ContentslistsavailableatScienceDirect
Drug
Resistance
Updates
j ou rn a l h o m e pa g e :w w w . e l s e v i e r . c o m / l o c a t e / d r u p
The
impact
of
Organic
Anion-Transporting
Polypeptides
(OATPs)
on
disposition
and
toxicity
of
antitumor
drugs:
Insights
from
knockout
and
humanized
mice
Selvi
Durmus
a,1,
Stéphanie
van
Hoppe
b,1,
Alfred
H.
Schinkel
b,∗aBilkentUniversity,DepartmentofMolecularBiologyandGenetics,06800Bilkent,Ankara,Turkey
bTheNetherlandsCancerInstitute,DivisionofMolecularOncology,Plesmanlaan121,1066CXAmsterdam,TheNetherlands
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received6February2016
Receivedinrevisedform7May2016
Accepted17June2016 Keywords: OATP1A/1Btransporters OATP1B1 OATP1B3 Drugdisposition Hepaticuptake Drug–druginteractions
a
b
s
t
r
a
c
t
Itisnowwidelyacceptedthatorganicanion-transportingpolypeptides(OATPs),especiallymembers oftheOATP1A/1Bfamily,canhaveamajorimpactonthedispositionandeliminationofavarietyof endogenousmoleculesanddrugs.Owingtotheirprominentexpressioninthesinusoidalplasma mem-braneofhepatocytes,OATP1B1andOATP1B3playkeyrolesinthehepaticuptakeandplasmaclearance ofamultitudeofstructurallydiverseanti-cancerandotherdrugs.Here,wepresentathorough assess-mentofthecurrentlyavailableOATP1AandOATP1Bknockoutandtransgenicmousemodelsaskey toolstostudyOATPfunctionsinvivo.Wediscussrecentstudiesusingthesemodelsdemonstratingthe importanceofOATPs,primarilyintheplasmaandhepaticclearanceofanticancerdrugssuchastaxanes, irinotecan/SN-38,methotrexate,doxorubicin,andplatinumcompounds.Wefurtherdiscussrecentwork onOATP-mediateddrug–druginteractionsinthesemousemodels,aswellasontheroleofOATP1A/1B proteinsinthephenomenonofhepatocytehopping,anefficientandflexiblewayofliverdetoxification forbothendogenousandexogenoussubstrates.Interestingly,glucuronideconjugatesofboththeheme breakdownproductbilirubinandtheproteintyrosinekinase-targetedanticancerdrugsorafenibare stronglyaffectedbythisprocess.TheclinicalrelevanceofvariationinOATP1A/1Bactivityinpatientshas beenpreviouslyrevealedbytheeffectsofpolymorphicvariantsanddrug–druginteractionsondrug toxic-ity.ThedevelopmentofinvivotoolstostudyOATP1A/1Bfunctionshasgreatlyadvancedourmechanistic understandingoftheirfunctionalroleindrugpharmacokinetics,andtheirimplicationsfortherapeutic efficacyandtoxicsideeffectsofanticancerandotherdrugtreatments.
©2016ElsevierLtd.Allrightsreserved.
1. IntroductiontoOATP1A/1Btransportersandgenetically
modifiedmousemodelstostudytheirfunctions
1.1. PropertiesofOATP1A/1Btransporters
Organicanion-transportingpolypeptide(OATP)uptake trans-porterscanplayamajorroleintheuptakeofnumerouscompounds, includingmanyanticancerdrugs,intocellsandorgans.Positioned
Abbreviations: ABC,ATP-bindingcassette;AUC,areaunderthe
concentration-timecurve;DDI,drug–druginteractions;E2G,estradiol17-d-glucuronide;i.v.,
intravenous;OATP,organicanion-transportingpolypeptide;TKI,tyrosinekinase
inhibitor;UGT1A1,UDP-glucuronosyltransferase1A1.
∗ Correspondingauthor.
E-mailaddress:a.schinkel@nki.nl(A.H.Schinkel).
1 Thesetwoauthorscontributedequallytothispaper.
in theplasma membrane, thesemultispanning transmembrane proteinscanmediatetheuptakeofastructurallyhighlydiverse rangeofsubstratesintothecell,byasyetincompletelyresolved mechanisms.Asaconsequence,theycanhaveamajorimpacton thepharmacokineticdispositionoftransporteddrugs,determining theiroralavailabilityandplasmaclearance,aswellastheir distribu-tiontoliverandotherorgans,andthemainroute(s)ofelimination (forrecentreviewssee:GongandKim,2013;Konigetal.,2013; Niemietal., 2011;Shitara etal.,2013; Stiegerand Hagenbuch, 2014).OATPscanthereforehaveastrongeffectonthetherapeutic efficacy,butalsothetoxicsideeffectsofsubstratedrugs.Moreover, severalOATPsarevariablyexpressedinarangeofhumancancers. Asthis mayobviouslyinfluencetheeffectiveintracellular expo-sureofthecancercellstoOATPsubstrateanticancerdrugs,this candirectlyaffectthetherapysusceptibilityofthesecancers(for recentreviewssee:NakanishiandTamai,2014;Obaidatetal.,2012; Sissungetal.,2012;Thakkaretal.,2015).Theactivityofthehuman http://dx.doi.org/10.1016/j.drup.2016.06.005
OATPsthatarethoughttobemostimportantforthegeneral phar-macokineticbehaviorofdrugs,OATP1A2,OATP1B1,andOATP1B3 (aswellaspossiblyOATP2B1,butseebelow),canfurthervary dra-maticallybecauseofgeneticpolymorphismsandmutationsthat affectdrugtransport,butalsobecauseofdrug–druginteractions withavarietyofco-administereddrugs(e.g.Durmusetal.,2015; Frankeetal.,2009;GongandKim,2013;Konigetal.,2013;Niemi etal.,2011;Obaidatetal.,2012;Shitaraetal.,2013;Stiegerand Hagenbuch,2014;vandeSteegetal.,2012).
Giventheirobviousmedicalimportance,itiscrucialtoobtain clearinsightintotheinvivopharmacological,toxicological,and physiologicalfunctionsoftheOATPproteins,especiallythoseofthe OATP1A/1Bfamily.Onewaytoachievethisgoalistogenerateand studymousestrainsthathavethemouseOatp1aandOatp1bgenes knockedout,orthathavereplacedthemouseOatp1a/1bgeneswith oneormoreoftheirhumananalogues(althoughtheformalgene namefortheOATP-encodinggenesisSLCO(forSoluteCarrierof OrganicAnions),forsimplicitywewillmostlyusetheOATP/Oatp nomenclatureinthis review).Thesemouse modelscanthenbe usedtoinvestigatetheimpactofthegeneticmodificationsonthe behaviorof,amongstothers,anticancerdrugs.Thisreviewfocuses onrecentstudiesonsuchmousestrainsandtheinsightsobtained foranumberofanticancerdrugs.Assomeaspectshavealready beenextensivelyreviewedpreviously(Iusufetal.,2012b,c;Sprowl andSparreboom,2014;Tangetal.,2013),wewillonlybrieflytouch uponthose.
1.2. OATP1A/1Bknockoutandtransgenicmousestrains characterizedtodate
Todate, mostcharacterizedknockoutand transgenicmouse modelsconcernmembersoftheOATP1AandOATP1Bsubfamilies, asthesearethoughttobemostrelevantforoverall pharmacoki-neticsinman.SomeinitialstudiessuggestedthathumanOATP1A2 wasexpressedintheintestinalepithelium,whichwould poten-tiallyindicateanimportantroleindrugabsorption(Glaeseretal., 2007).However,manyindependentlaterstudiescouldnot cor-roboratethesefindings,anditisnowprobablysafetoconclude thatnormallythereisnosubstantiallevelofOATP1A2presentin thesmallorlargeintestineofhumans(e.g.Drozdziketal.,2014). Oncurrentdata,OATP1A2issubstantiallyexpressedin cholangio-cytesliningthebileductsintheliver,inthehumanblood-brain barrier, in apical membranes of kidney tubules, and in a vari-etyof humantumors(vandeSteeg etal., 2013and references therein).IncontrasttoOATP1A2,humanOATP1B1andOATP1B3 arehighlyandprimarilyexpressedinthebasolateral(sinusoidal) membraneofhumanhepatocytes,wheretheycanmediatethe hep-aticuptakeofnumeroussubstratecompounds(e.g.Nakanishiand Tamai,2012).Asthesegenesarealsoknowntobesubstantially affectedbygeneticpolymorphismsandmutationsinhumans(e.g. Niemietal.,2011;vandeSteegetal.,2012),theyhaveattracted mostattention.ThefunctionallyrelatedOATP2B1proteinisalsoa broad-specificitymultidrug-uptaketransporter,especiallyatlower pH, and highly expressedin both intestine and the sinusoidal membraneofhepatocytes.Ithasthereforebeensuggestedthatit mightalsohaveconsiderablepharmacokineticimpact(Nakanishi andTamai,2012).However,todatenoOATP2B1mousemodels havebeenpublished,sowewillnotfurthercoverthistransporter here.
AcomplicationinstudyingmousemodelsfortheOATP1A/1B transporters is that there are no straightforward orthologues between the individual mouse and human Oatp1a/1b and OATP1A/1Bgenes.AsindicatedinFig.1,therearenolessthan4 differentOatp1agenesinthemouse,Oatp1a1,Oatp1a4,Oatp1a5 andOatp1a6,inadditionto2Oatp1a-likeelementsthatmaybe pseudo-genes. Thiscompareswiththesingle OATP1A2gene in
Table1
Oatp1a/bknockoutandtransgenicmousemodelsdescribedtodate.
Mousemodels Geneticbackground Primaryreferences
Oatp1a1knockout C57Bl/6N (Gongetal.,2011)
Oatp1a4knockout C57Bl/6N (Gongetal.,2011;
Oseetal.,2010)
Oatp1b2knockout(variant1) C57BL/6 (Luetal.,2008)
Oatp1b2knockout(variant2) DBA1/lacJ (Zaheretal.,2008)
Oatp1a/1bknockout FVB (vandeSteegetal.,
2010)
HumanizedhepaticOATP1A2
transgenic
FVB (vandeSteegetal., 2013)
HumanizedhepaticOATP1B1
transgenic
FVB (vandeSteegetal., 2009)
HumanizedhepaticOATP1B3
transgenic
FVB (vandeSteegetal., 2013)
HumanizedhepaticOATP1B1
andOATP1B3transgenic
FVB (Salphatietal., 2014)
humans.Ontheotherhand,themousehasonlyoneOatp1b2gene, contrastingwith thetwo humanOATP1B1and OATP1B3genes (Fig.1).Although the mouse and humanOATP1A proteinsare obviouslymoresimilartoeachotherthantothemouseandhuman OATP1Bproteins,andviceversa,theaminoaciddivergencewithin eachsubfamilyisstillconsiderable(aslowas67%aminoacid iden-titywithin theOATP1A subfamily, and 65%withinthe OATP1B subfamily).Consequently,withthesebroad-specificitymultidrug transporters,noreliablestatementscanbemadeonoverlapping substratesjustbasedonaminoacidsimilarity.Asthetissue distri-butionisalsonotconservedbetweenmembersofonesubfamily (for instance, mouse Oatp1a1 and Oatp1a4 are present in the sinusoidalmembraneofhepatocytes,whereashumanOATP1A2is not)itisclearthatonecannotusesingle-genemouseOatp1aor Oatp1bknockoutstrainstomakereliablepredictionsontheinvivo behaviorofhumanOATP1A2,OATP1B1,orOATP1B3.Thiswasan importantmotivationtogenerateacompleteOatp1a/1bknockout strain,anduseittospecificallyexpresshumanOATP1A2,OATP1B1, andOATP1B3inthisknockoutbackground(vandeSteegetal.,2012, 2013,2010).
