan bemeasuredthroughAFM. Colanicacidproduction andsurface lipopolysaccharidelengths,forexample,werepreviously demon-stratedtodeterminethestrengthofattachmentofE.colicells,as colanicacid-overproducingmutantswerefoundtoexhibitstronger attachmentwhileshortersurfacelipolysaccharideswere associ-atedwithalackofadhesivecapacity(Razatosetal.,1998).
BacterialbiofilmsarealsoofspecialinterestwithregardstoAFM characterization.Biofilmsareextracellularpolysaccharidesthatare secretedtofacilitatetheattachmentofbacteria(orotherunicellular organisms)toasurface,andprotecttheadheringcellsagainst hos-tileenvironmentalfactorssuchasantibiotics,detergentsandheavy metals(Daviesetal.,1998).Biofilmsareundesirableelementsin manysettings,andsuitablemeanstoinhibittheirformationis nec-essarytopreventpotentialhealthhazardsinfood,agriculturaland medicalindustries.Assuch,themechanicalpropertiesofbiofilms, as wellasthe mechanismsby which antifoulingmolecules act againstbiofilmproduction,havebeeninvestigatedbyusingAFM and othermechanical characterizationtechniques(Beech etal., 2002;ArnoldandBailey,2000).Corrosiondamageandadhesive capacity of biofilms on a variety of metal surfaces have been detailed intheliterature: Holdenet al.reportthat unsaturated andliquid-grownbiofilmsofPseudomonasputidarespond differ-entlytodrying,suggestingthatthebiofilmcompositionisaltered foroptimalgrowthindryandwetenvironments(Auerbachetal., 2000).Inanotherreport,Tayetal.detailtheeffectofsilverions onStaphylococcusepidermidisbiofilmsandproposeamechanism throughwhichsilverdestabilizesthebiofilmstructurebybinding to the electron donor groups provided by the biofilm compo-nents,therebyweakeningthehydrogenbondsthatholdthebiofilm matrixtogether(Chawetal.,2005).
4. Atomicforcemicroscopyofmammaliancellsandtissues AselectionofAFMstudiesonthestiffnesscharacterizationof eukaryoticcellsandtissuesisprovidedinTable2.AFMof mam-maliancellsandtissuesisoftenundertakenfordiseasediagnosis orstemcelldifferentiationstudies,asdiseasedtissuesoftendisplay mechanicalcharacteristicsdistinctfromtheirhealthycounterparts and stem cells are widely known to alter their differentiation pathwaysand mechanicalcharacteristicsdependingonexternal stimuli.Consequently,AFMofcancerandstemcellscanprovide greaterinsightintothepathwaysrequiredforeventssuchas metas-tasisandlineagecommitment.However,measurementofthese samplesismoredifficultthansingle-cellularorganismsor non-livingbiologicalmaterialssuchasbiofilmsandothersecretions,as mammaliancellsrequireaspecificsetofenvironmentalconditions tosurviveandenvironmentalstresscanhaveamajorimpacton theirmechanicalproperties.Inaddition,whilefixativetreatment canbeusedpriortoimaging,fixationmaygreatlyalterthe mechan-icalpropertiesofeukaryoticcells,andlivecellimagingisgenerally requiredtoacquirereliableelasticitydata(Fig.4).Nonetheless, cellcultureconditionscanbereplicatedtosomeextentwithinthe liquidcellofanAFM,andalargenumberofsuccessful investiga-tionshavebeenmaderegardingthemechanicalcharacterofliving
A.D. Ozkan et al. / Micron 89 (2016) 60–76 65 Table2
MechanicalcharacterizationofmammaliancellsandtissuesbyAFM.
Sample Tipproperties Imagingconditions Elasticproperties Reference
NIH3T3fibroblasts Siliconnitride,k=0.018N/m (calibratedbythermalmethod)
Contactmode,liquid(DMEM containingd-glucose(1000mg/L)and 10%fetalbovineserum,fresh,warmed Ringer’ssolutionusedasmedium replenishment),sampleon fibronectin-coatedglass
(Young’smodulus)4–100kPaoverthecellsurface,lower aroundthenucleusthanintheperiphery
Hagaetal.(2000)
Breastcancerlines(MCF-10A andMCF7)
Siliconnitride,k=0.01N/m(nominal) modifiedwitha4.5mdiameter polystyrenebead
Contactmode,liquid(culture medium),sampleonglass
(Young’smodulus)0.2–1.2kParange,malignantcellline (MCF7)1.4–1.8timessofterthanbenigncellline (MCF-10A)
Lietal.(2008)
Osteoblasts,mesenchymal stemcellsandosteosarcoma cells
Siliconnitride,k∼20N/m(calibrated usingthermalmethod)
Contactmode,liquid(25mMHEPES), fixedcellsonpolystyrene,glassor collagen-coatedglass
(Young’smodulus)0.7±0.1to2.6±0.7kParange,lower Young’smodulusforMG63osteosarcomacellsoncollagen
Dochevaetal.(2008)
Zonalarticularchondrocytes Sphericalgold-coatedborosilicatebead (5mdiameter),k∼0.065N/m (calibratedbythermalmethod)
Contactmode,liquid(DMEM),sample onpoly-l-lysine-coatedglass
(Young’smodulus)Instantaneousmoduliat0.55±0.23kPa forsuperficial,0.29±0.14kPaformiddle/deepcells; relaxedmoduliat0.31±0.15kPaforsuperficial, 0.17±0.09kPaformiddle/deepcells;apparentviscosities at1.15±0.66kPasforsuperficial,0.61±0.69kPasfor middle/deepcells
Darlingetal.(2006)
Cardiacmuscle,skeletal muscleandendothelialcells
Siliconnitride,k=0.03to0.05N/m (calibratedusingthermalmethod)
Contactmode,liquid(growth medium),sampleonglassslide
(Young’smodulus)Moduliof100.3±10.7kPaforcardiac muscle,24.7±3.5kPaforskeletalmuscleand1.4±0.1to 6.8±0.4kPadependingontheregiontestedforepithelial cells
Mathuretal.(2001)
Cardiacmyocytes Siliconnitride,k=0.06N/m(nominal) Contactmode,liquid(culture medium),sampleonlaminin-coated petridish
(Young’smodulus)35.1±0.7kPaforcardiomyocytesfrom 4-montholdrats,42.5±1.0kPaforcardiomyocytesfrom 30-montholdrats.
