A conducting composite of polypyrrole
I. Synthesis and characterization
F a t m a S e l a m p i n a r , U r a l A k b u l u t , T a l a t Y a l q i n , ~;efik Si.izer* a n d L e v e n t T o p p a r e * * Department of Chemistry, Middle East Technical University, 06531 Ankara (Turkey)
(Received December 29, 1992; accepted September 22, 1993)
Abstract
A conducting composite of polypyrrole was prepared via electrochemical methods. A polyamide was used as the insulating matrix polymer. The characterization of the composite was done by FT-IR, SEM, TGA, DSC and pyrolysis studies. Conductivity and solubility studies together with spectroscopic methods reveal that H bonding exists between the two polymers and a possible grafting to a certain extent.
Introduction
Electrically conducting polymers have attracted great attention in the past years because of their electronic properties. Many studies have focused on the synthesis of conducting polymers, such as polyacetylene, poly- pyrrole, polythiophene and their derivatives. Polypyrrole (PPy) can readily be obtained in its conducting form by electrochemical oxidation of pyrrole [1]. Several groups have reported that the electrochemical poly- merization of pyrrole can also take place on an electrode which is already coated with an insulating polymer [2-9]. The main purpose is to obtain homogeneous composites which retain the characteristics of both polymers, at least to a certain extent. In these studies low percolation thresholds were achieved with the aid of hydrogen bonding between the host matrix and polypyrrole [9] as well as graft polymers [10, 11]. The films obtained in the latter case showed interesting features, such as differing behaviour in DSC, SEM and FT-IR analyses compared to a simple mechanical mix- ture of the two polymers. The insolubility of the host matrix in regular solvents after the electrolytic poly- merization of the conducting element (e.g. pyrrole, aniline) suggested the generation of a new structure composed of the insulating and conducting polymers. Pyrolysis studies finally revealed that the so-called com- posite may be a graft copolymer rather than an intimate mixture of the two polymers.
In the above-mentioned technique, polymerization starts around the interface between the electrode surface
*Present address: Bilkent University, Ankara, Turkey. **Author to whom correspondence should be addressed.
and the host polymer film coated on the electrode. The resultant PPy grows inside the matrix forming an electrically conducting polymer alloy film. According to this method we have potentiostatically prepared polypyrrole-polyamide (PA) composites.
Experimental
PPy-PA composites were prepared by the electro- chemical polymerization of pyrrole onto a polyamide- coated electrode at a constant potential of +1.5 V versus Ag°/Ag ÷ (10 -2 M). The polyamide films were dip-coated from a chloroform solution (10 mg 1-1) of a commercial polyamide resin (Aldrich Co. 19, 101-9). The amounts of insulating and conducting polymer coatings were determined gravimetrically.
Potentiostatic polymerizations were carried out in a three-compartment cell equipped with Pt foils (1.5 cm 2 each) as the working and counter electrodes and a capillary reference electrode (Ag/Ag+). The sol- vent-electrolyte couple was acetonitrile-tetrabutyl- ammonium tetrafluoroborate (TBAFB). Blank runs were also carried out (i.e. in the absence of pyrrole in the electrolytic medium) with PA-coated electrodes to ensure that there were no changes either chemically or by weight in the polymer electrode. Details of the potentiostatic electrolyses have been given elsewhere
[121.
Conductivities of samples were measured via a four- probe technique. The composites were characterized by FI'-IR (Nicolet 510) and SEM (Cambridge Stereo-
202
scan $4-10). Thermal gravimetric analysis and DSC were recorded on a Du Pont 2100 instrument.
Results and discussion
The electrooxidation of pyrrole using a PA-coated Pt anode gave rise to black films which could be peeled off from the electrode surface. Almost equal conduc- tivities on both sides of the films suggest that a ho- mogeneity was achieved, at least in terms of conductivity (Table 1). It is also interesting to note that conductivity was not greatly affected by the percolation composition. There exists only a sixfold decrease between 24% pyrrole (by weight) and the pure polypyrrole obtained under the same conditions. Several percolation threshold val- ues have been cited elsewhere [9, 13-15].
