Full Terms & Conditions of access and use can be found at
http://www.tandfonline.com/action/journalInformation?journalCode=irnf20
Renal Failure
ISSN: 0886-022X (Print) 1525-6049 (Online) Journal homepage: http://www.tandfonline.com/loi/irnf20
Ionic High-Osmolar Contrast Medium Causes
Oxidant Stress in Kidney Tissue: Partial Protective
Role of Ascorbic Acid
Meltem Çetin, Erdinç Devrim, Sibel Serin Kiliçoglu, Imge B. Ergüder, Mehmet
Namuslu, Recep Çetin & Ilker Durak
To cite this article: Meltem Çetin, Erdinç Devrim, Sibel Serin Kiliçoglu, Imge B. Ergüder, Mehmet Namuslu, Recep Çetin & Ilker Durak (2008) Ionic High-Osmolar Contrast Medium Causes Oxidant Stress in Kidney Tissue: Partial Protective Role of Ascorbic Acid, Renal Failure, 30:5, 567-572, DOI: 10.1080/08860220802064739
To link to this article: https://doi.org/10.1080/08860220802064739
Published online: 07 Jul 2009.
Submit your article to this journal
Article views: 115
ISSN: 0886-022X print / 1525-6049 online DOI: 10.1080/08860220802064739
567
LRNF
LABORATORY STUDY
Ionic High-Osmolar Contrast Medium Causes Oxidant Stress
in Kidney Tissue: Partial Protective Role of Ascorbic Acid
Contrast Medium Causes Oxidant Stress in Kidney Tissue
Meltem Çetin
Ankara Oncology Teaching and Research Hospital, Radiology Clinics, Ankara, Turkey Erdinç Devrim
Ankara University Faculty of Medicine, Biochemistry Department, Ankara, Turkey Sibel Serin Kiliçoglu
Ufuk University Faculty of Medicine, Histology & Embryology Department, Ankara, Turkey
Imge B. Ergüder and Mehmet Namuslu
Ankara University Faculty of Medicine, Biochemistry Department, Ankara, Turkey Recep Çetin
Ankara Diskapi Teaching and Research Hospital, General Surgery Clinics, Ankara, Turkey
Ilker Durak
Ankara University Faculty of Medicine, Biochemistry Department, Ankara, Turkey
It has been known that contrast medium may cause con-trast-induced nephropathy in risk groups. This study sought to establish possible effects of ionic high-osmolar contrast medium administration with or without antecedent cisplatin treatment on oxidant/antioxidant status in rat kidney tissues, as well as to investigate a possible protective role of antioxidant ascorbic acid in this regard. Thirty-five female, 14-week-old Wistar-albino rats were used in this study. They were divided into five groups of seven rats (sham, contrast, contrast + ascorbic acid, contrast + cisplatin, and contrast + cisplatin + ascorbic acid). Ascorbic acid was given in a dose of 250 mg/kg/day orally throughout the study period, and cisplatin (10 mg/kg) as a single i.v. dose on the fourth day. Ionic high-osmolar contrast medium (3 gr/kg iodine as a single dose) was administered by i.v. route on the fifth day. After the animals were sacrificed on the sixth day, their kidney tissues were removed surgically to be used in the analyses. Malondialdehyde (MDA) level and
activities of antioxidant (superoxide dismutase [SOD], glutathione peroxidase [GSH-Px] and catalase [CAT]) and oxidant (xanthine oxidase [XO]) enzymes were measured in these samples. Serum urea and creatinine levels were mea-sured to evaluate kidney functions. Histopathological investi-gation of the tissues was also performed. It was observed that contrast medium administration caused increases in MDA levels in the kidney tissues, either alone or together with antecedent cisplatin treatment. However, ascorbic acid prevented the increases in MDA levels in the kidney tissues. Histopathological findings revealed that ionic high-osmolar contrast medium administration alone led to mild acute structural damage, but contrast medium administration together with antecedent cisplatin usage caused severe tubular necrosis. Ascorbic acid supplementation prevented these changes, to a great extent. The results suggest that ionic high-osmolar contrast medium administration, either alone or together with antecedent cisplatin treatment, leads to acceler-ated oxidative reactions in rat kidney tissues, and ascorbic acid protects in part the kidney tissues against this oxidant stress.