Lieberetal.,(2004a)
LLC-PK1andMDCKkidney epithelialcelllines
Siliconnitride,k=0.12N/m(nominal) Contactmode,liquid(artificialurine) (Young’smodulus)1.5±0.8MPaforLLC-PK1cells, 5±1.5MPaforMDCKcells,oxalatetreatmentdecreases Young’smodulusto1.2±MPaforLLC-PK1cells(other stiffnessparametersalsomeasured)
Rabinovichetal.(2005)
Neuronalgrowthcones Siliconnitride,k=0.006N/m(nominal) Contactanddynamicmodes,liquid (L15/ASWmedium),sampleon poly-l-lysine-coatedglass
(Young’smodulus)3–7kPafortheCdomain,7–23kPafor theTdomain,10–40kPaforthePdomain
Xiongetal.(2009)
Healthyandpathological erythrocytes
Siliconnitride,k=0.03(nominal) Contact,liquid(PBS),sampleon poly-l-lysine-coatedglassandfixedby glutaraldehyde
(Young’smodulus)Moduliof26±7kPaforhealthy erythrocytes,43±21kPaforhereditaryspherocytosis, 40±24kPaforthalassemiaand90±20kPaforG6PD deficiencysamples
Dulinskaetal.(2006)
Liverendothelialcells Siliconnitride,k=0.032N/m Contact,liquid(serum-freeendothelial cellmedium),cellsoncollagen-coated petridisheswithandwithout glutaraldehydefixation
(Young’smodulus)Moduliof2kPaforlivingcellsandover 100kPaforfixedcells
Braetetal.(1998)
Oralsquamouscellcarcinoma, normalandmalignantlines
Siliconnitridetip,k=0.01to0.1N/m; APTES-modifiedsiliconoxidesphere tip,k∼0.5N/m(calibratedusingSader method)
Contact,air,samplepre-fixedwith2% PFAandfixedwith3.7%PFA
(Young’smodulus)Medianvaluesof6.75MPafor“normal” and4.36MPaformetastaticcancercells.Elasticity measurementstakenusingsphere-modifiedtips.
Lasalviaetal.(2015)
PC-3prostatecancercells Siliconnitridetip,k=0.012N/m (calibratedusingthermalmethod)
Contact,liquid(culturemedium), samplestreatedwithanticancerdrugs orDMSOcontrolfor24hpriorto analysis.
(Young’smodulus)c.3kPaforuntreatedcells,increasedto c.6–12kPainadose-dependentmannerfollowingdrug treatment.Frequency-dependencyoftheelasticmodulus wasalsotestedandfoundtochangesignificantlyfor Celebrex,BAY,Totamine,TPAandVPAtreatment,butnot forDSF,MKandTaxol.Thiseffectislinkedtothefactthat theformerdrugsmayaltercrosslinkingratesof cytoskeletalfilaments,whilethelatteronlychangefiber lengthandthickness.
66 A.D. Ozkan et al. / Micron 89 (2016) 60–76 Table2(Continued)
Sample Tipproperties Imagingconditions Elasticproperties Reference
Normalandcancerousbladder epitheliumcells
Siliconnitridetip,k=0.011to0.018 (calibratedusingthermalmethod)
Contact,liquid(culturemedium), sampleonglass
(Adhesionenergy)averageof8.17×10−16Jfornormal,
26.95×10−16Jforcancercells
(Young’smodulus)averageof27.57kPafornormal, 2.46kPaforcancercells
Canettaetal.(2014)
Porcinearticularcartilage Siliconnitride,k=0.06N/m(nominal) andborosilicateglassbeadswith r=2.5m,k=0.06and13N/m (nominal)
Contact,liquid(PBS),tissueson poly-l-lysine-coatedglass
(Dynamicelasticmodulus)Ontheorderof2.6MPafor borosilicateglassbeads,about100-foldlowerforsharp tips
Stolzetal.(2004)
Articularcartilageofnormal andarthritic(Col9a1−/−
knockout)mice
Siliconnitride,k=0.06N/m(nominal) andmicrospheres,k=10and12N/m
Contact,liquid(PBS),bulktissuesglued onaroundTeflondisk
(Dynamicelasticmodulus)1.3±0.4MPaformicrospheres (nochangerecordedbetweenages);22.3±1.5kPa, 36.8±1.5kPaand50.9±4.7kPaforsharptipsin1-, 10-and19-montholdnormalmice;22.3±1.5kPa,25.5±kPa and27.7±1.1kPainthenon-thickened,intermediateand heavilythickenedcollagenfibersof1-montholdarthritic mice.
Stolzetal.(2009b)
Aorticintimaofrats Notlisted,tipmountedonacustom platformforinvivoAFMimaging
Anaesthetizedlivinganimals (Young’smodulus)0.4–0.5MParangeforbloodvessels withoutdruginfluence,raisedtoc.1.0MPainthepresence ofnitroglycerinanddecreasedbacktoc.0.3MPainthe presenceofnorepinephrine
Maoetal.(2009)
Anteriorhumancornealstroma Phosphorus-dopedsilicon,k=25and 33N/m(calibratedbyanoptical method,asdescribedbySaderetal.)
Contact,liquid(15%dextran),bulk tissueplacedonTefloncellwithout attachment
(Young’smodulus)Between1.14and2.63MPa,consistent acrosstheindentationdepths(between1.0and2.7m)
Lombardoetal.(2012),Sader etal.(1999)
Humancornealbasement membrane
Borosilicateglass,k=0.06N/m (nominal)
Contact,liquid(PBS),a3×3mmtissue piecedissectedandgluedontoawell onsteeldisk
(Young’smodulus)2–15KPa,meanof7.5±4.2kPaaverage fortheanteriorbasementmembrane;20–80KPa, 50±17.8kPaaveragefortheDescemet’smembrane
Lastetal.(2009)
Monkeylenses Gold-coatedtip,k=0.01N/m (nominal),calibratedbythe relationshipbetweenappliedvoltage andcantileverdeflection
Contact,liquid(BSS),lensesdissected andplacedonTeflonslide
(Young’smodulus)1.720±0.88kPa Ziebarthetal.(2007)
Humanbone Various Various (Young’smodulus)16.6±1.1to27.1±1.7fordryadult
tibiae(lowerforchildren),13.4±2.0to22.7±3.1fordry adultvertebrae(lowerforwetsamples),16.58±0.32to 26.6±2.1fordryadultfemoralmidshaft(lowerforwet samplesandinthefemoralneck),otherbone measurementsandtissuehardnessesalsonoted
Thurner(2009)
Bovineoculartendonfibers Siliconnitridetip,k=0.02N/m (calibratedusingthermalmethod)
Contact,air(samplingchamberkeptat 100%humidity),samplegluedonglass petridish
(Young’smodulus)60±2.69MPaforlateralrectus, 59.69±5.34MPaforinferiorrectus,56.92±1.91MPafor medialrectus,59.66±2.64MPaforsuperiorrectus, 57.7±1.36MPaforinferiorobliqueand59.15±2.03for superiorobliquetendons.Differencesbetweentendon elasticitiesarenotstatisticallysignificant.
Yooetal.(2014)
Breasttissuesections Borosilicateglasstip,k=0.06N/m (nominal),individualtipscalibrated usingthermalmethod
Contact,liquid(PBSsuppliedwith proteaseinhibitorsandpropidium iodide),samplessectionedby cryomicrotomy
(Young’smodulus)c.400Painhealthyandnon-invasive tumorregions,4-foldincreaseinaveragestiffnessin invasivetumorfront.Higheraveragestiffnessinthe invasivefrontiscausedbyhighlystiff(>5kPa)regionsin thisarea.Aggressivebreastcancersubtypesarealsofound toexhibithigherYoung’smoduli,quantifiedintermsof upper10%stiffness.