Table1liststhevariousOatp1aandOatp1bknockoutstrains, and OATP1A/1Bhumanized strainsthat havebeendescribed so far.Oatp1a1andOatp1a4knockoutstrainsweredescribedbyOse etal.(2010)andGonget al.(2011).Thesemouse strains, origi-nallymadein129/OlaEScellsbyDeltagen,werebackcrossed10 timestoaC57BL/6background,andsubsequentcharacterization oftheselineswasmainlydoneinthisgeneticbackground.Afew independentOatb1b2knockoutstrainsweregenerated.Luetal. (2008)describedthegenerationandinitialcharacterizationofan Oatp1b2knockoutstraingeneratedin129S1EScells,whichwas backcrossedfor7generationstoaC57BL/6background. Indepen-dently,Zaheretal.(2008)generatedOatp1b2knockoutmiceusing DBA1/lacJEScells,whichwerefurtherkeptandcharacterizedin a DBA1/lacJgeneticbackground.Combined Oatp1a/1bknockout mice,coveringallthemouseOatp1aandOatp1bgenes,were gen-eratedin129/OlaEScells.InitialcharacterizationofOatp1a/1b−/− micewasdoneinamixed(∼50%)129/OlaandFVBgenetic back-ground(vandeSteegetal.,2010),butthisstrainwassubsequently backcrossedforatleast7generationstoanFVBbackground,in which furthercharacterization took place (van de Steeg et al., 2012).TheseFVBbackgroundOatp1a/1b−/−micewerethenused togeneratethreedifferenthumanizedmousestrainswith predom-inantexpressionofhumanOATP1A2,OATP1B1,orOATP1B3cDNA, respectively,intheliverparenchymecells,againallinFVB back-ground(vandeSteegetal.,2012,2013).Moreover,acombined humanizedOATP1B1/OATP1B3strainwascreatedbycrossingthe separatetransgenicstrains(Salphatietal.,2014).
Fig.1. ComparisonofthehumanandmouseOATP1A/B/Cgeneclusters.ThehumanOATP1A/B/Cgeneclusterislocatedonchromosome12p.OATP1C1isfollowedby
OATP1B3,theOATP1B-likepseudogeneLST-3(OATP1B7accordingtoEnsembl),OATP1B1,andOATP1A2.OATP1A2istranscribedfromtheoppositestrandcomparedtothe
OATP1B-andOATP1C-typegenesinthecluster.ThemouseOatp1a/b/cgenesarelocatedonchromosome6pinasimilarorderasthehumangenes,startingwithOatp1c1
followedbyOatp1b2,Oatp1a-likepseudogeneA(Ensembl:Gm5724),Oatp1a4,Oatp1a1,Oatp1a-likepseudogeneB(Ensembl:Gm6614),Oatp1a6,andOatp1a5.Analogous
tothehumangenecluster,allOatp1a-typegenes(andpseudogenes)areorientedintheoppositetranscriptiondirectionoftheOatp1b-andOatp1c-typegenes.Therelative
sizesanddistancesofthegenesaredepictedroughlytoscale,alsobetweenthemouseandhumanchromosome,butwithoutshowingexon/intronstructures,andarrows
indicatethedirectionoftranscription.Pseudogenesandtheirputativedirectionoftranscriptionarerenderedhatched.Directionsofcentromeresandtelomeresandthe
approximatesizeofthehumanandmousegeneclustersareindicated.
1.3. CaveatsinusingOATP1A/1Bgeneticallymodifiedmouse models
Itshouldbenotedthatthegeneticbackgroundstrainmayat timesbeimportantfor theextenttowhich certainphenotypes are detectablein knockoutand transgenic mice. Although this hasnotbeensystematicallyanalyzedsofarinthevarious Oatp knockoutstrains,wehaveobservedmodestphysiologicalchanges inOatp1a/1b−/− miceinmixedOla/129/FVBbackground,which disappearedupon furtherbackcrossinginto anFVBbackground (unpublisheddata).
Alsotheenvironmentalconditions(e.g.,diet,bedding,intestinal microflora) may at times be important factors in the pene-tranceofcertainphenotypes.Forinstance,wehaveobservedthat FVBbackgroundOatp1a/1b−/− miceanalyzedinourfacility(The NetherlandsCancerInstitute,NKI)hadamarkedinductionof car-boxylesterasegenesrelativetowild-typeFVBmice(Iusuf etal., 2014).However,theverysamestrainofmiceobtainedbyother groups through a commercial supplier (Taconic) did not show adifferenceincarboxylesteraseexpressionwithwild-typemice (Salphati et al.,2014).Further analysissuggested thatthis was becausethewild-typeFVBmiceobtainedfromTaconicalreadyhad amuch higher endogenoushepatic carboxylesteraseexpression comparedtoFVBmicekeptattheNKI,mostlikelybecauseof envi-ronmental differences.The Oatp1a/1b knockoutfrom “Taconic” circumstancesdidnot furtherincrease carboxylesterase expres-sion.Interestingly,humanizingtheOatp1a/1bknockoutmicewith the OATP1B1 transgene did reverse the high carboxylesterase expression in both NKI and Taconic mice (Iusuf et al., 2014; Salphatietal.,2014),indicatingthatthiscouldapparentlyoverrule anyenvironmentalfactorscausingthedifferentcarboxylesterase expressioninFVBwild-typemice.Ontheotherhand,transgenic OATP1B3couldfullyreversethecarboxylesteraseoverexpression
intheNKIfacility,butnotelsewhere.Thisfurthersupportsthat, whateverthefactor(s)pushingupcarboxylesteraseexpressionin theOatp1a/1b−/−(andFVB)mice,theyweremoreprominentinthe otherfacilitiesthanintheNKIfacility.Itisimportanttokeepsuch complicationsinmindwhencomparingtheresultsingenetically modifiedmousestrainsobtainedbydifferentgroups.Moreover,as environmentalconditionsinmousefacilitiescannotalwaysbekept undercompletecontrol,itisalsopossiblethatshiftsinphenotypes occurovertimeevenwithinonefacility.
Thirdly,alsogendermaysubstantially affectresultsobtained withOatp1a/1bgeneticallymodifiedmice.Forinstance,Oatp1a1, Oatp1a4,andOatp1b2areallsubstantiallyexpressedinthe baso-lateral(sinusoidal)membraneofthemouseliver.JudgedbyRNA level,Oatp1a1ismoreabundantinmalethaninfemaleliver(about 2-fold),andOatp1a4infemaleliver(alsoabout2-fold),whereas Oatp1b2issimilarlyexpressedinbothgenders(Chengetal.,2005). Thiscandirectlyaffectwhethercertainpharmacokineticeffectscan bedetectedinknockoutstrains. Forinstance,Gongetal.(2011) foundthatOatp1a1deficiencysubstantiallyreducedtheclearance ofthediagnosticdyedibromosulfophthaleininmalemice,butnot infemalemice.
Insomecases,authorshaveassertedthattheknockoutofmouse Oatp1b2,asthesinglemouserepresentativeofthemammalian OATP1Bsubfamily(Fig.1),mightbemoreorlessequivalenttothe deficiencyofOATP1B1andOATP1B3inhumans(e.g.,Zaheretal., 2008).However,formanyOATPsubstratesthisassumptionclearly doesnotapply.Inhumans,OATP1B1andOATP1B3aretheonly membersoftheOATP1A/1Bfamilythatarepresentinthe basolat-eralmembraneofliverparenchymecells,andthusinvolvedinthe uptakeofsubstratesfrombloodintotheliver.HumanOATP1A2is alsoexpressedintheliver,butonlyfoundinthecholangiocytes, andthereforeirrelevantforthedirectuptakeofcompoundsfrom bloodintotheliver.Incontrast,inthemousetherearetwoOATP1A
proteinsthataresubstantiallypresentinthesinusoidalmembrane ofliverparenchymecells,Oatp1a1andOatp1a4.Thismeansthat foranysubstratethatissubstantiallytransportedbybothOatp1b2 andeitherorbothofOatp1a1orOatp1a4,resultsobtainedin sin-gleOatp1b2−/− micemayunderestimatetheeffectsofOATP1B1 and OATP1B3deficiency inhumans. Acase in point is therole ofOATP1B1andOATP1B3intheuptakeofconjugatedbilirubin. WhereassingleOatp1b2−/−miceshowedonlyamarginalincrease inplasmatotalandconjugatedbilirubinlevels(Luetal.,2008;Zaher etal.,2008),infullOatp1a/1b−/− micetherewasapronounced increaseinplasmabilirubinglucuronidelevels(vandeSteegetal., 2010).Thelatterobservationpointedtotheprominentrolethat humanOATP1B1andOATP1B3playinthereuptakeofconjugated bilirubininhumanliver,andprovidedthemechanistic explana-tionforthehumanRotorsyndromecausedbyfullOATP1B1and OATP1B3deficiency(vandeSteegetal.,2012).Clearly,the redun-dantroleofOatp1a1andOatp1a4relativetoOatp1b2inhepatic uptakeofconjugatedbilirubininthemouseobscuredthe impor-tantcontributionofthehumanOATP1Bproteinsinthisprocess. Greatcaremustthereforebetakenwhenextrapolatingfromresults inOatp1b2−/−micetothefunctionalroleofthehumanOATP1B1 andOATP1B3proteinsinliver.Forpharmacokineticstudies,given the very substantial but incomplete and unpredictable overlap in substratespecificity betweenthevarious mouse and human OATP1A/1Btransporters,everydrugshouldbecarefullyassessed initsownright,andgreatcautionshouldbeusedin extrapolat-ingfromresultsobtainedinsingleknockoutstrainstothehuman situation.
Keepingthesecaveatsinmind,inthisreviewwewillfocuson recentstudiesperformedwiththesemouse modelsfora range ofanticancerdrugs.Fig.2providesanoverviewofthestructures ofthesedrugs,alsoillustratingtheirstructuraldiversity.Theaim ofsuchstudieswastoobtainbasicinsightintothehandlingof the anticancer drugs by OATP1A/1B proteins, in the hope that thisknowledgemayultimatelybeusedtoimprovecurrent anti-cancerdrugtreatmentregimens,byenhancingtherapeuticefficacy ofthesedrugs,reducingtoxicsideeffects,andpossiblyboth.