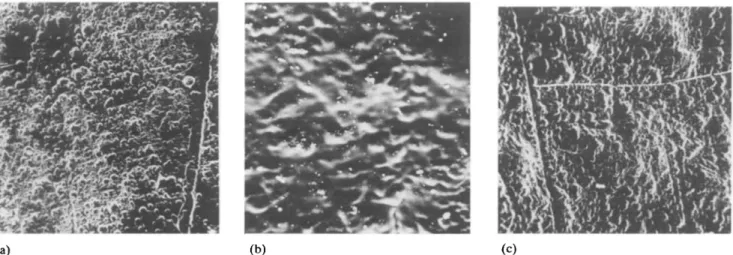
When the films were washed with chloroform (solvent of polyamide) no marked changes were measured in conductivity. The washing procedure was repeated for several weeks in order to see whether there was a loss in weight, since polyamide should easily be removed by chloroform. No weight loss could be detected. The surface appearance of the washed and unwashed films remained the same (Fig. 1). This behaviour can be
T A B L E 1. Conductivities of P A - P P y films
%PPy Electrode side Solution side
(S cm - : ) (S crn - l ) 24 4 4 36 10 10 47 12 15 62 14 19 100 22 25
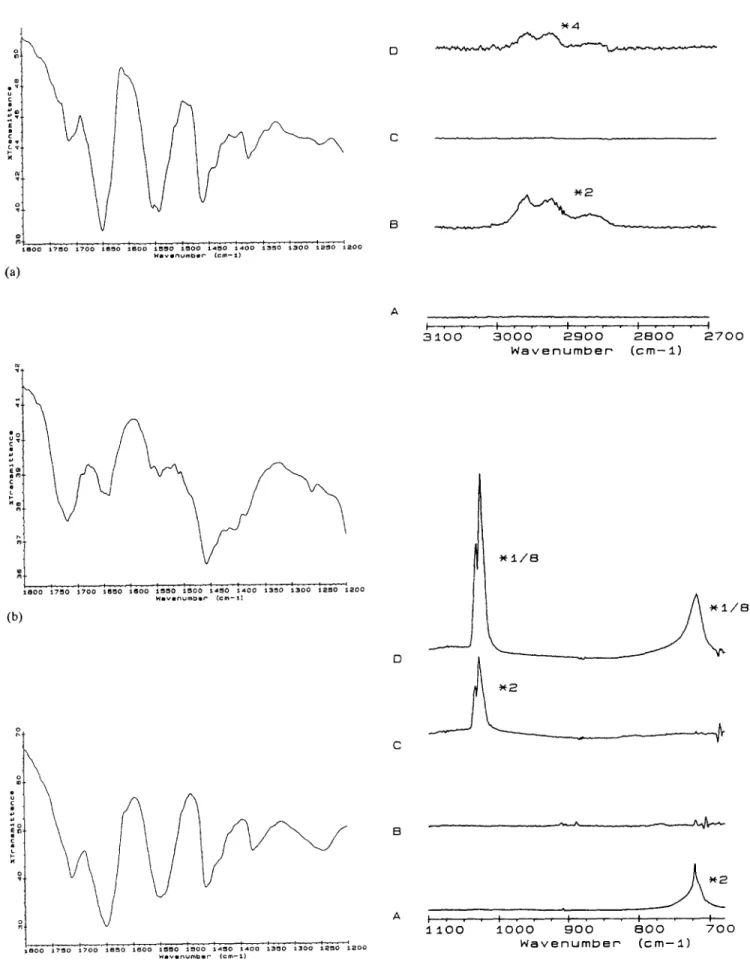
attributed to a chemical interaction between the two polymers rather than to a simple physical adhesion. FT-IR spectra of the two pure polymers and their mechanical mixture yield different features compared to the electrolytic film (Fig. 2).
In Fig. 3(b) the H-bonded carbonyl bond can be seen together with the free carbonyl group stretching. Yet such a chemical interaction cannot only be explained by a rather weak hydrogen bonding phenomenon. This matter was further elucidated by pyrolysis experiments. Figure 4 reveals the gas phase FT-IR of pure compounds, and the electrolytic film after pyrolysis. CO, CO2 and CH4 evolutions were clearly observed for the pristine polymers (Figs. 4(A) and (B)). It is remarkable that the spectrum of the film is not a simple addition of the previous two under the same conditions (300 °C, 10 -3 Yorr) (Fig. 4(C)). The pyrolysis FT-IR of the film at T=350 °C reveals different features in accordance with DSC studies (Fig. 4(D)). This temperature has significant meaning in terms of thermal characteristics. The glass transition temperature of PA lies around 82 °C, and DSC studies yield a different thermal be- haviour for the PA-PPy film (Fig. 5). The region 344-368 °C indicates a new thermal behaviour. A simple me- chanical mixture of the two polymers, on the other hand, shows the polyamide thermal characteristics as if only one phase existed. This is quite normal since under the given conditions we did not expect any thermal behaviour for polypyrrole.
Thermal gravimetric analysis of the potentiostatically obtained film reveals a single weight loss pattern sug- gesting a homogeneous single phase system (Fig. 6).
The reproducible response of the electrochemical film compared to pure PPy for several gases also suggests the generation of a new material. This will be the topic of an forthcoming article.