Keywords contrast medium, cisplatin, nephrotoxicity, oxidant/ antioxidant status, ascorbic acid
Address correspondence to Prof. Dr. Ilker Durak, Ankara Üniversitesi Tip Fakültesi Biyokimya Anabilim Dali, Dekanlik Binasi, Sihhiye, 06100 Ankara, Turkey; Tel.: +90 312 3116457; Fax: +90 312 3106370; E-mail: ilker_durak@yahoo.com
568 M. Çetin et al. INTRODUCTION
Contrast medium-associated or -induced nephropathy (CIN) is known as an important cause of acute renal failure requiring hospitalization. Contrast-induced nephr-opathy is defined as an acute worsening of renal function following the administration of a contrast medium in the absence of any other known reason. It is characterized by an increase in serum creatinine of more than 25% above baseline level within 48 hours. The risk factors for CIN are preexisting renal failure, presence of diabetes mellitus (DM), volume of contrast medium used, dehydration, congestive heart failure, advanced age, and simultaneous usage of nephrotoxic drugs.[1] Renal medullary ischemia following contrast-induced intrarenal vasoconstriction, direct cytotoxicity, oxidative tissue damage, and apopto-sis are possible pathophysiological mechanisms implied for CIN.[2] Recently, various physico-chemical properties of contrast media, such as osmolality, ionic/nonionic, and viscosity, have been thought to contribute to CIN.[3]Some antioxidant agents like N-acetylcysteine (NAC) and ascorbic acid have been thought to protect the kidney against CIN through their antioxidant properties, as oxidative tissue damage is one of the possible pathophysiological mechanisms.[1] In a recent study, it has been concluded that volume supplementation by sodium bicarbonate plus NAC is a better strategy than the combination of normal saline with NAC alone or together with ascorbic acid in patients at medium to high risk for CIN.[4]
There is a balance between oxidant agents and anti-oxidant defense mechanisms under normal conditions, and oxidative stress occurs if this balance is disturbed. Reactive oxygen species (ROS) such as superoxide anion radicals (O2−) are known as oxidant agents, and ischemia-reperfusion injury is an important cause of oxidative stress.[5] Malondialdehyde (MDA), a lipid peroxidation end product, is an indicator of oxidative stress.[6] Xanthine oxidase (XO) is an oxidant enzyme catalyzing oxidation of hypoxanthine and xanthine and can produce O2−.[7] Superoxide dismutase (SOD) catalyzes the dismu-tation of O2− to hydrogen peroxide (H2O2), and catalase (CAT) and glutathione peroxidase (GSH-Px) are the enzymes that catalyze the conversion of H2O2 to water. SOD, CAT, and GSH-Px are known as endogenous antioxidant enzymes.[8] Ascorbic acid is a water soluble antioxidant vitamin.[9]
This study sought to investigate the effects of ionic high-osmolar contrast medium administration with or without antecedent cisplatin treatment on oxidant/antioxi-dant status in rat kidney tissues. A possible protective role of ascorbic acid as an antioxidant agent against these effects was also investigated.
MATERIALS AND METHODS Contrast Medium
High osmolar and ionic sodium ioxithalamate + meglumine ioxithalamate (Telebrix 35 produced by
Guerbet AG) having 350 mg iodine per milliliter was used
as a contrast medium in this study.
Animal Care and Treatment
Thirty-five female Wistar-albino rats with an age of 14 weeks were obtained from Laboratory Animals Unite of Ankara Teaching and Research Hospital. Their mean weights were 200 ± 10 g. They were divided into five separate treatment groups of seven rats each (i.e., control, contrast, contrast+ascorbic acid, contrast + cisplatin and contrast+cisplatin+ascorbic acid; groups 1–5, respec-tively). Ascorbic acid was given in a dose of 250 mg/kg/day orally together with water to the animals in groups 3 and 5 throughout the study period.[10] Cisplatin (10 mg/kg) was administered in a single i.v. dose on the fourth day to the animals in groups 4 and 5.[11] The contrast medium was given as a single dose in a volume of 8.5 mL/kg (approxi-mately 3 gr/kg iodine load) by i.v. route on the fifth day except to the animals in the control group, which were given physiological serum as vehicle in the same volume.[12] The animals were given ketamine – HCl (100 mg/kg) and sacrificed on the sixth day. Then, their kidney tissues were removed surgically. Blood samples were also obtained from inferior vena cava of the animals just before sacrification.