Acerbietal.(2015)
Benignandaggressiveprostate tumors
Siliconnitridetip,k=0.06N/m (nominal),individualtipscalibrated usingthermalmethod
Contact,liquid(physiologicalbuffer), sampleslicedbyrazorandgluedon glass
(Young’smodulus)3.03±0.64kPaforbenign,1.727±1.22 forcanceroustissues.TheaverageYoung’smodulusfor cancersampleswithGleasonscoresinthe2–7rangewas 2.07±1.30,thisvaluewas1.39±0.48forsampleswith Gleasonscoresinthe8–10range.Inaddition,metastatic tumorshadanaveragemodulusof1.06±0.58,while non-metastaticcancertissuehadanaverageelasticity valueof1.99±1.24.Thesevaluesreflectthetissue microenvironmentandcontrastmacro-scaleelastography results,inwhichmoreaggressivecancersarestiffer.
A.D.Ozkanetal./Micron89(2016)60–76 67
Fig.3. Theeffectofanantimicrobialpeptide(CM15)onE.colicellwalls,asobservedbyhigh-speedAFM.Disruptionsbegintoappearoncellsurfacesasearlyast=13sand increaseinseveritywithtime(a).Althoughsomebacteriaresisttheeffectsofthepeptide(b,c),theseindividualsneverthelessreacttoprolongedtreatment(d,att=∼30min). ReplicatedwithpermissionfromFantneretal.(2010b).
mammaliancellsandtissues,eitherculturedexvivoorcollected immediatelypriortoimaging.
4.1. Cancerdiagnosisandcharacterization
Whilemedicaladvanceshaveledtosignificantdecreasesinthe incidencesofmanycancers,cancerstillremainstobeoneofthe mostimportantdiseasesinrecent history.Itis well-established thatcancercellsexhibitmarkeddifferencesinstiffnessand elas-ticitycomparedtotheirhealthycounterparts(Crossetal.,2007); however,diagnosticapplicationsof mechanicalcharacterization methodsshouldcurrentlybeconsideredlimited.Effective diagnos-tictechniquesmustascertainthepresence(orabsence)ofdisease withhighconfidenceandusingminimalamountsofsample tis-sueandtime,anddespitetheabilityofmechanicalmeasurements toeffectivelydistinguishbetweenhealthyand tumorcells,it is unlikelythatabiopsyatanearlystageofdiseasewouldyieldtumor cellsinnumbersnecessarytoperformdiagnosisbasedpurelyon mechanicaldata.Nonetheless,oncethepresenceofatumoris con-firmed usingmore conventional methods, AFM mayserve as a valuabletoolfor itscharacterization:Laidleretal.,forexample, reportthepossibilityofutilizingAFMtodeterminewhethera sus-pectedbreast orprostatetumoris malignantonthebasisofits
elasticproperties(Lekkaetal.,2012).Inaddition,AFMcantilevers canbefunctionalizedwithantibodiesfordisease-specificmarkers andusedinthedetectionofcancerandotherdisorders(Laurent etal.,2014);however,thistechniqueeffectivelyconvertstheAFM intoabiosensororsortingsysteminsteadofrelyingonitscapacity formechanicalcharacterization.
Greaterutilityliesin thecharacterization oftumorcellsand theirinteractionsbyAFM,whichmayfurtherthecurrent under-standingofcancerbiologyand allowthedesign andevaluation of novelcancerdrugs. Discrepanciesbetweentheelasticitiesof cancerandnon-malignantcells,forexample,havebeenrecorded inhumanlung,breast,pancreas,fibroblast,prostate, adenocarci-nomaandothercelllines(MullerandDufrene,2008;Crossetal., 2011).Thesechanges are suspectedtoincrease themobility of malignantcellsduringmetastasis,anddecreasesincellstiffness appear tobeprogressive, withmore malignantcells expressing lowerYoung’smoduli.Fuhrmannetal.reportedthatthedysplastic Barrett’sesophaguscelllinesarelessrigidcomparedto metaplas-ticcells,whichinturnaresofterthantheirhealthycounterparts (Fuhrmannetal.,2011).Otherchangesin cellmorphologymay alsooccurtofacilitatemetastasis,andthesetoocanbequantified byAFM.Sokolov etal.,forexample, havedeterminedthat can-ceroushumancervicalepithelial cellsdisplay twobrushlayers,
68 A.D.Ozkanetal./Micron89(2016)60–76
Fig.4.Effectoffixationoncellularelasticity.Unfixed(a,d)andGA-fixed(bandefor20min;candffor60min)cellshavedistinctappearancesandelasticmoduli;withGA fixationgreatlyincreasingcellularYoung’smoduliinatime-dependentmanner(g).ReplicatedwithpermissionfromShibata-Sekietal.(2015).
incontrasttothesingle-lengthmolecularbrushofnon-malignant cervicalepithelium,which mayalsoassistin invasion attempts bythesemalignantcells(Iyeretal.,2009).Inaddition,thetumor microenvironmentalsoappearstocontributesignificantlytothe alterationsintumorelasticity:Insteadoftheexpecteddecreasein Young’smoduli,Weaveretal.observedatime-dependentincrease intumorstiffnessduringmammarytumordevelopmentinPyMT mice,andthisincreaseinrigiditywasalsoreflectedinepithelial cellstakenfromthetumorsite.However,cellstakenoutoftheir nativeenvironmentandgrowninvitrohadlowerYoung’s mod-ulicompared totheirinvivocounterparts,andtheinhibitionof theECM-crosslinkingenzymelysyloxidasemitigatedthegradual increaseintheelasticmodulusofmammaryglandtumors(Fig.5) (Lopez etal.,2011).Assuch,tissue culturesamplesandinvivo tumorcellsmaynotnecessarilyagreeinelasticproperties,asthe stiffnessoftheformerdependsonthecytoskeletalpropertiesof thecells,whilethatofthelatterislargelymediatedbythetissue microenvironment.
DrugresponsesofcancercellscanalsobequantifiedbyAFM. Zhangetal.observedthatHeLa,HepG2andC6cellsexperience dose-dependentmorphologicalchangesontheircellmembranes followingtreatmentwithcolchicineorcytarabine.Surface rough-nessincreasedandporesappearedonthecellmembraneafterdrug administrationandbeforeMTT-quantifiabledecreasesinviability couldbeobserved,suggestingthatthesurfacealterations repre-sentanearlyresponsetodrugpresence(Wangetal.,2009).Such differencesmaybemonitoredtoevaluatetheeffectivenessofdrug candidates.Changesassociatedwithgenedeletionorrestoration canalsobeobservedthroughAFM:Zhouetal.reportedthatthe expressionofBRMS1(restoringbreastcancermetastasis suppres-sor1)inducesincreasesincelladhesioncapacity,cellularspring constantandYoung’smodulus,whichsupportstheideathatBRMS1 expressionisassociatedwithcytoskeletalrearrangementsthatare unfavorablefor metastaticactivity.Cell morphologyand rough-nesswerealsoalteredfollowingBRMS1expression,possiblyasa consequenceofcytoskeletalmodifications(Wuetal.,2010).
