ARTICLE
The determination of molecular scattering differential cross
sections for compounds of some essential elements at 3.38 Å
−1
photon momentum transfer
Burcu Akça and Salih Erzeneog˘lu
Abstract: Molecular scattering differential cross sections of 59.5 keV␥-rays have been measured for some compounds of Na, Mg,
Al, Ca, and Fe elements at 90°. The␥-rays of Am-241 were counted using a Si(Li) detector of EDXRF (energy dispersive X-ray fluorescence) system. The experimental results have been compared with non-relativistic and relativistic theoretical values. Key words: essential elements, molecular scattering differential cross sections, energy dispersive X-ray fluorescence system.
Résumé : Nous mesurons les sections efficaces différentielles pour des molécules bombardées par rayons␥ de 59.5 keV, pour des
composés de Na, Mg, Al, Ca et Fe a` 90°. Les rayons␥ émanant de241Am sont comptés par un détecteur Si(Li) d’un système EDXRF
(analyse dispersive en énergie en fluorescence X). Nos résultats expérimentaux sont comparés a` des valeurs théoriques non relativistes et relativistes. [Traduit par la Rédaction]
Mots-clés : élément essentiel, section efficace différentielle moléculaire, analyse dispersive en énergie en fluorescence X. PACS Nos.: 32.90.+a, 32.30.-r, 32.10.-f, 33.20.-t, 34.50.-s, 33.15.-e.
1. Introduction
The coherent and incoherent scattering interaction of photons with matter is crucial for obtaining information about the char-acteristics and structure of samples. The scattering of␥-rays by atoms has been the subject of significant theoretical and experi-mental interest. A knowledge of Rayleigh and Compton scattering of photons, especially at low energies (10–100 keV), has been added to the total cross section because of its application in various fields [1]. Determining differential scattering cross sections for X- and␥-rays is useful in studying radiation attenuation, transport, and energy deposition, and has an important role in medical physics, reactor shielding, and industrial radiography, in addition to X-ray crystallog-raphy [2].
According to the National Committee for Clinical Laboratory Standards (NCCLS); Na, Mg, Ca, and Fe are essential-major elements, but Al is a non-essential toxic element [3]. It is known that essential elements play an important role in a number of biological processes. An element is deemed essential firstly, if, without it, the species cannot achieve normal, healthy growth or complete its normal life cycle; and secondly, if it is part of a molecule of an essential constit-uent or metabolite. Moreover, the element must be specific and not be replaceable by another, and it must exert its effect directly on growth or metabolism and not by some indirect effect, such as an-tagonism of another element present at toxic levels [4]. Toxic ele-ments are heavy eleele-ments that should not be present in the human organism (e.g., Tl, Hg, Al, Cd, and Ba) [5]. According to the literature, the following several studies are relevant to experimental molecular scattering differential cross sections. Coherent and incoherent scat-tering cross sections of 59.5 keV␥-rays were measured using a HPGe detector on elements in the range of 13 ≤ Z ≤ 50 [2]. Abnormal scat-tering effects in elastic photon–atom scatscat-tering for biomedically im-portant elements have been discussed in the energy range 1–100 keV
[6]. The low-angle scattering properties of some tissues and tissue-equivalent materials have been studied [7]. The molecular scattering differential cross sections of 59.5 keV␥-rays for La2O3, CeO2, and Nd2O3were determined in the angular range of 60°–120° [8]. The angular distribution of photons scattered by healthy and cancerous human breast tissue and tissue-equivalent materials have been ob-tained for 17.44 and 6.93 keV photon energies [9]. The incoherent scattering functions have been calculated from the measured differ-ential incoherent scattering cross sections of Fe, Cu, Zr, Sn, Ta, W, Au, and Pb at 661.6 keV photon energy [10]. The new small-angle coherent scattering measurements have been presented to empha-size the need to update available open-access databases [11]. The mass attenuation coefficients have been determined for low atomic num-ber samples based on Rayleigh to Compton scattering ratio (R/C) and the effective atomic number [12]. The Rayleigh to Compton scatter-ing cross section ratios have been determined for 6 ≤ Z ≤ 82 elements at 145 keV photon energy and 50°, 70°, and 90° scattering angles [13]. According to the literature, there are no experimental data for molecular scattering differential cross sections for these com-pounds at 59.5 keV photon energy and 90° scattering angle; this study presents the first experimental data. The aim of this work is to ameliorate this deficiency of the literature and create a basis for other studies. Our experimental results have been compared with relativistic theory (RT) of F(q, Z) and non-relativistic theory (NRT) of F(q, Z) and S(q, Z). In these accounts, F(q, Z) and S(q, Z) values were obtained using the interpolation method from tables in the liter-ature [14–16] at 3.38 Å−1photon momentum transfer.
2. The theoretical basis of molecular scattering
differential cross sections
The resulting measurements for a compound or mixture can be expressed in terms of a molecular differential scattering coefficient.