Biochemical Analysis
MDA level and activities of antioxidant (SOD, GSH-Px and CAT) and oxidant (XO) enzymes were measured in the kidney tissues. The tissues were first homogenized in physiological saline (1 gram in 5 ml) and then were centri-fuged at 4000 × g. Upper clear supernatants were removed and used in the analyses. Protein levels of the supernatants were measured by using the Lowry’s method and adjusted to equal concentrations before analyses.[13] MDA levels were measured by the thiobarbituric acid reactive sub-stances (TBARS) method.[14] XO activity was determined by measuring uric acid formation from xanthine substrate at 293 nm.[15] GSH-Px activity was measured by following changes in NADPH absorbance at 340 nm.[16] CAT activity was determined by measuring the decrease of H2O2 absor-bance at 240 nm.[17] In the activity calculations (IU, international unit), extinction coefficients of uric acid,
H2O2 and NADPH were used for XO, CAT, and GSH-Px, respectively. SOD activity was measured by a method based on nitro blue tetrazolium (NBT) reduction rate. One unit for SOD activity was expressed as the enzyme protein amount causing 50% inhibition in NBT reduction rate.[18] Serum urea and creatinine levels were measured by routine spectrophotometric methods.
Histopathological Investigation
The tissue samples were obtained from the kidneys and fixed in 10% neutral buffered formalin solution for five days. Tissues were washed in flowing water and dehy-drated with increasing concentrations of ethanol (50%, 75%, 96%, and 100%). After dehydration, specimens were put into xylene to obtain transparency and were then infiltrated with and embedded in paraffin. Histological sections (4 μm) of the kidney from all the treated groups were stained with hematoxylin and eosin. Histopathological examinations were performed and photographed by Nikon eclipse E 600. The microscopic scoring of the kidney sec-tions was carried out in a blinded fashion by a histologist who was unaware of the treatment groups and assigned a score that represents the approximate extent of necrotic area in the cortical proximal tubules on a scale of 0–4 (0: no necrosis, 1: a few focal necrotic spots, 2: about half of the area is necrotic, 3: about two-thirds of the area is necrotic, 4: almost the entire area is necrotic).[19]
Statistics
Data were expressed as arithmetic mean ± standard deviation (mean ± SD). In the statistical evaluation of the results, one-way analysis of variance (ANOVA) and post-hoc LSD tests were used to determine the differences between the groups. Values of p lower than 0.05 were considered significant.
RESULTS
The biochemical results are presented in the Table 1. As shown in the table, MDA levels were found to increase significantly in groups 2 and 4 as compared to the control group. When animals as in groups 3 and 5 were under treatment with ascorbic acid, this increase could be prevented. However, there were no significant differences between the enzyme activities in the groups. It was observed that contrast medium, when given together with cisplatin, caused important increases in serum creatinine (approximately 36%) and urea (approximately 20%,
though this is not significant) levels as compared with those of the control group.
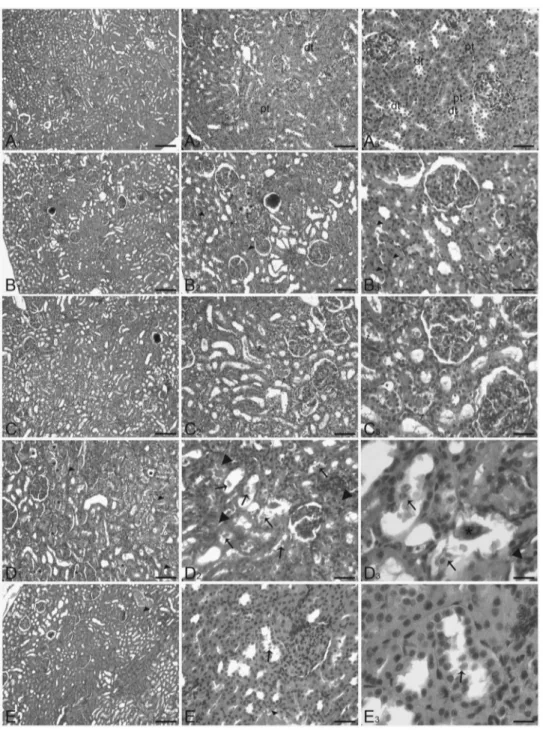
Histopathological findings are shown in Figure 1A–1E. Compared with the control group (see Figure 1A), renal tis-sue sections from the contrast medium-administered group exhibited increased evidence of acute structural damage, characterized by tubular necrosis, degeneration, casts, and red blood cell extravasations. The desquamation of epithe-lial cells and necrosis occurring predominantly in the proximal convoluted tubules were in a few focal necrotic spots (see Figure 1B). Rats treated with both contrast medium and ascorbic acid showed no difference from the control group with the common structure of brush-bordered cuboidal epithelial cells of the proximal convo-luted tubule. The well-preserved histology of the tubules was observed in this group (see Figure 1C). The histopathological changes observed in the kidneys from rats treated with contrast medium and cisplatin revealed severe and widespread tubular necrosis, with a dilation of proximal tubules, desquamation of renal tubular cells, and cast forma-tion in the lumen. Widespread red blood cell extravasaforma-tions were seen in this group (see Figure 1D). A significant reduction in the extent of tubular damage was found in kidney tissue from rats administered both contrast medium and cisplatin and treated with ascorbic acid (see Figure 1E). Qualitative scoring of histopathology for the various treatment groups is summarized in Table 2.