2. RecentpharmacologicalandtoxicitystudieswithOATP
knockoutandhumanizedmice
2.1. Taxanes
Thetaxanespaclitaxelanddocetaxelarecytotoxicanticancer drugsthatbindtomicrotubulesanddisrupttheirfunctionby stabi-lizingGDP-boundtubulin,thusinterferingwithpropercelldivision. Paclitaxelanddocetaxelarecurrentlyappliedintravenously(i.v.) inthetreatmentofseveraltypesofcancer,suchasnon-smallcell lungcancer,ovarian,breast,gastric,prostate,andhead-and-neck cancer(GligorovandLotz,2004;Koolenetal.,2010).Both pacli-taxelanddocetaxelarequitelarge,veryhydrophobic,uncharged molecules(Fig.2).Itwasthereforethoughtthattheycouldpass cellmembranesprimarilybypassivediffusion,soitwasinitiallya bitofasurprisethatthesemoleculesweresubstantiallytakenup intocellsbyOATP1B1andOATP1B3inseveral(butnotall,seealso Section4)invitroexpressionsystems(Bakeretal.,2009;deGraan etal.,2012;Nieuweboeretal.,2014;Smithetal.,2006;Svoboda etal.,2011).Toassessthepossibleinvivorelevanceofthisuptake transport,anumberofstudieswithpaclitaxelanddocetaxelwere performedinOATP/Oatpknockoutandtransgenicmice.
2.1.1. Paclitaxel
VandeSteegetal.(2011)foundthatthepaclitaxelplasmaAUC wasmorethan2-foldincreasedinOatp1a/1b−/−relativeto wild-type miceafter i.v.administration at 10mg/kg. Conversely,the
liverAUCwas2-foldlowerintheOatp1a/1b−/−mice.Clear dif-ferencesinplasma andliverpaclitaxelconcentrationswerenot yetapparentat3.5min,butemergedfrom7.5minafter admin-istration.Thissuggeststhattheveryearlypaclitaxeldistribution, whenplasmaconcentrationswereveryhigh(>20mg/l),wasnot muchdependentonOatps.However,withplasmaconcentrations below20mg/l,paclitaxelliveruptakeandhenceplasmaclearance becamestronglydependentonOatp1a/1btransporters.This sug-gestedthatatplasmalevelsabove20mg/lthesinusoidalOatp1a/1b proteinsweresaturated,andotheruptakeprocesses,perhaps pas-sivediffusion,dominatedtheliveruptakeofpaclitaxel.Thiswas supportedby limited paclitaxeldistributionstudies ata higher dosage (50mg/kg), which yielded reduced differences between Oatp1a/1b−/− andwild-typemicein plasmaconcentration (1.7-fold) and liver concentration (1.5-fold) 30min after paclitaxel administrationcomparedtothe10mg/kgdose(1.9-foldand 2.2.-fold,respectively).Overall,thedataindicateasubstantialroleof hepatic sinusoidalOatp1a/1bproteinsintheclearance of pacli-taxelfromplasma,atplasmaconcentrationsthatcanalsooccur in patients. Followinghigh-dose paclitaxelchemotherapy, peak plasma concentrations range from 2 to 11mg/l (Rowinsky and Donehower, 1995), i.e. well below the saturation level of the Oatp1a/1b proteins. A later, independent study byNieuweboer etal.(2014)foundquantitativelysimilareffectsofasingleOatp1b2 knockoutontheplasmaAUCandliveraccumulationofi.v. pacli-taxeldosedat5mg/kginmice.Thiscouldindicatethatmostof theeffectsonpaclitaxelobservedintheOatp1a/1b−/−micewere primarilymediatedbyOatp1b2deficiency,althoughdifferencesin geneticbackgroundstrain(DBA/1lacJvs.FVB),exactpaclitaxel for-mulationused,laboratoryandevenperiodofexperimentation,may allaffectthedetailedoutcome ofsuchstudies(e.g.Nieuweboer etal.,2014;SparreboomandMathijssen,2014).
Ina follow-upstudy,thepharmacokineticsofpaclitaxelwas analyzed in “humanized” Oatp1a/1b−/− mice with transgenic expression of human OATP1B1, OATP1B3, or OATP1A2 in the liver parenchyme cells (van de Steeg et al., 2013). The trans-genicpromoter/enhancerusedwaschosentoobtainpreferential expressionofthehumanproteinsinliverparenchymecells,which wassuccessful,althoughminorOATP1B1andOATP1B3transgene expressionwasalsofoundinkidney,butnotsmallintestine.Based onproteinimmunoblotanalysisandproteinmassspectrometry, thehepaticlevel oftransgenicOATP1B1wasinthesameorder asthatobservedinhumanliversamples,andthatoftransgenic OATP1B3 somewhat higher, with estimated humanized/human ratiosof0.5- to1-foldand ∼3-foldfor OATP1B1and OATP1B3, respectively(Higginsetal.,2014;Salphatietal.,2014).Transgenic OATP1A2expressionwasmuchhigherthaninhumanliver,but thisrelatesmostlytothefactthatOATP1A2inhumanliverisonly expressedincholangiocytes,whereasinthetransgenicmiceitis expressedinthefarmoreabundantliverparenchymecells.This alsomeansthattheOATP1A2humanizedmousestraindoesnot representaphysiologicallycorrectmodelofthenormalOATP1A2 functionin humanliver.However,it doesallowanassessment of the in vivo functioning of OATP1A2in uptake of drugs and othercompoundsfromplasma.Thismay,interalia,berelevant asOATP1A2issubstantiallyexpressedinthehumanblood-brain barrier,inapicalmembranesofkidneytubules,andinavarietyof humantumors(vandeSteegetal.,2013andreferencestherein). Immunohistochemically, transgenic OATP1B1 was foundin the sinusoidalmembraneofhepatocytesandexpressedthroughoutthe liverlobule,albeitwithstrongerstainingaroundtheportalveinin humanliver,whereasthistransporterisvariouslyreportedtobe expressedthroughouttheliverlobule,orprimarilyincentrilobular hepatocytes(vandeSteegetal.,2009,2013).TransgenicOATP1B3 waslikewisefoundinthehepatocytesinusoidalmembrane, show-ingsomewhatdisperseddistributionthroughouttheliverlobules
Fig.2. StructuresofanticancerdrugsclearlyaffectedbyOatp1a/1bactivityinvivo.Drugsthatarestructurallycloselyrelated,butthatarenotappreciablyaffectedby
Oatp1a/1b-mediatedtransportinvivoarenamedinitalicsandmarkedwithanasterisk.Whilepaclitaxelanddocetaxelareclearlytransported,cabazitaxel,whichdiffersin
justtwomethoxygroupsfromdocetaxel,isnotappreciablytransportedbyOatp1a/1binvivo.Oatp1a/1bactivityinmiceclearlyaffectsdispositionofbothirinotecanand
itsactivemetaboliteSN-38.SorafenibitselfisnotnoticeablytransportedbyOatp1a/1binvivo,butitsglucuronateconjugatesorafenib--D-glucuronideis.Forcisplatinit
shouldbenotedthatthisdrugishighlyreactive,andonlytotalPtlevelsaremeasuredininvivoexperiments.Itisthereforepossiblethatstructurallydifferentcomplexes
(vandeSteegetal.,2013).InhumanliverOATP1B3ispreferentially foundinthesinusoidalmembranesofcentrilobularhepatocytes. AlthoughthetransgenicOATP1B1andOATP1B3thereforedonot exactlyreflectthelobularsubdistributionofOATPsinhumanliver, subsequentstudiesrevealedgoodfunctionalityoftheseproteins (aswellastransgenicOATP1A2)intheliveruptakeandplasma clearanceofarangeofcompounds.
Strong support for the functional activity of the hepatic OATP1B1,-1B3and -1A2transportersinthehumanized strains camefromthereversalofthehighlyincreasedplasmabilirubin levelsfoundintheOatp1a/1b−/−micerelativetowild-typemice (vandeSteegetal.,2013).Theincreasedplasmalevelsof biliru-binmonoglucuronideanddiglucuronidewerereversedtonearly wild-typelevels(>15-foldand>7-foldreduction,respectively)by bothOATP1B1andOATP1B3expression,whereasOATP1A2caused amoremodest2-foldreduction.Interestingly,transgenicOATP1A2 wastheonlytransporterthatcouldsignificantlyreducethe2-fold increasedlevelsofunconjugatedbilirubinbacktowild-typelevels. ThissuggeststhatOATP1A2is arelativelymore efficient trans-porterofunconjugatedbilirubincomparedtoconjugatedbilirubin, whereastheinverseistrueforOATP1B1andOATP1B3.Whatthis meansforthenormalbiologicalfunctionofhumanOATP1A2in cholangiocytes is as yet unclear.Perhaps it plays a role in the resorptionofhighlyinsolubleunconjugatedbilirubininadvertently formedinbile,thusreducingthechanceofformationof bilirubin-containinggallstones,butforthemomentthispossibilityremains speculative.
A limited pharmacokinetic study of paclitaxel in the OATP humanizedmicerevealedamodest,∼1.6-fold,buthighly signifi-canteffectofOATP1B3andOATP1A2inenhancingtheliveruptake ofpaclitaxeldosedi.v.at2mg/kgcomparedtothatinOatp1a/1b knockoutmice.Asmallereffectwasobservedat10mg/kgi.v. pacli-taxel(onlysignificantforOATP1A2).TransgenicOATP1B1didnot elicitsignificantchangesrelativetoOatp1a/1b−/− miceateither dose(vandeSteegetal.,2013).Collectively,thesedatasuggestthat OATP1B3andperhapsOATP1B1mayonlyhavemodesteffectson paclitaxelliveruptakeandclearanceinhumans.Itshouldbekeptin mind,though,that,unlikethesituationinsinglehumanizedmice, inhumanliverOATP1B1andOATP1B3usuallyfunction simulta-neously,whichcouldwellenhancetheiroverallpharmacokinetic impact by additiveeffects. Such an additiveeffect was indeed observed for another drug in recently generated OATP1B1/1B3 double-transgenicmice,wheresingleOATP1B1orOATP1B3 trans-genesdidnot markedlyreducetheAUCofpravastatinafteri.v. administration,butthecombinationreversedtheAUCtocloseto thatseeninwild-typemice(Salphatietal.,2014).