A.D.Ozkanetal./Micron89(2016)60–76 69
Fig.5. Changesassociatedwithmammarytumorformation,asquantifiedbyAFM.Normalmammaryducttissueislessstiffcomparedtotumortissue(a),andthetissueelastic modulusincreaseswithtumorage(b).Inaddition,theinhibitionofcollagencross-linkingbyBAPN(whichblockstheactivityoflysyloxidase)preventsthetumor-associated increaseinstiffness,suggestingthatthetumorenvironmentismodifiedthroughchangesintheextracellularmatrix.ReplicatedwithpermissionfromLopezetal.(2011).
4.2. Diagnosisofotherdiseases
Whilecancer-associatedchangesincellelasticityare particu-larlydrastic,canceris by nomeanstheonlycondition toalter themechanicalpropertiesofaffectedtissues.Avarietyofother conditions,includingmalaria,sicklecellanemia,hepaticfibrosis, cardiovasculardisease,renalstiffness,musculardystrophiesand bonedisorders,havealsobeenassociatedwithnotablechangesin theelasticpropertiesoftheaffectedtissues(Fig.6)(Costa,2003; Nagaoetal.,2000;Lieberetal.,2004b;Bozecetal.,2005;Engler etal.,2004).Mechanicaldiagnosismethodshavebeendevisedfor somesuchconditions,andAFMcanbeutilizedtostudythe struc-turalandmechanicalpropertiesofaffectedtissues;butitmustbe notedthat,aswithcancer,AFM-baseddiagnosticmethodsforthese diseasescurrentlyappeartobemoresuitedtowardssupporting conventionaldiagnosis.However,thehigh-resolutionimagingand mechanicalprobingcapacityofAFMisidealforthedetermination ofthecausesunderlyingtheprogressionofthediseasesinquestion. SeveralreportsonAFM-baseddiseasecharacterizationexistin theliterature.Szymo ´nskietal.,forexample,demonstratethatred bloodcellsbelongingtopatientswithhereditaryblooddiseases generally bear a higher Young’s modulus compared to healthy erythrocytes(Dulinskaetal.,2006).Inparticular, gluteraldehyde-fixed, poly-l-lysine-immobilized erythrocytes of patients with hereditary spherocytosis, thalassemia or G6PD deficiency were
stiffercomparedtonormalcells,whilepatientswithanisocytosis displayedtwodistinctpeaksintheirhistogramofYoung’s mod-uli,correspondingtohealthyanddiseasedpopulationsofcells.In patientswithhereditaryspherocytosis,changesincell morphol-ogywerealsoobserved.Similarly,Vatneretal.haveinvestigated theeffectsofagingoncardiacmyocytesbyAFMindentation, com-paring4month-and30month-oldratsinordertoassesswhether myocytehealthinfluencestheaging-associateddiastolic dysfunc-tionof theleftventricule(Lieber etal., 2004b).Myocytes were foundto significantly increase in stiffness withage,suggesting thattheobserveddysfunctioncanbelinkedatleastinparttothe malfunctionofindividualmyocytes.Theeffectofagingoncellular Young’smoduliwasalsonotedbySokolovetal.,whohaveshown thatolderhumanepithelialcellsaremorerigidthantheiryounger counterparts,andhavefurtherdemonstratedthatanincreaseinthe densityofcytoskeletalelementsisresponsiblefortheage-related increaseinstiffness(Berdyyevaetal.,2005a,b).Theearly detec-tionofosteoarthritiswasalsoperformedonthearticularcartilage ofnormalandarthriticmice,andnanoindentation(butnot micro-scaletips)wasshowntoresolvethegradualchangesincartilage stiffnessbetweenarthriticandnon-arthriticanimalsofthesame age(Stolzetal.,2009b).
AnotherinterestingfrontierinAFM-based disease character-ization is theinvestigation of malfunctioning proteinsthat are involvedinthepathogenesisofneurodegenerativedisorderssuch
70 A.D.Ozkanetal./Micron89(2016)60–76
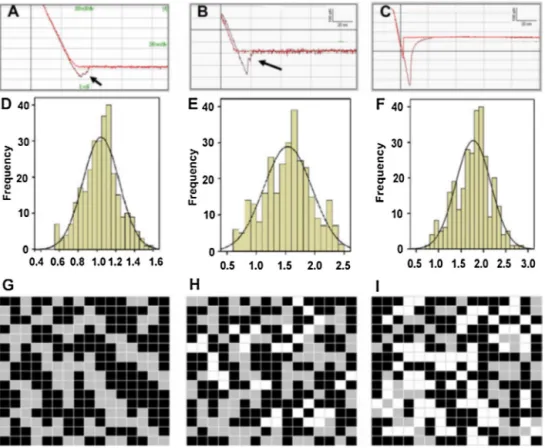
Fig.6.Young’smodulusmeasurementsoferythrocytesfromyoungandhealthy(YHP;a,dandg);oldandhealthy(OHP;b,eandh)andoldandtype-IIdiabetic(ODP; c,fandi)individuals.ErythrocytesexhibitedhigherstiffnessinODPindividualscomparedtoeitherYHPorOHP(1.78±0.39×105N/m2v.1.04±0.19×105N/m2and
1.53±0.41×105N/m2,respectively).Inaddition,OHPandODPerythrocytesexhibitedhigheradhesioncomparedtoYHPerythrocytes(420±25pNand510±63pNv.
200±38pN,respectively).ReplicatedwithpermissionfromJinetal.(2010). as Alzheimer’s disease, Parkinson’s disease, Huntington’s dis-easeandamyotrophiclateralsclerosis.Thesedisordersseverely decreasethequalityoflife,areexceptionallycommonamongthe elderlyandhavenodefinitivecures,whichrendersitcrucialtogain furtherinsightintothenatureoftheircausativeagents.Assuch, theformationofamyloidplaqueshasbeeninvestigatedindetail usingavarietyofbiological,chemicalandmaterialcharacterization methods,includingseveralAFM-basedstudies.Lansburyetal.,for example,reportedontheinvitroformationofmetastableA amy-loidfibrilprecursorsthatmaylaterdevelopintocompleteamyloid assemblies,andsuggestthatthehaltingofthismechanismmay preventtheonsetofAlzheimer’sdiseasebyretainingthe precur-sorfibrils(“protofibrils”)intheirbenignintermediateform(Harper etal.,1997).Inaddition,KowalewskiandHoltzmandemonstrated thatthesize,shapeandproductionkineticsofAaggregateswere altereddependingonthesurfaceonwhichtheaggregationoccurs (KowalewskiandHoltzman,1999)(i.e.particulateassemblieswere generatedonthehydrophilicmicasurface,while-sheetsformed onthehydrophobicgraphite),andsuggestedthatthe-sheet form-ingbehaviorofAongraphitemayyieldusefulinformationonhow proteinfoldingoccursinvivo.TheabilityofApeptidestoform ion-channellikestructureswithoutinteractingwithother mem-branecomponentswasalsoconfirmedusingAFM,withtrimeric, tetrameric,pentamericandhexamericporestructuresbeing iden-tifiedin topographic images. BothD-and L-enantiomers of A peptideswereabletoformthesechannels,suggestingthatthepore formationmechanismisnotstereospecific(Connellyetal.,2012).