DISCUSSION
It has been known that CIN is an important and increas-ing cause of acute renal injury needincreas-ing hospitalization.[20] Oxidative damage is one of the pathophysiological mecha-nisms thought to play a role in CIN.[2] In the recent years, two important antioxidant agents (i.e., acetylcysteine and ascorbic acid) have been investigated as to whether they have the potential to prevent CIN.[21] In one of those stud-ies, Spargias et al. investigated the protective effects of ascorbic acid against CIN. They showed that CIN occurred in 20% of patients who underwent coronary angiography and/or intervention, whereas only in 9% of patients who were given ascorbic acid before and after the procedure (odds ratio = 0.38; p = 0.02). As a result of that study, the authors concluded that prophylactic usage of ascorbic acid might show protection against CIN in high-risk patients undergoing coronary angiography.[22] We thought that this finding gives promise for the future, but that further studies are needed regarding the protective effects of ascorbic acid against CIN. It is also known that the CIN Consensus Working Panel put ascorbic acid into potentially beneficial drugs in patients at high risk accord-ing to the present evidence.[23]
570 M. Çetin et al.
In this regard, as far as we know, our study is the first one in the literature investigating the possible protective effects of ascorbic acid against CIN in rats administered contrast medium and cisplatin through evaluating oxidant/ antioxidant status. With this aim, oxidative stress markers and histopathological changes in kidneys from rats were examined in the present study. A previous study per-formed in our laboratory showed that cisplatin administra-tion in rats caused significant oxidant stress in the kidney, and black grape as a natural antioxidant can protect the kidneys against cisplatin toxicity.[11] In that study, histo-pathological examination results also revealed significant damage in the kidney tissues from the cisplatin-treated rats, and supplementation of natural antioxidants caused significant improvements.[11] Regarding the subject, Durak et al. previously observed significant increases in serum urea and creatinine levels in guinea pigs seven days after cisplatin treatment.[24] The present study sought to establish the possible effects of contrast medium use on oxidant/antioxidant status in kidneys from rats given cispl-atin as a nephrotoxic drug simultaneously. Additionally, ascorbic acid has been used as an antioxidant agent to evaluate possible protective effects of antioxidants in this situation.
In the current study, it was found that contrast medium administration—either alone or together with antecedent cisplatin treatment—led to significant oxida-tive stress in rat kidney tissues, as shown in Table 1 (increased MDA levels in groups 2 and 4 as compared to that of control group). However, there were no significant changes in the activities of oxidant and antioxidant enzymes, which pointed out that this oxidant stress was a
direct result of contrast medium and cisplatin usage. It has been observed that ascorbic acid protected the kidney tis-sues against this oxidant stress by preventing MDA increases without any change in the enzyme activities, as presented in Table 1. We also found that ionic high-osmolar contrast medium administration together with antecedent cisplatin usage led to increases in serum creatinine and urea levels (approximately 36% and 20%, respectively), indicating an occurrence of CIN in rats at high risk. How-ever, ascorbic acid administration could not prevent the increases in serum creatinine and urea levels. All of these results show that renal failure due to the contrast medium administration is the result of not only oxidant stress, but also of some other factors unknown in detail yet.