2.1.2. Docetaxel
Althoughquantitativelydivergentresultshavebeenobtained for the impact of Oatp1b2 and Oatp1a/1b knockouts, and of various OATP1A2/1B1/1B3 humanized transgenes on docetaxel pharmacokinetics, all studies to date support a role of these OATP/Oatpsindocetaxelclearance.DeGraanetal.(2012)reported that after i.v. administration of docetaxel at 10mg/kg to wild-typeandOatp1b2−/−mice,theplasmaAUCwas26.3-foldhigher in Oatp1b2−/− mice, indicating a very large effect of Oatp1b2 removal.Asexpected,theliver-to-plasmaAUCratiowasreduced inOatp1b2−/−mice,by6.2-fold.However,somewhatsurprisingly, theliverdocetaxelAUCitselfwassubstantiallyhigher(4.3-fold)in Oatp1b2−/−comparedtowild-typemice,ratherthanlower(or per-hapsequal,seebelow).Thisresultwasunexpectedincasetheliver uptakeofdocetaxelwasstronglyreducedintheknockoutmice. Ananalysisofpotentialcompensatoryexpressionchangesof func-tionallyrelatedgenesintheknockoutmicedidnotyieldobvious alternativecausesofthechangesindocetaxelpharmacokinetics.A simplemechanisticexplanationoftheobservedincreasedliverAUC
ofdocetaxelintheOatp1b2−/−miceisforthemomenttherefore stilllacking.
Thepotentialexperimentalvariabilityofthesedocetaxel phar-macokineticstudieswasillustratedbytheoutcomeofverysimilar experimentsthatwereperformedafewyearslaterbythesame group,inthesamemousestrains,underapparentlythesame con-ditions(Huetal.,2014;SparreboomandMathijssen,2014).Instead ofa 26-foldhigherplasma AUCof docetaxelintheOatp1b2−/− mice,onlya1.6-foldincreasewasobserved,whichwas,however, stillstatisticallysignificant.Themaindifferencewasnotachange overtimeintheplasmaAUCoftheOatp1b2−/−mice(∼7400vs. ∼8800ngxh/ml),butalmostentirelyattributabletoamuchhigher plasmaAUCofdocetaxelinthewild-typemicecomparedtothe ear-lierexperiments(4500vs.336ngxh/ml).Despiteextensivelater analysesbythesamegroup,thecauseofthesedivergentresultsis stillunclear(SparreboomandMathijssen,2014).Althoughinour experienceitisnotuncommontoseesomevariationinabsolute druglevels(AUC)measuredinpharmacokineticstudiesperformed afewyearsapartunderotherwiseseeminglyidenticalconditions, thisusuallyconcernslessthanabout2-folddifferences.However thismaybe,collectively,thedatastillindicatearoleforOatp1b2in theclearanceofi.v.docetaxel,mostlikelybymediatinguptakeof docetaxelfrombloodintotheliver.Thiswasfurthercorroborated byindependentstudiesbytwoothergroups.
UsingthesamestrainofOatp1b2−/−mice,Leeetal.(2015)very recentlyreporteda limited,30minpharmacokineticstudywith i.v.[3H]-docetaxeldosedat1mg/kg.Bydeterminingplasmaand liverradioactivity,theyfounda5.5-foldhigherplasmaAUCof[3 H]-docetaxelequivalentsinOatp1b2−/−mice(340±149vs62±8ngx h/ml,P<0.05),nosignificantdifferenceinliverconcentrations,but a3-folddecreasedliver-to-plasmaratio(P<0.05).While concern-ingtotalradioactivitymeasurements,whichmaybecomplicated byextensiveandpossiblydifferentialdocetaxelmetabolism,these resultsqualitativelysupportthefindingsofHuetal.(2014)andDe Graanetal.(2012),indicatingaroleofOatp1b2inplasmaclearance ofdocetaxelbymediatinguptakeintotheliver.
Iusuf et al. (2015) studied docetaxel pharmacokinetics in Oatp1a/1b−/− miceof anFVB geneticbackground,usinga low-polysorbate80formulationtominimizepossibleinhibitoryeffects onOatps(deGraanetal.,2012; Nieuweboeret al.,2014).After i.v.administrationofdocetaxelat10mg/kg,theplasmaAUCwas 2.9-foldhigherintheOatp1a/1b−/−mice(609vs212gxmin/ml, P<0.001).SimilartothefindingsofLeeetal.(2015),theliver expo-surewasnotsubstantiallyalteredintheOatp1a/1b−/−mice,butthe liver-to-plasmaratiowasmarkedlyloweratallexcepttheearliest timepoints(atleast3-foldormoreafter15min).Alimited pharma-cokineticstudy(15–60min)ofi.v.docetaxel(dosedat10mg/kg) inOATP1B1,OATP1B3,andOATP1A2humanizedmiceindicated a4-foldincreaseindocetaxelplasmaAUCinOatp1a/1b−/− com-pared to wild-type mice, which was largely reversed in both OATP1B1- and OATP1A2-transgenic mice (P<0.001), and more modestly(P<0.01)inOATP1B3-transgenicmice(Iusufetal.,2015) (Fig.3AandB).InaccordancewiththeunalteredliverAUCinthe Oatp1a/1b−/− mice,theliverAUCs inallthehumanized strains werenotsignificantlydifferentfromthoseinthewild-typeand Oatp1a/1b−/−mice.
TheseunalteredliverAUCdataarereadilyexplainedbya phys-iologicallybasedpharmacokineticmodeldevelopedbyWatanabe etal.,2009,2010).Thisshowsthatastrongreductioninhepatic uptake of drugs that have little alternative extrahepatic clear-ance(e.g.,renalclearance),willoftenresultinmarkedlyincreased plasma levels of the drug, but only very small changes in the liverAUC.Accordingly, liver-to-plasmaconcentrationratioswill decrease,butmainlyduetotheincreasedplasmaconcentrations ofthedrug.Thisisexactlythebehaviorthatwasobservedfor doce-taxelinthestudiesofIusufetal.(2015)andLeeetal.(2015),butalso
Fig.3.ImpactofmouseandhumanOATP1A/1Btransportersontheplasmaandliverdispositionofvariousanti-cancerdrugs.Plasmadisposition(A,C,andE)and
liver-to-plasmaratios(B,D,andF)ofdocetaxel,SN-38anddoxorubicinareshowninthefigureatvarioustimepointsafteri.v.administrationof10mg/kgdocetaxel,10mg/kg
irinotecan(pro-drugofSN-38)or5mg/kgofdoxorubicin,respectively.Drugswereadministeredtowild-typeandOatp1a/1b−/−mice,andtoOATP1B1-,OATP1B3-,and
OATP1A2-transgenicmiceinanOatp1a/1b−/−background.Dataarepresentedasmean±SD[*P<0.05;**P<0.01;***P<0.001whencomparedwithwild-type;#P<0.05;
##P<0.01;###P<0.001whencomparedwithOatp1a/1b−/−mice].Notethatdifferentunitsareusedtoindicatetheplasmaconcentrationsofeachdrug.Thisfigurewas
modifiedwithpermissionfrompreviouslypublishedexperimentaldataofIusufetal.(2015,2014)andDurmusetal.(2014).
earlierforrosuvastatin,anotherOATPsubstrate(Iusufetal.,2013). OnlytheearlierdataofDeGraanetal.(2012),whichappearedto showa>4-foldincreaseinliverAUCofdocetaxelinOatp1b2−/− micearenotyetadequatelyexplained.
Nonetheless,thecollectivedatainOatpknockoutandvarious OATP1B-humanizedstrainsprovide strongsupportforthe con-ceptthatmouseOatp1b2andhumanOATP1B1andOATP1B3in thesinusoidalmembraneofthelivercancontributesubstantially totheliveruptakeofdocetaxel,andthusitsplasmaclearanceas showninFig.3AandB.Itisfurthernoteworthythatthe contribu-tiontodocetaxelclearanceofOATP1B1andOATP1B3inthehuman liverwilllikelybeatleastadditive.Itcouldthereforebethat co-administrationofdocetaxelwithstronginhibitorsofOATP1B1and OATP1B3mightresultinunwarrantedoverexposureofpatientsto docetaxel,whichonlyhasanarrowtherapeuticwindow.
2.1.3. Cabazitaxel
Thetaxanecabazitaxelwasrecentlyintroducedintheclinicas a second-linetreatmentof hormone-refractory prostate cancer. Amongstothersthiscompoundislessaffectedbymultidrug resis-tancecausedbytheMDR1P-glycoproteinthandocetaxel(Figgand Figg,2010;Kathawalaetal.,2015;PallerandAntonarakis,2011) However,recentstudiesdemonstratedthatstepwiseselectionof MCF-7cellstocabazitaxelresultedinP-gpoverexpression(Duran etal.,2015).(Interestingly,cabazitaxelhasaverysimilarstructure as docetaxel, withthe only difference being that two hydroxy groups in docetaxel are replaced by methoxy groups (Fig. 2). However,inspiteofthisgreatsimilaritytodocetaxel,cabazitaxel appearsnottobetransportedbyhumanOATP1B1andOATP1B3 ormouseOatp1b2inHEK293cells,orinvivoinOatp1b2knockout mice(Nieuweboer etal., 2014).Thisillustrateshow apparently minor structural modifications can sometimes have dramatic effects onwhethera compound is transported by OATPs,as is alsothecaseforMDRtransportersoftheABCsuperfamilysuchas ABCG2(Brametal.,2009).Onthepositiveside,thisprobablyalso meansthatforthisdrug,variationsinOATP1Bactivityinpatients duetogeneticpolymorphisms,drug–druginteractionsorvariable
expression in tumors, are less likely to affect the therapeutic efficacyortoxicity.
2.2. Irinotecan/SN-38
Theanticancerdrugirinotecanis atopoisomeraseIinhibitor widelyusedinthetreatmentofcolorectal,ovarianandlungcancer. Itstherapeuticindexisquitelow,inpartowingtoitscomplex phar-macokineticsinvolvingavarietyofmetabolicenzymesanddrug transporters.Irinotecan,essentiallyaprodrug,ishydrolyzedtoits primarypharmacodynamicallyactivemetabolite,SN-38,by vari-ouscarboxylesterasesoccurringinliver,intestine,and,inmice,also inplasma(Innocentietal.,2009;Mathijssenetal.,2002).Severe toxicsideeffectsofirinotecantherapyincludediarrheaand neu-tropenia,andthesegenerallycorrelatewiththesystemicexposure toSN-38(Smithetal.,2006).Low-activitypolymorphicvariantsof OATP1B1,whichmayleadtoimpairedhepaticclearanceof irinote-canand/orSN-38,areassociatedwithincreasedsystemicexposure toSN-38and life-threateningtoxicity (Han etal.,2008;Takane etal.,2009;Xiangetal.,2006).Itwasthereforeofgreatinterest toimproveourunderstandingoftheinvivoimpactofOATPson irinotecan/SN-38pharmacokineticsandtoxicityusingapanelof Oatp/OATPknockoutandtransgenicmice.Asitturnedout, how-ever,thisstudyalsorevealedapotentiallyimportantconfounder instudieswiththesemousestrainsfordrugsthatcanbeaffected byplasmacarboxylesterases.