(a) (b) (c)
o ' 0 ~ o o g o E a ~ g
E
°
o:i
g --- ~. 0 N o ~ ~.. O'~ ~o
< ~ C O~ ~o
^ ; I 0 7 ~ T P I I n l l m i t t a n c e 3 0 4 0 5 0 S O 7 0/
- - - ~ fO~: 8 01
o~~T
° t.it
@ w,- C O' ~o, 'D ~ 0 I < ~ o n O 3 0 I o 0 o 3 4 t ~ T p a n a m l t t @ n c @ 3 6 3 8 4 0 4 2204 g
i;
s e o o 1 7 m o XTO0 t s m o a 6 o o (a) W a v ~ n u m b B r ( c r n - - 11 D C B ~ 4 ~ . I . I . t t . q I ~ . I i I I . - 1 8 0 0 1 7 ~ 0 t 7 0 0 x B ~ O 1 6 0 0 1 ~ 0 1 5 0 0 1 4 ~ 0 ~ 4 0 0 1 3 5 0 x 3 0 0 ~ 2 5 0 1 2 0 0 W m V l n o m ~ l r ( c m ~ J ) (b) o o tl ,a 3 8 0 0 a 7 5 0 t 7 0 0 ~ B m o 1 6 0 0 1 8 5 0 1 5 0 0 1 4 5 0 1 4 0 0 1 3 5 0 1 3 0 0 ~ 5 0 1 2 0 w a v e ~ m b a r ( c m - l ) (c)Fig. 3. FT-IR spectra of (a) mechanical mixture, (b) film and (c) pure PA.
A
. I I I I
3 a. oo' 3060' 296o'
2eoo"
2 7 0 0W a v e n u m D e m ( c m - - l ) D C w i / 8 B r I :11130' 9 0 0 8 0 ( 3 7 0 0 W a v e n u m D e r ' - ( c m - - : l . )
Fig. 4. Gas phase FT-IR spectra (300 °C, 10 -3 Torr) of (A) polypyrrole, (B) polyamide, (C) electrolytic film and (D) elec- trolytic film at 350 °C, 10 -3 Torr.
0 . 3 3= o [..L l - O" - ! 3 4 4 4 . 2 4 7 ° C 0 . 2 O . i o b.. ¢J 0 . 0 o - 0 . I l O 0 i 5 0 2 0 0 2 5 0 3 0 0 35O 4 0 0 4 5 0 T a m o a r a t u P e f°C) I - 0 . 2 5 0 0
Fig. 5. Differential s c a n n i n g calorimetry results for the P A - P P y electrolytic film.
~-20 O . B Y= ! 0 0 - 80 60 ¸ 2 0 - 0 • 94. B3 X I 280 480 . . .
6~o
800 ' T e m p e r a t d r e (°C)Fig. 6. T h e r m a l gravimetric analysis of t h e P A - P P y electrolytic film•
0 . 6 0 . 4 4J Jc ' 0 . 2 > ¢7 . 0 . 0 - 0 . 2 t 0 0 0
206
Conclusions
W e have e n o u g h evidence to believe that the P A - P P y p o l y m e r alloy film contains c o p o l y m e r s o f the two e l e m e n t s ( P A and PPy) to a certain extent. H b o n d i n g is also evident, b u t it c a n n o t explain the pyrolysis results alone.
Acknowledgements
This w o r k was p e r f o r m e d as p a r t o f the T B A G - 9 7 0 project s p o n s o r e d by t h e Turkish N a t i o n a l Science a n d R e s e a r c h C o u n c i l ( T U B I T A K ) . W e also thank the M E T U R e s e a r c h F u n d (92-05-01-04) and t h e V W F o u n - d a t i o n (1/67 069) for s u p p o r t o f this work.
References
1 A.F. Diaz, K. Kanazawa and G.P. Gardini, J. Chem. Soc.,
Chem. Commun., (1979) 635.
2 M. De Paoli, R.J. Waltmann, A.F. Diaz and J. Bargon, J.
Chem. Soc., Chem. Commun., (1984) 1015.
3 O. Niwa and T. Tamamura, J. Chem. Soc., Chem. Commun., (1984) 817.
4 X. Bi and Q. Pei, Synth. Met., 22 (1987) 145. 5 G.P. Zhang and X. Bi, Synth. Met., 41-43 (1991) 251. 6 B. Zinger and D. Kijel, Synth. Met., 41-43 (1991) 1013. 7 Y. Chen, R. Qian, G. Li and Y. Li, Polym. Comrnun., 32
(1991) 189.
8 B. Tieke and W. Gabriel, Polymer, 31 (1990) 20.
9 H.L, Wang, L. Toppare and J.E. Fernandez, Macromolecules,
23 (1990) 1053.
10 S. Do/gan, U. Akbulut and L. Toppare, Synth. Met., 53 (1992) 29.
11 S. Do/gan, U. Akbulut and L. Toppare, Synth. Met., 60 (1993) 27.
12 L. Toppare, S. Eren, 6. Ozel and U. Akbulut, J. MacromoL
Sci., Chem., 21 (1984) 1281.
13 G. Beggiato, G. Casalbore-Miceli, V. Fattori, A. Geri, A. Berlin and G. Zotti, Synth. Met., 55-57 (1993) 3495. 14 S.W. Byun and S.S. Im, Synth. Met., 55-57 (1993) 3501. 15 K. Yoshino, S. Marita, X.H. Yin, M. Onoda, H. Yamamato,