According to the histopathological findings of this study, ionic high-osmolar contrast medium administration alone led to mild acute structural damage, but the contrast medium administration together with antecedent cisplatin treatment caused severe tubular necrosis, indicating an occurrence of CIN in rats at high risk. However, ascorbic acid supplementation prevented these changes to a great extent in the both groups (see Table 2 and Figure 1). As emphasized above, in our previous study, we observed histopathologically that cisplatin administration led to sig-nificant damage in kidney tissues, and that the supplementa-tion of natural antioxidants prevented this damage.[11]
All of these biochemical and histopathological find-ings in the present study are in agreement with the results of CIN Consensus Working Panel on the beneficial role of ascorbic acid in the contrast treated patients at high risk.[23] However, as established from increased blood urea and creatinine levels in the ascorbic acid- and
Table 1
Parameters in serum and kidney tissues from rats
Parameters Control (1) Contrast (2)
Contrast + ascorbic
acid (3) Contrast + cisplatin (4)
Contrast + cisplatin + ascorbic acid (5) Kidney MDA (nmol/mg) 0.553 ± 0.068*† 0.706 ± 0.127 ‡§ 0.540 ± 0.065 0.699 ± 0.143储 0.549 ± 0.100 XO (mIU/mg) 0.153 ± 0.011 0.158 ± 0.007 0.159 ± 0.013 0.150 ± 0.013 0.143 ± 0.014 SOD (U/mg) 47.87 ± 3.61 44.25 ± 4.18 45.03 ± 3.62 43.04 ± 1.69 40.94 ± 9.07 GSH-Px (mIU/mg) 92.03 ± 12.06 95.39 ± 12.46 85.01 ± 11.37 92.03 ± 7.22 92.44 ± 8.25 CAT (IU/mg) 85.30 ± 8.22 77.44 ± 13.11 76.16 ± 8.14 68.03 ± 7.43 69.31 ± 8.99 Serum Creatinine (mg/dL) 0.44 ± 0.09† 0.39 ± 0.03§ 0.36 ± 0.07 0.60 ± 0.21 0.64 ± 0.08 Urea (mg/dL) 10.91 ± 1.48 8.93 ± 2.64§ 7.94 ± 2.37 13.04 ± 5.53 13.69 ± 5.69 Mean ± SD; n = 7 in each group. Note: To convert serum creatinine in mg/dL to μmol/L, multiply by 88.4; urea in mg/dL to mmol/L, multiply by 0.167.
*1 vs. 2, †1 vs. 4, ‡2 vs. 3, §2 vs. 5, 储4 vs. 5: p < 0.05 according to ANOVA and post-hoc LSD test.
Abbreviations: CAT = catalase, GSH-Px = glutathione peroxidase, MDA = malondialdehyde, SOD = superoxide dismutase, XO = xanthine oxidase.
contrast-treated groups, ascorbic acid could not prevent contrast-induced renal failure, which showed that oxidant stress was not a single factor in this ailment.
In light of these findings, it is suggested that the supplementation of antioxidant agents such as ascorbic acid before and during ionic high-osmolar contrast medium
Figure 1. Light microscopy section of kidney. (A) Control rats (control group) showed no abnormalities, proximal tubules (pt), distal tubules (dt). (B) Rats treated with contrast medium showed epithelial cell swelling, desquamation (arrow), casts (*), necrosis, and red blood cell extravasations (arrow head) occurring predominantly in the proximal convoluted tubules. (C) Rats treated with contrast medium and ascorbic acid revealed a very mild degeneration of tubular epithelial cells (arrow). (D) Rats treated with contrast medium and cisplatin showed typical severe tubular necrosis with swelling, desquamation (arrow), casts (*) of proximal tubules. Red blood cell extravasation (arrow head) was clear. (E) Rats that received ascorbic acid treated with contrast medium and cisplatin showed a marked reduction in the extent of tubular damage. Very few epithelial desquamation (arrow) of proximal tubules epithelial cells. Scale bar: A1, B1, C1 = 40 μm, A2, B2, C2, D1, E1 = 20 μm, A3, B3, C3, D2, E2 = 10 μm, D3, E3 = 4 μm.
572 M. Çetin et al.
administration to ones at high risk may protect in part against the occurrence of CIN by preventing oxidative reactions.
REFERENCES
1. Soma VR, Cavusoglu E, Vidhun R, Hrishman W, Sharma SK. Contrast-associated nephropathy. Heart Disease. 2002;4:372–379.
2. Oudemans-van Straaten HM. Contrast nephropathy, pathophys-iology and prevention. Int J Artif Organs. 2004;27:1054–1065. 3. Davidson C, Stacul F, McCullough PA, et al. Contrast
medium use. Am J Cardiol. 2006;98:42K–58K.