Initialstudiesofi.v.administeredirinotecan(10mg/kg)in wild-typeandOatp1a/1b−/− miceshowedconsistentlyhigherplasma levelsofirinotecan(AUC1.7-foldhigher)andSN-38(AUC2.9-fold higher)intheknockoutmice,and roughlysimilarliver concen-trations,resulting in clearlydecreased liver-to-plasma ratiosof bothcompoundsintheknockoutsatvirtuallyalltimepoints(Iusuf etal.,2014).Thisisconsistentwithreduceduptakeofboththese compoundsfrombloodintotheliverintheabsenceofOatp1a/1b transporters,causinghigherplasmalevels.Theamountofplasma SN-38 as a fraction of plasma irinotecan was ∼10% in wild-typemice,and∼23%inknockoutmice.Amedium-termtoxicity
experiment,withdailyi.v.administrationsofirinotecan(30mg/kg) over 6 days resulted in more extensive weight loss in the Oatp1a/1b−/−mice,andamorepronouncedreductioninthewhite bloodcellcountthaninthewild-typemicemeasuredattheendof theexperiment(day7).Assessedatthissameday7,therewassome toxicitytobonemarrow,thymus,andsmallintestineinwild-type mice,butthiswasmuchmorepronouncedinOatp1a/1b−/−mice (Iusuf etal.,2014).Thesedatawereinlinewitha muchhigher plasmaconcentrationofSN-38intheOatp1a/1b−/−micemeasured 24hafterthelastirinotecanadministration,whereastheirinotecan concentrationsatthistimepointwerenotsignificantlydifferent fromwild-typemice.It seemsvery likelythatthehigher expo-suretoSN-38intheOatp1a/1b−/−micewasthemaincauseofthe increasedtoxicity.Themostobviousexplanationforthehigher SN-38exposurewasstronglyreducedhepaticclearanceofSN-38,and likelyalsoofitsprecursoririnotecan,byeliminationofthemouse Oatp1a/1btransporters.
2.2.1. UpregulationofplasmacarboxylesteraseinOatp1a/1b−/− mice
Furtheranalysesrevealed,however,thatthehigherSN-38 expo-surewas not caused solely by a decreased clearance of SN-38 directlyduetoadeficiencyofOatp1a/1btransporters.Itturnedout that,testedexvivo,plasmaofOatp1a/1b−/−micecontainedahighly increasedlevelofirinotecanhydrolaseactivity,whichcausedafar morerapidformationofSN-38fromirinotecanthanobservedin wild-typeplasma. Thisdifference wasmostlikely theresult of thehighlyincreasedexpressionofanumberofcarboxylesterase (Ces)genesintheliverofOatp1a/1b−/−mice,namelyCes1b,Ces1c, Ces1dandCes1e.Ces1c,whichisrelativelyabundant,displayed an80-foldincreaseinRNAlevels(Iusufetal.,2014).Interestingly, themouseCes1cenzyme isknowntobemainlysecretedfrom liverintoplasma,duetotheabsenceofa C-terminal endoplas-micreticulumretentionsignalinitsaminoacidsequence(Holmes etal.,2010).Applicationofthecarboxylesteraseinhibitor bis(4-nitrophenyl)phosphate(BNPP)supportedthatincreasedplasma carboxylesterase activity was responsible for the hydrolysis of irinotecaninOatp1a/1b−/−plasma.Moreover,variousothertested Ces1,Ces2,andCes3familygenes,andgenesforothercandidate plasmaesteraseenzymes likebutyrylcholinesterase,Aadac, and Pon1-3,werenotupregulatedintheOatp1a/1b−/−liver.Altogether, Ces1cupregulationisthusthemostlikelycauseoftheincreased hydrolysisofirinotecaninOatp1a/1b−/−mice.
ThattheincreaseinCes1cexpressionwasadirectconsequence of the functional Oatp1a/1b deficiency wasstrongly supported byanalysisof Cesgene expressionin OATP1B1-and OATP1B3-humanizedtransgenicderivativesofOatp1a/1b−/−mice:thehighly increasedliverexpressionofCes1c,butalsoofCes1b,Ces1dand Ces1e,weremarkedlyreduced,forCes1candCes1dtoevenbelow thelevelofexpressionseeninwild-typemice,bybothtransgenic OATP1B1andOATP1B3(Iusufetal.,2014).Thisindicatesthatthe functionoftheremovedOatp1a/1bgenesresponsiblefor keep-ingliverCes1expressionlowcouldbetakenoverbybothhuman OATP1B1andhumanOATP1B3.Still,theexactmechanism under-lyingtheseCes-regulatoryfunctionsofOatp/OATP1proteinsisas yetunclear.Anobviouspossibilityappearstobeadetoxifying func-tionoftheseproteins,normallyinvolvedinremovingoneormore Ces-inducingcompoundsfromthebody.Suchcompoundsmight beofendogenousorexogenousorigin(dietary,ormetabolitesor evenprimaryproductsformedbytheintestinalmicroflora),or pos-siblyboth.Theirprolongedretentioninthebodymightincrease theoverallliverexposureandthusCesinductionintheknockout strain.Furthersupportfortheinvolvementofadetoxifyingroleof theOatp1a/1bproteinsinCesgeneexpressionemergesfromthe findingthattheknockoutofanumberofotherbroad-specificity detoxifyingproteinsinFVBmice,includingthemultidrugefflux
transportersAbcb1a/1bandAbcg2,andthemultidrug metaboliz-ingCyp3acomplex,causesimilarlevelsofupregulationofhepatic Ces1b,Ces1c,Ces1d,andCes1eexpression(Lagasetal.,2012;Tang etal.,2015,2014).Alternatively,afunctionoftheOATPsinliver uptakeofCes-repressingcompoundsisalsoapossibility.Ifthese compoundsthengetefficientlyclearedthroughalternativeroutes, mayberenalorbymetabolism,theeffectiveliverexposuretothese compoundsmight bereducedin theOatp1a/1bknockoutmice. However,forthemomenttheseoptionsremainspeculative. 2.2.2. IrinotecanstudiesinOATP1B1andOATP1B3humanized mice
Whatever themechanismof Cesupregulation,theincreased plasmalevelsofCes1cinOatp1a/1b−/−micemightconfound inter-pretation of the irinotecan and SN-38 disposition and toxicity resultsinthesemice.Theincreasedconversionofirinotecan to SN-38 could reduceplasma irinotecan levels,and increase SN-38levelsindependentofdirectOatp-mediatedtransport.Forthis reason,amongothers,i.v.irinotecanstudieswerealsoperformed intheOATP1B1andOATP1B3humanizedmice,asthesehavea mostlynormalizedplasmaCes1cactivity.Theresultsobtainedfor irinotecan and SN-38 were strikingly different: the humanized miceshowedhigherplasmalevelsofirinotecancomparedto wild-typemice,butsimilarliverconcentrations,resultinginmarkedly reducedliver-to-plasmaratiosofirinotecan.Asthesedifferences cannotbeattributedtoCesupregulation,theresultssuggestthat thetransgenicOATP1B1andOATP1B3arefarlessefficient(ifat all)intakingupirinotecanintotheliverthanthemouseOatp1a/1b proteins(whichareabsentfromthehumanizedmice).Thisalso explainsthehigherplasmalevelsofirinotecaninthehumanized micecomparedtowild-typemice.
In contrast,for SN-38afteri.v. irinotecan administrationwe foundthattheplasmalevelsinthetransgenicmiceweremarkedly reducedcomparedtoOatp1a/1b−/−mice,butsimilartothosein wild-typemice(Fig.3CandD).Intheorythiscouldeitherbecaused bytheup-anddown-regulationofCes1c,orbychangesinSN-38 clearance.Interestingly,theliverlevelsinalltestedstrainswere thesame,andtheliver-to-plasmaratiosofSN-38wereidentical betweenthewild-typeandtransgenicstrains,butmarkedlyhigher thaninOatp1a/1b−/−mice.Thelatterresultstronglysuggeststhat theliveruptakeofSN-38wascompromisedintheOatp1a/1b−/− mice,andmostlyrestoredbyOATP1B1andOATP1B3inthe human-izedstrains(Fig.3CandD).
2.2.3. SN-38dispositioninOatp1a/1b−/−mice
Inanalternativeapproachtocircumventthecomplicationsof Cesupregulationin theOatp1a/1b−/− mice,SN-38was directly administeredi.v.tothesemice,at1mg/kginviewofthe consider-ablesolubilityandformulationlimitationsofSN-38.Thisresulted insignificantlyhigherplasmalevelsofSN-38shortlyafter admin-istration,andsignificantlylowerliverconcentrationscomparedto levelsinwild-typemice.Areducedshort-termliveruptakeof SN-38wasfurthersupportedbyatwo-folddecreaseinbiliaryexcretion ofSN-38intheOatp1a/1b−/−mice(Iusufetal.,2014).
Collectively,themousestudiesindicatethatbothirinotecanand SN-38areclearedfromplasmabyuptakeintotheliverthrough oneormoreofthemousehepaticOatp1a/1btransporters.In con-trast,thetransgenichumanOATP1B1andOATP1B3canmediate substantialliveruptakeandplasmaclearanceofSN-38,butnotof irinotecan.Theseresultsfitwellwithinvitrostudies,whichfound thatSN-38isreadilytransportedbyhumanOATP1BandOATP1B3, whereasirinotecanisnot(Nozawaetal.,2005;Oostendorpetal., 2009; Yamaguchiet al.,2008).The resultsindicatespecies dif-ferencesinsubstratespecificitybetweenthemouseandhuman hepaticOATP1A/1Bproteins,asexpressedinvivoinmouse hepa-tocytes.Theirinotecan/SN-38toxicitydatainthemousestrainsare
moredifficulttointerpretinviewofthealteredCes1clevelsand rateofSN-38formationinOatp1a/1b−/−mice.However, extrapo-latingfromthedelayedhepaticSN-38clearanceinOatp1a/1b−/− miceanditsreversalbyhumanOATP1B1andOATP1B3,itseems verylikelythatalsoinhumanstheseproteinsareinvolvedinthe detoxification ofSN-38. Thiswould be in linewiththeclinical observationsthatpartialdeficienciesinOATP1B1duetogenetic polymorphismscorrelatewithincreasedirinotecan/SN-38toxicity (Hanetal.,2008;Takaneetal.,2009;Xiangetal.,2006).Thedataof Iusufetal.(2014)stronglysuggestthatthishasprimarilytodowith delayedSN-38clearanceinthesepatientsduetothecompromised functionofOATP1B1.