4.3. Stemcelldifferentiation
Fluctuationsinmechanicalpropertiesdonotnecessarilysuggest aging-relateddeteriorationoradiseasestate,ashealthycellsmay
alsorespondtoenvironmentalsignalsbyalteringtheirmembrane integrity.Thisismostobviouslyobservedinstemcells,as differen-tiationinducesfundamentalchangesnotonlyincellmorphology andexpressionpatterns,butalsoinmembranecontentand stiff-ness(Discher,2006;Evansetal.,2009;ReillyandEngler,2010). Since themaintenance, recruitmentand differentiationof stem cellsareintimately linkedtothemechanicalpropertiesof their immediateenvironment,itisfeasibletouseAFMmeasurementsin ordertodeterminethefactorsthatdrivethedifferentiationprocess inthesecells.Suchfactorsarerelativelyclearinsomecases,such asthemesenchymalstemcelldifferentiationintomyogenic, chon-drogenicorosteogeniclineages,butthemechanicaltriggersbehind thedifferentiationofothercellsarewelllessunderstood.In addi-tiontostemcelldifferentiation,changesinthemicroenvironmental conditionsoftissuescanbeassessedusingAFMorother mechani-calcharacterizationmethods,especiallyinsituationsinvolvingthe slowrecoveryofadamagedsystem,asinthecasesofbonefracture healing.Otherprocesses,suchasthelocalremodelingand extra-cellularmatrixsecretionofcellsinatissuecultureenvironment, orthecapacityofbiomimeticmaterialstoimitatethemechanical environmentoftheirtissuemodel,alsofallwithinthepurviewof AFM.
Thedifferencesbetweenstemandderivedcellshavealsobeen investigated by AFM. Guilak et al. have confirmed that chon-drocytes,osteoblastsandadipocytes,theprimarydifferentiation productsof mesenchymalstemcells, displaydifferentrigidities, anddeterminedthatundifferentiatedmesenchymalstemcellsare similartoadipocytesintheirmechanicalcharacteristics(Darling etal.,2008).Schiekeretal.reportedthatthetwosubcategories of humanmesenchymal stem cells, flat cells and rapidly self-renewing cells, can also be differentiated on thebasis of their morphologicalandadhesivecharacteristics(Dochevaetal.,2008),
A.D.Ozkanetal./Micron89(2016)60–76 71 withtheformertypeappearingtopographicallysimilartohuman
osteoblastsand displayinga highadhesiontothesurface,while thelatterexhibitedcharacteristicssimilartoMG63osteosarcoma cells anddisplayeda smoothertopography. Evenstemcellfate canbepredictedbymechanical propertiesprior tothe appear-anceofvisibleindicators.Gonzalez-CruzandDarlingreportedthat adipose-derivedstemcellscanbeclassifiedaccordingtotheir dif-ferentiationpotentialtoadipogenic, osteogenicorchondrogenic lineagesonthebasisoftheirmechanicalbehavior(Gonzalez-Cruz etal.,2012).Ascouldbeexpected;softer,largerand more pli-antadipose-derivedstemcellsaremorelikelytodifferentiateinto adipocytes,whilesmallerandmorerigidstemcellsareinclined towardsosteogenicor chondrogenicdifferentiation.Inaddition, theeffectofstemcellsonaninvivoenvironmentcanbedetermined followingtheirimplantation:Mesenchymalstemcellsinjectedinto thesiteofamyocardialinfarctionhavebeenrecordedtodecrease therigidityofthemyocardium,whichwasassociatedwithreduced fibrosisandabetterprognosisforthepostinfarctedheart.
Theimportanceoftheextracellularmatrix(ECM)forthe main-tenance and differentiation of stem cells is both obvious and paramount(Bosnakovskietal.,2006;Suzukietal.,2003).Itis there-foreunsurprisingthatthemechanicalpropertiesofECMelements arevitalinprovidingthesignalsresponsibleforinducingstemcells todifferentiate,orforretainingthemintheirquiescentstate(Reilly andEngler,2010;Guilaketal.,2009).Greatdifferences existin theECMrigiditiesofadulttissues,from0.1kPainbrainto>30kPa inbone(Huangetal.,2012),andexcessivelysoftorrigidtissues mayresultinsuboptimaldifferentiation(seee.g.Engleretal.foran accountofaberrantmyotubeformationassociatedwith unsatisfac-torysurfacestiffness(Engleretal.,2004)).Inadditiontoadultstem cellsinmaturetissues,thereisalsoevidencethatendo-,meso-and ectodermalprogenitorsarearrangedintodistinctivegermlayers withtheassistanceofdifferencesintensilestrength(Discheretal., 2005;Puechetal.,2005):Cell-cortextensionsofendodermal pro-genitorsingastrulatingzebrafishembryosarehigherthanthatof themesodermalprogenitors,whichinturnarelargerthanthatof ectodermalprogenitorcells(Fig.7)(Kriegetal.,2008b).Inasimilar vein,cardiacloopinginchickenembryoswassuggestedtooccur asaresultofastiffness-mediatedasymmetryinthedeveloping cardiacjelly(Zamiretal.,2003).
4.4. Extracellularsecretionsandtissuemicroenvironments
AFMcanbeusedtomeasurethenativestiffnessoftissue micro-andnanoenvironments,althoughothermethods,suchas microin-dentation,canalsobeusedforthemicromechanicalinvestigation oflargerareas oftissues. Bone,cartilageand theoculartissues havebeenfrequentlyinvestigatedusingAFM,andage-or disease-relatedeffectshavebeenfoundtobereflectedintissuestiffness.As withbacterialandeukaryoticcells,themethodofsample prepara-tioncangreatlyaltertheelasticmodulusoftissues,andsampling locationlikewisehasasubstantialeffectonmechanicalproperties (Stolzetal.,2009b;Thurner,2009).Itmustalsobekeptinmind thatthemicro-andnanoscalestiffnessesofagiventissuemay dif-fergreatly,e.g.themicrostiffnessofporcinearticularcartilagewas observedtobeover100-foldgreaterthanitsnanostiffness,and theage-relatedeffectsofosteoarthritisonthearticularcartilage ofmicecouldonlybeobservedthroughnanoscaletips(Stolzetal., 2009a,2004).Assuch,caremustbetakennottocomparetheresults ofcell-ortissue-basedAFMstudiestoliteratureexamplesthatuse dissimilarconditionsofmeasurement.