4. Briguori C, Airoldi F, D'Andrea D, et al. Renal Insufficiency Following Contrast Media Administration Trial (REME-DIAL): A randomized comparison of three preventive strate-gies. Circulation. 2007;115:1211–1217.
5. Shah AM, Channon KM. Free radicals and redox signalling in cardiovascular disease. Heart. 2004;90:486–487.
6. Jackson MJ. An overview of methods for assessment of free radical activity in biology. Proceedings of the Nutrition Soci-ety. 1999;58:1001–1006.
7. Fang Y-Z, Yang S, Wu G. Free radicals, antioxidant, and nutrition. Nutrition. 2002;18:872–879.
8. McCord JM. The evolution of free radicals and oxidative stress. Am J Med. 2000;108:652–659.
9. Carr AC, Frei B. Toward a new recommended dietary allow-ance for vitamin C based on antioxidant and health effects in humans. Am J Clin Nutr. 1999;69:1086–1107.
10. Ueta E, Tadokoro Y, Yamamoto T, et al. The effect of cigarette smoke exposure and ascorbic acid intake on gene expression of antioxidant enzymes and other related enzymes in the livers and lungs of Shionogi rats with osteo-genic disorders. Toxicol Sci. 2003;73:339–347.
11. Çetin R, Devrim E, Kiliçoglu B, Avci A, Çandir Ö, Durak I. Cisplatin impairs antioxidant system and causes oxidation in rat kidney tissues: protective roles of natural antioxidant foods. J Appl Toxicol. 2006;26:42–46.
12. Lee H-C, Yen H-W, Sheu S-H. Effects of different contrast media on glutathione peroxidase and superoxide dismutase activities in the heart and kidneys of normal and streptozotocin-induced diabetic rats. J Formos Med Assoc. 2006;105:530–535.
13. Lowry O, Rosenbrough N, Farr L, Randall R. Protein measurement with folin phenol reagent. J Biol Chem. 1951;182:265–275.
14. Dahle LK, Hill EG, Holman RT. The thiobarbituric acid reaction and the autoxidations of polyunsaturated fatty acid methyl esters. Arch Biochem Biophys. 1962;98:253–261. 15. Hashimoto S. A new spectrophotometric assay method of
xanthine oxidase in crude tissue homogenate. Anal Biochem. 1974;62:426–435.
16. Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–169.
17. Aebi H. Catalase. In: Bergmayer HU, ed. Methods of enzymatic analysis. New York and London: Academic Press Inc.; 1974:673–677.
18. Durak I, Canbolat O, Kavutcu M, Öztürk HS, Yurtarslani Z. Activities of total, cytoplasmic and mitochondrial superox-ide dismutase enzymes in sera and pleural fluids from patients with lung cancer. J Clin Lab Anal. 1996;10:17–20. 19. Baliga R, Ueda N, Walker PD, Shah SV. Oxidant
mecha-nisms in toxic acute renal failure. Drug Metab Rev. 1999;31:971–997.
20. Feldkamp T, Baumgart D, Elsner M, et al. Nephrotoxicity of iso-osmolar versus low-osmolar contrast media is equal in low risk patients. Clin Nephrol. 2006;66:322–330.
21. Briguori C, Marenzi G. Contrast-induced nephropathy: pharmacological prophylaxis. Kidney Int Suppl. 2006; 69:S30–S38.
22. Spargias K, Alexopoulos E, Kyrzopoulos S, et al. Ascorbic acid prevents contrast-mediated nephropathy in patients with renal dysfunction undergoing coronary angiography or inter-vention. Circulation. 2004;110:2837–2842.
23. Stacul F, Adam A, Becker CR, et al. Strategies to reduce the risk of contrast-induced nephropathy. Am J Cardiol. 2006;98:59K–77K.
24. Durak I, Özbek H, Karaayvaz M, Öztürk HS. Cisplatin induces acute renal failure by impairing antioxidant system in guinea pigs: Effects of antioxidant supplementation on the cisplatin nephrotoxicity. Drug Chem Tox. 2002;25:1–8.
Table 2
Semi-quantitative evaluation of histopathological changes in renal tubules of kidneys from rats
Group Treatment Score
1 Control 0+
2 Contrast medium 1+
3 Contrast medium + ascorbic acid 0+
4 Contrast medium + cisplatin 3+