2.3. Methotrexate
Methotrexate is a folate antimetabolite that is used in the treatmentofseveralimportantcancertypes(breastcancer,lung cancer,head-andneckcancer,non-Hodgkin’slymphoma)(Assaraf, 2007;GonenandAssaraf,2012).Itisalsoused,albeitatusually lower dosage,and mostly orally, totreat non-malignant disor-derssuchasrheumatoidarthritisandpsoriasis(vanOutryveetal., 2002;Wesselsetal.,2008).Methotrexateisabicarboxylicorganic anion,that isknowntobetransported invitrobya number of OATP1A/1Bproteins, includinghuman OATP1B1,OATP1B3, and OATP1A2(Abeetal.,2001;Badagnanietal.,2006;Sasakietal., 2004).Inaddition,agenome-wideassociationstudyshowedthat SNPsinSLCO1B1associatedwithreducedtransportactivitywere linkedtodecreasedplasmaclearanceanddecreased gastrointesti-naltoxicityinchildrenwithacutelymphoblasticleukemia(ALL) treatedwithhigh-dosei.v.methotrexateinfusions(Trevinoetal., 2009).
2.3.1. MethotrexatepharmacokineticsinOatp1a/1bknockout mice
Initialcharacterizationofi.v. methotrexatepharmacokinetics inOatp1a/1b−/−micerevealeddramaticeffectsonplasma clear-anceandliveruptakeofthedrug(vandeSteegetal.,2010).The plasmaAUCwasincreased5-foldinOatp1a/1b−/−mice,andliver concentrationswereabout20-foldreduced.Hepatic methotrex-ateuptakewasveryrapid,with>50%ofthedoseaccumulatingin thewild-typeliverwithin3.5minafteri.v.dosing,whereasonly ∼2%ofthedrugaccumulatedintheliverofOatp1a/1b−/−mice. Ascouldbeexpectedfromthedramaticallyreducedliveruptake ofmethotrexate,thetotalintestinalcontentofi.v.methotrexate, whichmostly derivesfrombiliaryexcretion,wasabout17-fold reduced in the Oatp1a/1b−/− mice. Accordingly, theamount of unchangedmethotrexateexcretedinthefeceswasreducedfrom ∼20%to∼2% ofthedoseadministered.In contrast,theurinary excretionrosefrom∼40%ofthedoseinwild-typemiceto∼100%in Oatp1a/1b−/−mice,indicatingasubstantialreroutingfrom hepa-tobiliarytorenalexcretion.
AssomeOatp1a/1bproteinsarealsoexpressedinthemouse smallintestine(Slco1a4,andtoalowerextentSlco1a5andSlco1a6 RNAweredetected(Chengetal.,2005),anoralmethotrexatestudy wasalsoperformedtoassesspossiblechangesinoralavailability. However,whereasthesystemicplasmaandliverconcentrationsof methotrexatefollowedsimilarpatternsasseenafteri.v. adminis-tration,short-termhepaticportalveinsamplingdidn’tprovideany indicationforareducedrateofintestinaluptakeofmethotrexatein theOatp1a/1b−/−mice.Apparentlyotherintestinaluptakesystems areprimarilyinvolvedintheuptakeofthisbi-anioniccompound.
Infact,despiteextensiveefforts,noonehasthusfardirectly demonstratedforanyOATPsubstratedrugthatitsintestinaluptake is detectably dependentonOatp1a/1b proteins. Directly tested drugsinclude,inadditiontomethotrexate,fexofenadine, pravas-tatin,androsuvastatin(Iusufetal.,2012a,2013;vandeSteegetal.,
2010),aswellasanumberofother,asyetunpublished,drugs.This raisesthebroaderquestionwhetherornotOatp1a/1bproteinsplay anysignificantroleintheintestinaluptakeoforallyadministered substratedrugs.However,therecanbemanycausesfornegative resultsinthisrespect,includingthepossibilityofextensive redun-dancywithoneormoreotherintestinaluptaketransportsystems. Furtherexperimentation willbe neededto resolve the intrigu-ingquestionwhattransportsystemsareprimarilyinvolvedinthe intestinaluptakeofaspectrumofrelativelypolarorchargeddrugs. TheOatp1a/1b−/−micecanalsobeusedtoassesstheefficacy andspecificityofOATP-inhibiting drugs,forinstanceasacause ofdrug–druginteractions. Forthis purposerifampicin,aknown OATPinhibitor,wasadministeredi.v.3minbeforei.v. methotrex-atetowild-typeandOatp1a/1b−/−mice,andmethotrexateplasma and liverconcentrations weredetermined 15minlater (vande Steegetal.,2010).The methotrexateplasmaconcentrationwas increasedthreefoldbyrifampicintreatmentinwild-typemice,to thesamelevels asseen inOatp1a/1b−/− mice.In theknockout mice,rifampicintreatmenthadnoeffectontheplasmalevelsof methotrexate.Theliverconcentrationofmethotrexateinwild-type micewas4-folddecreasedbyrifampicin,toabout9%ofthe admin-istereddose.TheliverconcentrationinOatp1a/1bknockoutmice wasstilllower(∼1%ofthedose),andnotaffectedbyrifampicin treatment.Theseresultsindicatethatrifampicincouldlargely,but notcompletely,inhibitOatp1a/1b-mediatedmethotrexateuptake intotheliver,andthusitsassociatedplasmaclearance.Thelack ofeffectofrifampicinonmethotrexatepharmacokineticsinthe Oatp1a/1b−/−miceindicatesthatitdidnotsignificantlyaffectother methotrexate clearance mechanisms, attesting to its specificity undertheseconditions.
2.3.2. MethotrexatepharmacokineticsinOATP-humanizedmice In a follow-up study, methotrexate pharmacokinetics was analyzed in OATP1B1-, OATP1B3- and OATP1A2-humanized Oatp1a/1b−/−mice(vandeSteegetal.,2013).Asexplained else-wherein thisreview (Section2.1.1),thesethree transgenesare primarilyexpressedinliverparenchymecellsinthesemice,andthe proteinsaresituatedinthebasolateral(sinusoidal)membrane.For OATP1B1andOATP1B3thisisthephysiologicallyrelevant local-ization,butfor OATP1A2it isnot,asin humanliveritis found primarilyincholangiocytes,theepithelialcellsliningthebileducts. Still, inclusionof thelatterstrain allowsanalysisof theinvivo functioningofOATP1A2indruguptake,whichcanberelevantfor assessmentofanydruguptakethatitmaymediateinothertissues andintumorcells.
Methotrexatewasadministeredi.v.attwodifferentdoses(10 and2mg/kg)towild-type,Oatp1a/1b−/−,andthethree human-izedmousestrains, andplasma,liver,andintestine (tissueplus contents) levels of methotrexate were measured 15min after administration(vandeSteegetal.,2013).Asobservedpreviously, at10mg/kgintheknockoutmice,methotrexateplasmalevelswere increased5-fold,whereasliverlevelsweredecreased24-fold,and smallintestinelevels20-fold(Table2).Eachofthethreehumanized transgenespartiallyreversedallofthethreemeasured parame-ters,albeitnottothelevelsseeninwild-typemice.Plasmalevels ofmethotrexatewerereduced∼2-fold,andliverlevelsincreased by4-to9-fold.Smallintestinallevelswere2-to4-foldincreased. Qualitativelyvery similarresults wereobtained atthe 2mg/kg methotrexatedose(Table2).Ingeneral,transgenicOATP1B3and OATP1A2causeda∼2-foldmoreeffectivereversalofliverandsmall intestinalconcentrationsthanOATP1B1,whereastheplasma rever-saleffectsweresimilarbetweenthethreetransgenes.Thepartial reversalbythehumanizedOATP1B1andOATP1B3proteins com-paredtowild-typeparameterscouldrelatetospeciesdifferencesin substratepreferencebetweenmouseandhumanOatp/OATP pro-teins,but alsoto differencesin effective expressionlevel. Also,
Table2
ImpactofOATPtransportersinOatp1a/1bknockoutandOATP1A/1BtransgenicmiceonplasmaconcentrationandtissuedistributionofintravenouslyadministeredMTX(at
10and2mg/kg,15minafteradministration).
Dose Mousestrains Plasmaconcentration(g/ml) Liveramount(%ofdose) Smallintestineamount(%ofdose)
10mg/kg WT 1.55±0.24 30.0±4.1 20.9±1.4 Oatp1a/1b−/− 7.59±0.76 1.24±0.14 0.94±0.17 Oatp1a/1b−/−;1B1tg 4.66±0.38 4.54±1.24 2.48±0.60 Oatp1a/1b−/−;1B3tg 4.35±0.52 9.62±0.69 5.21±1.03 Oatp1a/1b−/−;1A2tg 4.03±1.00 11.3±2.1 5.81±2.56 2mg/kg WT 0.50±0.07 33.0±5.1 12.0±0.6 Oatp1a/1b−/− 1.90±0.29 1.65±0.14 1.62±0.26 Oatp1a/1b−/−;1B1tg 0.93±0.18 5.09±0.41 2.56±0.60 Oatp1a/1b−/−;1B3tg 1.11±0.14 10.2±1.2 3.93±0.51 Oatp1a/1b−/−;1A2tg 0.88±0.11 9.07±0.96 3.76±0.51
in humanliver OATP1B1 and OATP1B3 would presumably act additivelytowardsmethotrexate,andthusmaycauselarger phar-macokineticeffectsthansuggestedbytheeffectsseeninthesingle transgenicstrains.Regardless,thedataclearlyshowthathuman OATP1B1andOATP1B3canhavemajoreffectsontheplasmalevel, hepaticuptakeclearance,andsubsequenthepatobiliary/intestinal excretionofmethotrexate.Moreover,asOATP1B3isalsoexpressed invariousgastrointestinal,hepatocellular,breast,andlungcancers, itmayfurtheraffectsusceptibilityof thesecancerstoOATP1B3 substratedrugs(Abeetal.,2001;Cuietal.,2003;Monksetal., 2007;Mutoetal.,2007).ThedataobtainedforhumanOATP1A2 suggestthatthisproteincouldsubstantiallyaffectmethotrexate uptakeinvivoinotherrelevanttissuesandbarriers,suchasthe blood-brainbarrier,kidneytubules,andtumorcellsthatexpress OATP1A2(Gaoetal.,2000;Leeetal.,2005).
2.3.3. Impactofrifampicinandtelmisartanco-administrationon OATP-mediatedmethotrexatedisposition
Thehumanizedmousestrainsweresubsequentlyusedto fur-therinvestigateOATP-dependentdrug–druginteractions,starting with the inhibitory effect of rifampicin on in vivo methotrex-atetransport by humanOATP1B1 and OATP1B3. Using various humanOATP-overexpressingHEK293cells,Durmusetal.(2015) showedthat rifampicin inhibited methotrexateuptake invitro, withIC50 levelsrangingfrom0.3to0.9M.Inthemouse mod-els,rifampicin(20mg/kg,i.v.)substantiallyinhibitedbothmouse andhumanOATP-mediatedhepaticuptakeandplasmadisposition ofmethotrexate(10mg/kg,i.v.)atclinicallyachievable concentra-tionsforeachofthedrugs.AsshowninTable3,liver-to-plasma ratiosofmethotrexateweredecreased6-to8-foldbyinhibitingthe mouseOatp1a/1bproteins,∼4-foldbyinhibitinghumanOATP1B1 and11-to18-foldbyinhibitinghumanOATP1B3.Thisalsoledto increasedplasmalevelsofmethotrexateby4-to5-foldinmouse Oatp1a/1bbackgroundandupto2-foldinhumanizedOATP1B1or OATP1B3background(Table3).