Theextentofnatural heterogeneitywithina givencell pop-ulationortissue samplecan alsobeestablishedusingAFM.An AFMapparatusadaptedforuseinlivingbraintissue,forexample, hasbeenusedtodemonstratethattherat hippocampusis het-erogeneous,andthathippocampalsubregionsareassociatedwith
differentelasticmoduli(Elkinetal.,2007).Thewoundhealing pro-cesshasalsobeencharacterizedusingAFM,andtheleadingedge ofthewoundwasfoundtoexhibitaspatiallylimitedincreasein stiffnesstorecruitfibroblaststothewoundsite.Thislocalizedpeak iscreatedbycytoskeletalchangesinthecellsofthewoundedge, anddisappearsiftheexpressionoftheactinfiber-regulating pro-teinRhoAisdisabled,resultinginalackoffibroblasticrecruitment tothesiteofinjury(Waghetal.,2008).Inadditiontotissues,an individualcellcanalsobeanalyzedtoidentifydistinctregionsonits surface:Scheuringetal.havecharacterizedthemechanismsused byredbloodcellstocontortduringtheirpassagethroughblood ves-sels,utilizingamethodinwhichthefunctionofknownstructural proteinsarealteredandtheresultantchangesonthecellsurface aremeasuredtomaptheseproteinsontothemechanically het-erogeneousregionspresentontheerythrocyte(Picasetal.,2013). TheadditionofMgATPwasutilizedtoelicitchangesonthe mem-braneelementsontheextracellular(“outwards”)andcytoplasmic (“inwards”)sidesoftheerythrocyte,whichbehavedintwo dis-tinctwaysandthereforeallowedthedetailedcharacterizationof membranestructuresandmolecularcompositionsonbothsides.
In addition to characterizing the structure of previously depositedmatrixmicroenvironments,AFMcanalsodeterminehow cellularexudatesaresecretedoutsidecells,especiallywithregards tothemembranebuddingandfusioneventsthatoccurduringthe secretionprocess.Researchinthisdirectionfocusesmostlyoncells involvedinthesecretionofhormonesordigestivefluids,suchas pancreaticacinarcells(Schneideretal.,1997b)orgrowthhormone (GH)-secretingcells(Choetal.,2002).Secretioninacinarcellswas foundtodependonlarge(500–2000nm)apical“pits”thatcontain 3–20smaller(100–180nm)“depressions”or“fusionpores”,the diametersofwhichincreaseduringamylasesecretion(Fig.8). Simi-larpitsanddepressionswerealsopresentinGH-secretingcells,and theexistenceofGHwithinthesestructureswasconfirmedusing gold-taggedGH antibodies,demonstrating that thedepressions weresecretoryinnature.Acombinationofatomicforcemicroscopy andconfocalmicroscopywasalsousedtodemonstratethatthe secretoryvesicleswellingprocessisregulatedbyaGTP-binding protein(Jenaetal.,1997).
5. Futuredirections
AFMisaversatiletechniqueandcanbeusedinthemechanical characterizationofabroadrangeofbiomaterials,rangingfrom sin-glemoleculestobulktissues.Itsnanometer-scaleresolutionallows thedirectobservationofproteins,nucleicacidsandother biologi-callyimportantmolecules,whileitsabilitytocharacterizematerial stiffnessinliquidsisacrucialadvantageforinvestigatingthe nat-uralmechanicalenvironmentofcells.However,themethodsused forthepreparationofbiologicalsamplesforAFMimagingarehighly diverseandmayalterthemechanicalpropertiesofbiomaterials. Environmentalconditionssuchastemperatureandbuffersalinity canaltertheresponseofcellstomechanicalstimuli,whilefixation andimmobilizationprotocolsmaydirectlychangethechemical compositionofthetreatedcells.Theseeffectsinturnmakeit diffi-culttocompareresultsbetweenstudiesperformedunderdifferent conditions.Whilecountermeasures,suchasliquidcellswhich sup-plytheenvironmentnormallyexperiencedbymammaliancellsin cellculture,havebeendeveloped,agreatdealofprocess optimiza-tionandstandardizationisstillnecessaryforAFMtotrulyemerge asabiologicaltool.
Nevertheless, the mechanical environment of cells remains largelyunexplored,andAFMisanidealmethodforcharacterizing thebehaviorsofcellsandtissuesunderclose-to-naturalconditions. Thetechniquewillnodoubtremainindispensableforstudyingthe mechanicalcomponentsofsignalingpathwaysand theeffectof
72 A.D.Ozkanetal./Micron89(2016)60–76
Fig.7.Endo-andmesodermalcellsofthedevelopingzebrafishembryopreferentiallyadheretocellssharingthesamegermlayerinacadherin-dependentmanner.Adhesive forcesbetweentwocellscanbemeasuredbyattachingonecelltotheprobe(a).Endo-andmesodermalcellsexhibitstrongerhomotypicadhesioncomparedtoectodermal cells,andheterotypicadhesionisgenerallyweakerthanendoderm–endodermandmesoderm–mesoderminteractions(bandc,dusedascontroltoensurecontactstiffness didn’taccountforthedifferencesobserved).Calciumdepletionandsuppressionofcadherinblockstheobservedeffects,andcadherinexpressionishigherinendo-and mesodermalcells,suggestingthatadhesioniscadherin-dependent(e,f).ReplicatedwithpermissionfromKriegetal.(2008a).
Fig.8.Inducedsecretionofamylasefromacinarcellsofthepancreas,showing“depressions”inasingle“pit”(a)andtheirsecretoryresponses5min(b)and30min(c) aftersimulationbythesecretion-stimulatingpeptideMas7.Secretionisassociatedwithincreasesinthediametersanddepthsofthesecretorydepressions.Replicatedwith permissionfromSchneideretal.(1997a).
A.D.Ozkanetal./Micron89(2016)60–76 73 physicalsignalsonmetabolicfunctionsundernormalanddisease
states,whichisanareathathasremainedlargelyuninvestigated untilnow,forlackofadequatemethodsofanalysis.
References
Acerbi,I.,Cassereau,L.,Dean,I.,Shi,Q.,Au,A.,Park,C.,Chen,Y.Y.,Liphardt,J., Hwang,E.S.,Weaver,V.M.,2015.Humanbreastcancerinvasionandaggression correlateswithECMstiffeningandimmunecellinfiltration.Integr.Biol. (Camb.),http://dx.doi.org/10.1039/c5ib00040h.
Allen,S.,Chen,X.Y.,Davies,J.,Davies,M.C.,Dawkes,A.C.,Edwards,J.C.,Roberts,C.J., Sefton,J.,Tendler,S.J.B.,Williams,P.M.,1997.Detectionofantigen-antibody bindingeventswiththeatomicforcemicroscope.Biochemistry36(24), 7457–7463,http://dx.doi.org/10.1021/Bi962531z.
Ando,T.,Uchihashi,T.,Kodera,N.,2013.High-speedAFMandapplicationsto biomolecularsystems.Annu.Rev.Biophys.42,393–414,http://dx.doi.org/10. 1146/annurev-biophys-083012-130324.