Althoughthisspecificcombinationofdrugs(methotrexateand rifampicin)wouldberareintheclinic,theseresultsstillsuggest thatmorethanoneOATP-substrateor-inhibitordrugsmightbring theriskofdrug–druginteractionsatthesystemicandhepaticlevel. Thiswouldbeespeciallyimportantforpatientschronicallytaking OATP-interactingdrugs, suchasstatinsandhypertensiondrugs. Moreimportantly,peoplewhoalreadyhaveactivity-reducing poly-morphismsintheirOATPgenes(NakanishiandTamai,2012)might beatincreasedriskforaltereddrugdispositionduetothistype ofdrug–druginteractions,andconsequentlyineffectivetreatment and/orincreasedtoxicity.
Thepossibledrug–druginteractioneffectofaclinicallymore commondrug combination wasinvestigated using a hyperten-sivedrug,telmisartan,andmethotrexate.Thisalsoallowedfurther evaluationoftheapplicabilityofknock-outandhumanizedmouse strainsin drug–druginteractionstudies.Asimilarset-uptothe
rifampicin and methotrexate experiments was chosen, but the doseof telmisartanhad tobekeptlower(7mg/kg)due to sol-ubility issues. In vitro cellular uptake studies resulted in fairly butnotverylowIC50levelsfortelmisartaninhibiting methotrex-ateuptake (<11M),and in vivostudies in the mouse models showedthat telmisartanpre-treatmentdidnot yieldimportant changesinmethotrexatedisposition.Therewasonlyaweak inhi-bitionofOATP1B1-mediatedhepatic uptakeofmethotrexateby telmisartan,leadingto∼2-folddecreasedliver-to-plasmaratiosof methotrexate.However,comparingthesystemictelmisartan lev-els(40–200nM)inpatients(Stangieretal.,2000)withthelevels achievedin mouseplasma (7–13M)suggestsonly avery low risk ofadverse OATP-mediateddrug–druginteractions between telmisartanandmethotrexateintheclinic.Thesefindingsare help-fulforpatientswhoaresimultaneouslytreatedwithmethotrexate andtelmisartan.However,additionalclinicallyrelevant combina-tionsofdrugsthatareOATP-substratesand/or-inhibitorsshouldbe testedtoevaluatetherisksofOATP-mediateddrug–drug interac-tionsintheclinic,especiallyforthesystemicandhepaticeffectson drugconcentrationsduetothefunctionofOATPsinliveruptakeof theirsubstrates.Wesuggestthatthehumanizedtransgenicmouse modelswithliver-specificOATPexpressioncouldbeusedtoobtain amorerealisticviewofthehumansituation.However,itshouldbe notedthatthesemousemodels,whileveryusefultosupportthe developmentofbasicmechanisticinsights,shouldnotbeusedas simpleone-to-onemodelsofdrugbehaviorinhumans.
2.4. Doxorubicin
Doxorubicin,animportanttopoisomeraseIIinhibitordrugused incancertreatment,wasrecentlyshowntobeanOATPsubstrate (Durmusetal.,2014).Doxorubicinisananthracyclineantibiotic, commonly used in the treatment of several cancers, includ-ingHodgkin’sandnon-Hodgkin’slymphoma,multiplemyeloma, leukemia,sarcomaandbreast,ovarian,thyroid,gastricandlung cancers(Cortes-FunesandCoronado,2007;Gewirtz,1999). Vari-ousmodesofactionhavebeendescribedfordoxorubicin,ofwhich twomajormechanismsareintercalationofDNAthatleadsto inhi-bitionofthetopoisomeraseIIenzyme,requiredforsmoothDNA transcriptionandreplication,and,perhapsmorecontroversially, generationoffreeradicalsthatleadstoDNAandcellmembrane damage (Gewirtz,1999).Cardiotoxicity is the majorand dose-limiting side effect of doxorubicin both in adult and pediatric cancerpatients(GrenierandLipshultz,1998).Thus,recenteffortsto improvedoxorubicintreatmentfocusonimprovedanti-tumor effi-cacyanddecreasedcardiotoxicitybydevelopingtumor-targeting strategiessuchasliposomalformulationsofdoxorubicinor devel-opingnewdoxorubicinanalogs(Cortes-FunesandCoronado,2007; Minottietal.,2004).Anotherstrategyforloweringthetoxicityand increasingtheefficacyofdoxorubicinistounderstandthefactors affectingitstissuedisposition,suchasitsinteractionswithdrug
Table3
Impactofrifampicinpre-treatmentontheplasmalevelsandliver-to-plasmaratiosofmethotrexateinWT,OatpknockoutandOATP1Bhumanizedtransgenicstrains.
Mousestrains
Timepoint Parameter Rifampicin WT Oatp1a/1b−/− Oatp1a/1b−/−;1B1tg Oatp1a/1b−/−;1B3tg
5min
Plasmaconcentration(nmol/ml) − 11.9±1.7 46.6±7.4 49.0±15.3 32.2±3.7
+ 50.2±7.8 67.7±3.5 53.6±9.4 62.3±6.9
Rifampicin-inducedfoldincrease 4.2 1.5 1.1 1.9
Liver-to-plasmaratio − 15.5±2.0 0.2±0.0 0.5±0.1 1.7±0.3
+ 1.9±0.3 0.1±0.0 0.2±0.0 0.2±0.0
Rifampicin-inducedfolddecrease 8.2 2.0 2.5 8.5
15min
Plasmaconcentration(nmol/ml) − 6.6±2.7 31.2±13.4 43.1±4.2 19.5±10.3
+ 30.7±12.9 43.9±14.1 46.4±10.0 43.1±18.8
Rifampicin-inducedfoldincrease 4.7 1.4 1.1 2.2
Liver-to-plasmaratio − 27.0±5.9 0.2±0.0 1.1±0.2 5.1±1.4
+ 4.7±2.5 0.2±0.0 0.3±0.1 0.3±0.0
Rifampicin-inducedfolddecrease 5.7 1.0 3.7 17.0
transporters,andutilizetheseinsightstomodifythe pharmacoki-netics,possiblywithtransporterinhibitors.Althoughdoxorubicin hasbeenusedintheclinicforaverylongtime,informationonits interactionwithdruguptaketransportershasbeenverylimited. Okabeetal.(2005)suggestedforthefirsttimethatdoxorubicin mightentercellsviaorganiccationtransporter(OCT6)-mediated activetransport.
Very recently, it was shown that both mouse and human OATP1A/1Bfamilymemberscanmediatecellularuptakeof doxo-rubicin(Durmus etal., 2014).This wasrathersurprising, asits structure,withpropertiesofaweakbase,wasnotconsidereda typ-icalOATPsubstrate.InhumanOATP1A2-overexpressingHEK293 cells,theuptakeofdoxorubicinwas∼2-foldincreasedcompared tocontrolcells,buttherewasnonoticeabletransportbyhuman OATP1B1orOATP1B3invitro.MouseOatp1a/1bproteins trans-porteddoxorubicininvivo,asevidencedbyupto∼2-foldincreased plasmaconcentrationsandupto4-folddecreasedliver-to-plasma ratiosinOatp1a/1b−/−miceafteri.v.doxorubicinadministration (Fig. 3E and F) (Durmus et al., 2014).Moreover,the doxorubi-cin levelsin thesmall intestinal content were alsoreduced in theknockoutmice,suggestingadecreasedbiliaryoutput, proba-blyduetothelowerliverlevels.Interestingly,additionofoneof thehumanOATP1Aor1Btransportersintransgenicmice(on a mouseOatp1a/1bknockoutbackground)rescuedthealtered lev-els seen in the knockout mice to various extents (Fig. 3E and F).Transgenicliver-specificexpressionofhumanOATP1A2could completelyreverse thehepatic uptake and intestinal excretion ofdoxorubicinbacktowild-typelevels,whereasthatofhuman OATP1B1orOATP1B3resultedin partial,but substantialrescue of these phenotypes (Durmus et al., 2014). Only the OATP1B1 orOATP1B3transportersalonewerepresentinthesetransgenic mouselivers,insteadofbothsimultaneouslyasinhumans.The substantialimpactofeithertransporteralonethereforesuggests an even stronger impact of OATP1Bs on doxorubicin pharma-cokineticsin humans,especially affectingplasmaclearance and hepaticuptakecharacteristics.Obviously,fora drugwitha nar-rowtherapeuticindexlikedoxorubicin,thismightbecriticalforits therapeuticefficacyandtoxicityinpatients.Itmaythereforealso beofinteresttoinvestigatepossibleassociationsbetweenOATP transportactivityanddoxorubicinpharmacokinetics,therapeutic efficacy,andtoxicityinpatientcohorts.
2.5. Platinumchemotherapeutics
Cisplatinand morerecent platinum chemotherapeuticssuch ascarboplatinandoxaliplatinareusedtotreatawidespectrum of cancers, including testicular,bladder, lung, ovarian, colorec-tal,cervical,andbreastcancers,aswellassomelymphomasand sarcomas.InaCOMPAREanalysisof60humantumorcelllines(the
well-characterizedNCI-60panel),Lancasteretal.(2013)foundthat highOATP1B3expression,asjudgedbyReal-timeRT-PCR,was sig-nificantlylinkedwithsensitivitytothecytotoxicityof9anticancer drugs,amongstwhichcisplatin,carboplatin,andaplatinum com-poundstructurallyrelatedtooxaliplatin.UnlikeOATP1B1,which wasfoundtobeonlyexpressedinliverand livertumortissue, OATP1B3wasfoundexpressedinarangeofdifferenttissuesand derivedtumortissuesamples,suggestingthatitmightplayarole intumorsensitivitytoplatinumcompounds.