Arnold,J.W.,Bailey,G.W.,2000.Surfacefinishesonstainlesssteelreducebacterial attachmentandearlybiofilmformation:scanningelectronandatomicforce microscopystudy.PoultrySci.79(12),1839–1845.
Auerbach,I.D.,Sorensen,C.,Hansma,H.G.,Holden,P.A.,2000.Physicalmorphology andsurfacepropertiesofunsaturatedPseudomonasputidabiofilms.J.Bacteriol. 182(13),3809–3815,http://dx.doi.org/10.1128/Jb.182.13.3809-3815.2000. Bakker,D.,Huijs,F.,deVries,J.,Klijnstra,J.,Busscher,H.,vanderMei,H.,2003.
Bacterialdepositiontofluoridatedandnon-fluoridatedpolyurethanecoatings withdifferentelasticmodulusandsurfacetensioninaparallelplateanda stagnationpointflowchamber.ColloidsSurf.B32(3),179–190,http://dx.doi. org/10.1016/S0927-7765(03)00159-0.
Beckmann,M.,Venkataraman,S.,Doktycz,M.,Nataro,J.,Sullivan,C., Morrell-Falvey,J.,Allison,D.,2006.Measuringcellsurfaceelasticityon enteroaggregativeEscherichiacoliwildtypeanddispersinmutantbyAFM. Ultramicroscopy106(8–9),695–702,http://dx.doi.org/10.1016/j.ultramic. 2006.02.006.
Beech,I.B.,Smith,J.R.,Steele,A.A.,Penegar,I.,Campbell,S.A.,2002.Theuseof atomicforcemicroscopyforstudyinginteractionsofbacterialbiofilmswith surfaces.ColloidSurf.B23(2–3),231–247.
Berdyyeva,T.K.,Woodworth,C.D.,Sokolov,I.,2005a.Humanepithelialcells increasetheirrigiditywithageinginvitro:directmeasurements.Phys.Med. Biol.50(1),81–92.
Berdyyeva,T.,Woodworth,C.D.,Sokolov,I.,2005b.Visualizationofcytoskeletal elementsbytheatomicforcemicroscope.Ultramicroscopy102(3),189–198. Berquand,A.,Mingeot-Leclercq,M.,Dufrene,Y.,2004.Real-timeimagingof
drug-membraneinteractionsbyatomicforcemicroscopy.Biochim.Biophys. Acta1664(2),198–205,http://dx.doi.org/10.1016/j.bbamem.2004.05.010. Best,R.B.,Li,B.,Steward,A.,Daggett,V.,Clarke,J.,2001.Cannon-mechanical
proteinswithstandforce?Stretchingbarnasebyatomicforcemicroscopyand moleculardynamicssimulation.Biophys.J.81(4),2344–2356.
Bosnakovski,D.,Mizuno,M.,Kim,G.,Takagi,S.,Okumura,M.,Fujinaga,T.,2006. Chondrogenicdifferentiationofbovinebonemarrowmesenchymalstemcells (MSCs)indifferenthydrogels:influenceofcollagentypeIIextracellularmatrix onMSCchondrogenesis.Biotechnol.Bioeng.93(6),1152–1163.
Bowen,W.,Fenton,A.,Lovitt,R.,Wright,C.,2002.ThemeasurementofBacillus mycoidessporeadhesionusingatomicforcemicroscopy,simplecounting methods,andaspinningdisktechnique.Biotechnol.Bioeng.79(2),170–179, http://dx.doi.org/10.1002/bit.10321.
Bozec,L.,deGroot,J.,Odlyha,M.,Nicholls,B.,Nesbitt,S.,Flanagan,A.,Horton,M., 2005.Atomicforcemicroscopyofcollagenstructureinboneanddentine revealedbyosteoclasticresorption.Ultramicroscopy105(1–4),79–89,http:// dx.doi.org/10.1016/j.ultramic.2005.06.021.
Braet,F.,Rotsch,C.,Wisse,E.,Radmacher,M.,1998.Comparisonoffixedandliving liverendothelialcellsbyatomicforcemicroscopy.Appl.Phys.A66,
S575–S578,http://dx.doi.org/10.1007/s003390051204.
Butt,H.J.,Downing,K.H.,Hansma,P.K.,1990.Imagingthemembrane-protein bacteriorhodopsinwiththeatomicforcemicroscope.Biophys.J.58(6), 1473–1480.
Canetta,E.,Riches,A.,Borger,E.,Herrington,S.,Dholakia,K.,Adya,A.K.,2014. Discriminationofbladdercancercellsfromnormalurothelialcellswithhigh specificityandsensitivity:combinedapplicationofatomicforcemicroscopy andmodulatedRamanspectroscopy.ActaBiomater.10(5),2043–2055,http:// dx.doi.org/10.1016/j.actbio.2013.12.057.
Carvalho,F.A.,Martins,I.C.,Santos,N.C.,2013.Atomicforcemicroscopyandforce spectroscopyontheassessmentofproteinfoldingandfunctionality.Arch. Biochem.Biophys.531(1–2),116–127,http://dx.doi.org/10.1016/j.abb.2012. 11.007,S0003-9861(12)00400-6[pii].
Chaw,K.C.,Manimaran,M.,Tay,F.E.H.,2005.Roleofsilverionsindestabilizationof intermolecularadhesionforcesmeasuredbyatomicforcemicroscopyin Staphylococcusepidermidisbiofilms.Antimicrob.AgentsChemother.49(12), 4853–4859,http://dx.doi.org/10.1128/Aac.49.12.4853-4859.2005. Cho,S.J.,Jeftinija,K.,Glavaski,A.,Jeftinija,S.,Jena,B.P.,Anderson,L.L.,2002.
StructureanddynamicsofthefusionporesinliveGH-secretingcellsrevealed usingatomicforcemicroscopy.Endocrinology143(3),1144–1148. Cohen,S.R.,Kalfon-Cohen,E.,2013.Dynamicnanoindentationbyinstrumented
nanoindentationandforcemicroscopy:acomparativereview.BeilsteinJ. Nanotechnol.4,815–833,http://dx.doi.org/10.3762/bjnano.4.93.
Connelly,L.,Jang,H.,Arce,F.T.,Capone,R.,Kotler,S.A.,Ramachandran,S.,Kagan, B.L.,Nussinov,R.,Lal,R.,2012.AtomicforcemicroscopyandMDsimulations revealpore-likestructuresofall-d-enantiomerofalzheimer’sbeta-amyloid peptide:relevancetotheionchannelmechanismofADpathology.J.Phys. Chem.B116(5),1728–1735.
Costa,K.D.,2003.Single-cellelastography:probingfordiseasewiththeatomic forcemicroscope.Dis.Markers19(2–3),139–154.
Cross,S.E.,Jin,Y.S.,Rao,J.,Gimzewski,J.K.,2007.Nanomechanicalanalysisofcells fromcancerpatients.Nat.Nanotechnol.2(12),780–783,http://dx.doi.org/10. 1038/nnano.2007.388.
Cross,S.E.,Jin,Y.S.,Lu,Q.Y.,Rao,J.Y.,Gimzewski,J.K.,2011.Greenteaextract selectivelytargetsnanomechanicsoflivemetastaticcancercells.
Nanotechnology22(21),http://dx.doi.org/10.1088/0957-4484/22/21/215101 (Artn215101).
Dammer,U.,Popescu,O.,Wagner,P.,Anselmetti,D.,Guntherodt,H.J.,Misevic,G.N., 1995.Bindingstrengthbetweencell-adhesionproteoglycansmeasuredby atomic-forcemicroscopy.Science267(5201),1173–1175,http://dx.doi.org/10. 1126/science.7855599.
Danino,D.,2008.Advancesinatomicforcemicroscopyinvestigationsof biomolecules.Curr.Opin.ColloidInt.13(5),315,http://dx.doi.org/10.1016/j. cocis.2008.07.001.
Darling,E.,Zauscher,S.,Guilak,F.,2006.Viscoelasticpropertiesofzonalarticular chondrocytesmeasuredbyatomicforcemicroscopy.OsteoarthritisCartilage 14(6),571–579,http://dx.doi.org/10.1016/j.joca.2005.12.003.
Darling,E.M.,Topel,M.,Zauscher,S.,Vail,T.P.,Guilak,F.,2008.Viscoelastic propertiesofhumanmesenchymally-derivedstemcellsandprimary osteoblasts,chondrocytes,andadipocytes.J.Biomech.41(2),454–464,http:// dx.doi.org/10.1016/j.jbiomech.2007.06.019.
daSilva,A.,Teschke,O.,2005.DynamicsoftheantimicrobialpeptidePGLaaction onEscherichiacolimonitoredbyatomicforcemicroscopy.WorldJ.Microbiol. Biotechnol.21(6–7),1103–1110.
Davies,D.G.,Parsek,M.R.,Pearson,J.P.,Iglewski,B.H.,Costerton,J.W.,Greenberg, E.P.,1998.Theinvolvementofcell-to-cellsignalsinthedevelopmentofa bacterialbiofilm.Science280(5361),295–298,http://dx.doi.org/10.1126/ science.280.5361.295.
Discher,D.E.,Janmey,P.,Wang,Y.L.,2005.Tissuecellsfeelandrespondtothe stiffnessoftheirsubstrate.Science310(5751),1139–1143.
Discher,D.E.,2006.BIOT463-Matrixelasticityissensedwithnon-musclemyosinII anddirectsstemcelllineagespecification.Abstr.Pap.Am.Chem.Soc.,232. Docheva,D.,Padula,D.,Popov,C.,Mutschler,W.,Clausen-Schaumann,H.,Schieker,
M.,2008.Researchingintothecellularshape,volumeandelasticityof mesenchymalstemcells,osteoblastsandosteosarcomacellsbyatomicforce microscopy.J.Cell.Mol.Med.12(2),537–552,http://dx.doi.org/10.1111/j. 1582-4934.2007.00138.x.
Doktycz,M.J.,Sullivan,C.J.,Hoyt,P.R.,Pelletier,D.A.,Wu,S.,Allison,D.P.,2003.AFM imagingofbacteriainliquidmediaimmobilizedongelatincoatedmica surfaces.Ultramicroscopy97(1–4),209–216,http://dx.doi.org/10.1016/ S0304-3991(03)00045-7.
Dorobantu,L.S.,Goss,G.G.,Burrell,R.E.,2012.Atomicforcemicroscopy:a nanoscopicviewofmicrobialcellsurfaces.Micron43(12),1312–1322,http:// dx.doi.org/10.1016/j.micron.2012.05.005,S0968-4328(12)00156-4[pii]. Dufour,D.,Levesque,C.M.,2013.Bacterialbehaviorsassociatedwiththe
quorum-sensingpeptidepheromone(’alarmone’)instreptococci.Fut. Microbiol.8(5),593–605,http://dx.doi.org/10.2217/fmb.13.23.
Dulinska,I.,Targosz,M.,Strojny,W.,Lekka,M.,Czuba,P.,Balwierz,W.,Szymonski, M.,2006.Stiffnessofnormalandpathologicalerythrocytesstudiedbymeans ofatomicforcemicroscopy.J.Biochem.Biophys.Methods66(1–3),1–11, http://dx.doi.org/10.1016/j.jbbm.2005.11.003.
Ebner,A.,Wildling,L.,Kamruzzahan,A.S.,Rankl,C.,Wruss,J.,Hahn,C.D.,Holzl,M., Zhu,R.,Kienberger,F.,Blaas,D.,Hinterdorfer,P.,Gruber,H.J.,2007.Anew, simplemethodforlinkingofantibodiestoatomicforcemicroscopytips. Bioconjug.Chem.18(4),1176–1184,http://dx.doi.org/10.1021/bc070030s. Elkin,B.S.,Azeloglu,E.U.,Costa,K.D.,Morrison,B.,2007.Mechanicalheterogeneity
oftherathippocampusmeasuredbyatomicforcemicroscopeindentation.J. Neurotrauma24(5),812–822,http://dx.doi.org/10.1089/neu.2006.0169. Engler,A.J.,Griffin,M.A.,Sen,S.,Bonnetnann,C.G.,Sweeney,H.L.,Discher,D.E.,
2004.Myotubesdifferentiateoptimallyonsubstrateswithtissue-likestiffness: pathologicalimplicationsforsoftorstiffmicroenvironments.J.CellBiol.166 (6),877–887,http://dx.doi.org/10.1083/jcb.200405004.
Evans,N.D.,Minelli,C.,Gentleman,E.,LaPointe,V.,Patankar,S.N.,Kallivretaki,M., Chen,X.Y.,Roberts,C.J.,Stevens,M.M.,2009.Substratestiffnessaffectsearly differentiationeventsinembryonicstemcells.Eur.CellsMater.18,1–14. Fang,H.,Chan,K.,Xu,L.,2000.Quantificationofbacterialadhesionforcesusing
atomicforcemicroscopy(AFM).J.Microbiol.Methods40(1),89–97,http://dx. doi.org/10.1016/S0167-7012(99)00137-2.
Fantner,G.E.,Barbero,R.J.,Gray,D.S.,Belcher,A.M.,2010a.Kineticsof antimicrobialpeptideactivitymeasuredonindividualbacterialcellsusing high-speedatomicforcemicroscopy.Nat.Nanotechnol.5(4),280–285,http:// dx.doi.org/10.1038/nnano.2010.29.
Fantner,G.E.,Barbero,R.J.,Gray,D.S.,Belcher,A.M.,2010b.Kineticsof antimicrobialpeptideactivitymeasuredonindividualbacterialcellsusing high-speedatomicforcemicroscopy.Nat.Nanotechnol.5(4),280–285,http:// dx.doi.org/10.1038/nnano.2010.29.
Fernandes,J.C.,Eaton,P.,Gomes,A.M.,Pintado,M.E.,Malcata,F.X.,2009.Studyof theantibacterialeffectsofchitosansonBacilluscereus(anditsspores)by