SubsequentinvitrostudiesrevealedthathumanOATP1B3and OATP1B1 canmediate thecellular uptake of Ptupon exposure to cisplatin, and additionally OATP1B3 can mediate cellular Pt uptakeuponexposuretocarboplatinandoxaliplatin.Invivo stud-ieswithcisplatinadministeredintraperitoneallytowild-typeand Oatp1b2−/−miceshowedupto35%reducedliverlevelsofPtinthe knockoutmiceshortlyafteradministration,and2.5-foldhigher uri-naryexcretionofPt,thelatteramountingtomorethan60%ofthe administereddose(Lancasteretal.,2013).Thedatasuggestarapid andsubstantialliveruptakeofafractionofPtbeingmediatedby Oatp1b2inwild-typemice.IntheOatp1b2−/−mice,thisPtfraction mayinsteadbeexcretedprimarilyintotheurine.Asaconsequence, theplasmalevelsofPtarenotmuch differentbetweenthetwo strains.Itshouldbenoted,however,thatcisplatinishighlyreactive, forinstancewithcarbonate,andtheOatp1b2-mediatedPtuptake maythereforewellrepresentuptakeofvariousresultingnegatively chargedPtcomplexesinsteadofcisplatinitself.Cisplatinalsobinds irreversiblytoplasmaproteins,furthercomplicating pharmacoki-netic analyses.Howeverthat maybe, invivo Oatp1b2function doesclearlyaffectthepharmacokinetics ofcisplatin-derived Pt. Extrapolatingtohumanpatients,thiscouldmeanthatvariationsin OATP1B1andOATP1B3expressionandtransportactivityinliver,in othertissues,andespeciallyintumors,mightaffecttheefficacyand toxicsideeffectsofcisplatin-basedchemotherapy.Itwilltherefore beofinteresttoextendpreclinicalstudiesofcisplatinto OATP1B1-and OATP1B3-humanized Oatp1a/1b knockoutstrains, and also includestudies ofcarboplatin andoxaliplatin. It willfurtherbe worthwhiletoassesswhetherpartofthehighinter-individual vari-ationinefficacyandtoxicityofthesedrugsseeninpatientscanin partbeattributedtovariationsinOATPfunction.
2.6. Tyrosinekinaseinhibitors
Tyrosinekinaseinhibitors(TKIs),withtheprimeexample imat-inib (Gleevec), have revolutionized the treatmentof a number ofmalignancies (Drukeretal.,2001).Asof 2015,more than28 TKIs have been FDA-approved (Wu et al., 2015). These ratio-nallydesigned,targetedanticancerdrugscanmakeuseofspecific vulnerabilities of tumor cells, for instance bytargeting specific oncogenicmutationsinproteintyrosinekinases,thusincreasing
disease-specificityandreducingtheriskoftoxicsideeffects. Under-standably,therehasbeensignificantinterestinwhetherTKIsare OATPtransportsubstrates,asthiscouldaffecttheiraccumulation intumorcellsaswellasinvariousimportanttissues,andcontrol theirplasmapharmacokineticsandelimination.Suchparameters canbedecisiveintheoveralltherapeuticefficacyandtolerability ofanticancerandothertherapeuticagents.
Whilemany TKIs havenowbeen testedfor interactionwith OATPs,the invivo relevance for transport of the unconjugated parentcompoundsappearstobelimited.InRNA-injected Xeno-pusoocytes,humanOATP1A2mediatesmodestimatinibuptake, OATP1B3alittle,andOATP1B1none(Huetal.,2008).Initialtests failedtoshowsignificantuptakeofsorafenibandsunitinib medi-atedbyOATP1A2,OATP1B1,andOATP1B3inRNA-injectedXenopus laevisoocytes(Huetal.,2008).However,laterstudieswithhighly OATP-overexpressingHEK293cellsreportedsignificantuptakeof crizotinib, nilotinib,pazopanib, and sorafenib by OATP1B1 and OATP1B3ofimatinib,gefitinib,dasatinib,vandetanib,and vemu-rafenib by OATP1B3 but not by OATP1B1; and of sunitinib by OATP1B1butnotbyOATP1B3(Zimmermanetal.,2013).Itshould benoted,however,thatoftenrelativelevelsofuptakeofTKIs medi-atedbyOATPin thevariousinvitromodelsystemsweresmall comparedtothoseoftheanionicOATPcontrolsubstrates. Mod-erntargetedTKIsarerarely,ifatall,designedtobeanionic,given theusualrequirementsofgoodoralavailability(intestinal absorp-tion)andefficienttumorcellpenetrationofthesecompounds.A possiblefurthercauseofdiscrepanciesbetweeninvitroandinvivo OATP-mediatedtransportresultsforcertaindrugsisdiscussedlater inSection4ofthisreview.
2.6.1. Invitroandinvivostudieswithsorafenib
TheTKI sorafenibwas furtherinvestigatedin vivoin mouse models because it was readily transported by both OATP1B1 andOATP1B3invitro.Unlikeitsconjugate,sorafenib-glucuronide, which will be discussed later, the plasma pharmacokinetics of parent sorafenib, or its relative accumulation in the liver,was notsubstantially altered in eitherOatp1b2−/− mice(DBA back-groundstrain),orinOatp1a/1b−/−mice(FVBbackgroundstrain) afteradministrationof10mg/kgoralsorafenib(Zimmermanetal., 2013).Apartialexplanationmightbethatinvitrosorafenibwas poorlytransportedbymouseOatp1b2.However,sorafenib trans-portbythetwootherprominentsinusoidaluptake transporters inthemouseliver,Oatp1a1andOatp1a4,wasnottestedinvitro, soitremainsuncertainwhetherornotthereisaninvitro/invivo discrepancy(Zimmermanetal.,2013).
SomeTKIshavebeenfurtherevaluatedfortheirabilitytoinhibit humanOATP1B1invitroandinvivo(Huetal.,2014).These stud-iesweretriggeredbyclinicalobservationsthatco-administration ofseveralTKIscanincrease thesystemic exposuretodocetaxel incancerpatients.Theunderlyingmechanismwaspoorly under-stood,but,asdocetaxelclearance mayin partdependonOATP function(seeSection2.1.2),itmightinvolveinhibitionofOATPsby theseTKIs(Huetal.,2014).Nearlyall16testedTKIsinhibitedE2G uptakebyhumanOATP1B1invitrowhenappliedat10M,and4 oftheseTKIs(axitinib,nilotinib,pazopanib,andsorafenib)bymore than10-fold.Interestingly,threeofthese(axitinib,pazopanib,and sorafenib) are known to increase the docetaxel AUC by up to 50%ormorewhenco-administeredinpatients(clinicaldatafor nilotinib/docetaxel co-administrationare not available).Further analysisofsorafenibshowedthatitinhibiteddocetaxeluptakeby OATP1B1invitrowithanIC50below100nM,andveryextensively whenappliedat10M.MouseOatp1b2showedsimilarinhibition ofE2Ganddocetaxeltransportbysorafenibinvitro.Invivo, how-ever,nosignificanteffectofhigh-dose(60mg/kg)oralsorafenib co-administrationcouldbedemonstratedon10mg/kgi.v. doce-taxelplasmaCmaxorAUCinwild-type,Oatp1b2−/−,Oatp1a/1b−/−,
or in OATP1B1-humanized mice. Moreover, multiple sorafenib administrationsdidnotelicitsignificanteffects(Huetal.,2014). Ofnote,inthesespecificexperiments(seealsoSection2.1.2),the effectsofOatp1b2knockoutorOATP1B1transgenicsondocetaxel AUCwererelativelymodest(about2-fold).Thismayhaverendered theseexperimentslesssensitivetopickingupsmall pharmacoki-neticeffectsofsorafenib-mediatedinhibition.Theabsenceofan obviouspharmacokineticinteractionbetweensorafeniband doce-taxelinthevariousmousestrainssuggeststhattheremaybeother factorsinmice,perhapsalternativeorcompensatorymechanisms that can sufficientlyoffset anychanges in OATP1B-like activity towardsdocetaxelclearance.Heretoo,onehastoconsiderthe pos-sibilitythatinteractionsofOATPswithcertaindrugsmaydepend onthecellularcontextinwhichtheyareexpressed(inthiscase mousehepatocytes).Howeverthatmaybe,atthepresenttimeit remainsuncertainwhetherornottheobservedclinical pharma-cokineticinteractionbetweensorafenibanddocetaxelismediated throughOATP1B1,orratherbysomeothermechanism(s).
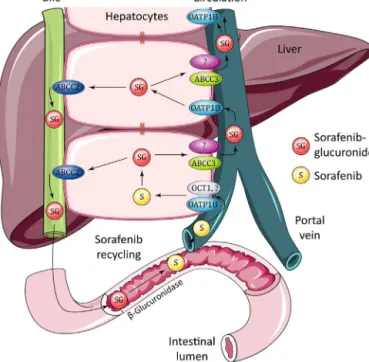
3. Hepatocytehoppingofaconjugatedanticancerdrug:
Thecaseofsorafenibglucuronide
In contrast to the nearly absent effect of Oatp1b2 or Oatp1a/1bdeficiencyonthepharmacokineticsoforally adminis-teredsorafenib,inthesameexperimentsapronouncedincrease in plasma levels of sorafenib glucuronide was observed, with a 5.5-fold increased AUC in Oatp1b2−/− mice, and 29-fold in Oatp1a/1b−/−mice;althoughthebiggerrelativeincreaseinthe lat-tercasemainlyarosefromasubstantiallylowerAUCofsorafenib glucuronideinwild-typeFVBmicethaninwild-typeDBAmice, withtheplasmaAUCsinboththeknockoutstrainsbeingsimilar (Zimmerman et al., 2013). The plasma levels of sorafenib glu-curonidein theknockoutstrainsequaled orsurpassed thoseof parentalsorafenib itself, indicating thequantitativeimportance of this process. Subsequent invitro experiments indicated that sorafenibglucuronidewasefficientlytakenupbymouseOatp1b2 and humanOATP1B1 and OATP1B3expressed inHEK293 cells. Furthermore,transgenicexpressionofeitherhumanOATP1B1or OATP1B3intheliverof“humanized”Oatp1a/1b−/−miceresulted inapartialreversaloftheincreasedplasmasorafenibglucuronide levels(Zimmermanetal.,2013).Thisconfirmedaprominentrole ofthemouseandhumansinusoidalOATPtransportersinhepatic uptakeofsorafenibglucuronide.
An important question wasthe origin of the sorafenib glu-curonide,asit isknownthat both enterocytesandhepatocytes can have substantial drug glucuronidation capacity, and oral sorafenib might undergo rapid glucuronidation in the intesti-nalwalluponabsorption.However,mouseintestinalmicrosomes showedonlymarginal sorafenibglucuronidationcapacity, com-paredtomouselivermicrosomes(>50-folddifference),suggesting that most sorafenib glucuronide had been formed in the liver (Zimmerman et al., 2013). Taken together, these findings sug-gestedstronglythatundernormalconditionsasubstantialpartof sorafenibglucuronideformedintheliverisextrudedacrossthe sinusoidalmembraneintotheblood,andthentakenupagaininto theliverbyOatp1b2,OATP1B1,andOATP1B3.
3.1. Hepatocytehoppingofbilirubinglucuronide
This process inferred to explain the behavior of sorafenib glucuronidewashighly reminiscent ofthepreviously proposed “hepatocyte hopping” process for the endogenous metabolite bilirubinglucuronide(Iusufetal.,2012c;vandeSteegetal.,2012, 2010).The hydrophobic,very poorly water-soluble,and poten-tiallyhighlytoxicunconjugatedbilirubin,theprimarybreakdown