Received 3 December 2015. Accepted 2 March 2016.
B. Akça. Computer Engineering, Faculty of Engineering, Ardahan University, Ardahan, Turkey. S. Erzeneog˘lu. Department of Physics, Faculty of Sciences, Atatürk University, Erzurum, Turkey. Corresponding author: Burcu Akça (email address:burcuakca@ardahan.edu.tr).
This quantity represents the probability that a photon with inci-dent energy E is scattered at an angle per unit length of the photon beam path.
When a sample is reputed to be mono-molecular with a molec-ular weight M, the theoretical molecmolec-ular differential scattering coefficient, which is the probability of a photon being scattered per unit length of beam path, takes the form [17]
s(E, q)⫽ NA
冋
dTH() d⍀兺
i wi AiFi 2 (q)⫹dKN(E,) d⍀兺
i wi AiSi(q)册
(1) where NAis the Avogadro constant, is the sample density, Fi(q) isthe molecular form factor of the ith element in the material, Si(q)
is the molecular incoherent scattering function, and q is the pho-ton momentum transfer given by
q⫽sin(/2)
(2)
The photon momentum transfer defines transferred momen-tum to the atom by the scattered photon. Here is the angle of scattering, and is the wavelength of the incident beam. In(1), the first term in the main brackets refers to the coherent and the second to the incoherent interaction. Rayleigh or coherent scat-tering gives information about a sample from its atomic number, while the Compton interaction of bound electrons or incoherent scattering gives information on the physical or electronic density [18]. Subscripts TH and KN stand for the well-known Thomson and Klein–Nishina expressions, respectively. Lastly, the experimental molecular scattering differential cross section is given as [8], s(E, q)⫽ N()
TK()B() (3)
where N() is the total area of coherent and Compton peaks for target sample, K() is a constant characteristic of the experimental geometry, B() is a constant changing with scattering angle and connected with linear attenuation coefficients of the target sam-ple, and T is collecting time.
2.1. The effective atomic number
The ratio of total atomic cross section (t,a) and electronic cross section (t,el) is the effective atomic number (Zeff), and takes the form [19]
Zeff⫽ t,a
t,el (4)
The experimental effective atomic numbers were determined and the theoretical effective atomic numbers calculated using values of WinXCom and FFAST [20].
3. Experimental process
The experimental geometry in the present study is shown in
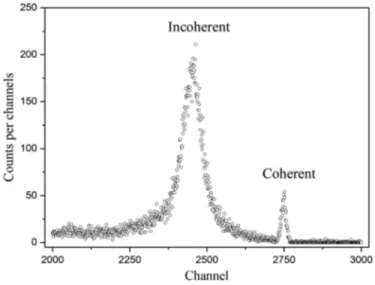
Fig. 1. The 59.5 keV␥-rays were emitted from a filtered low-energy photon point source of Am-241. The intensity of the source was 100 mCi. The spectra were collected for a period of 3600 s. The source and detector were shielded with a lead collimator. The hole radius of the source–collimator was 1 mm. The length of source– collimator was 32 mm. The detector–sample and source–sample distances were set at 25 mm. Coherent and Compton peak areas were measured. An exemplary spectrum of 59.5 keV␥-rays scat-tered by FeCl2are shownFig. 2. To reduce the statistical error, the
measurements were repeated at least three times. The errors in the peak areas were less than 3%. The errors in the calculation of sample thickness were about 1.94%. The radii of the samples were 6.5 mm. The samples were prepared in pellet form. In the scattering experi-ment, compounds of some essential elements were used, namely, NaO2C2H3, NaNO3, Na2CO3, NaF, Na2SO4, Na2SO3, NaCl, Mg(NO3)2, MgO, Al(NO3)3, AlCl3, C6H10CaO6, CaHPO4, CaSO4, CaF2, Fe2(SO4)3, FeCl3, and FeCl2.
4. Results and discussion
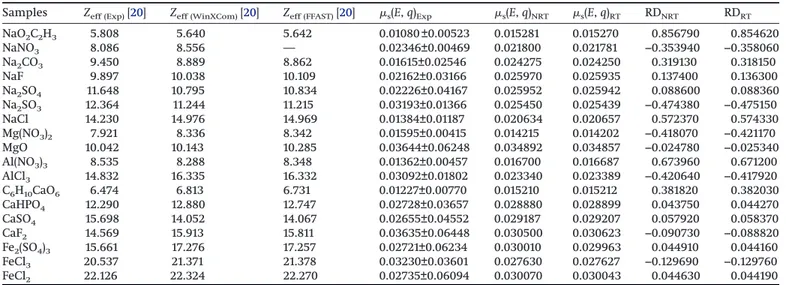
The aim of this study was to measure the molecular scattering differential cross sections of NaO2C2H3, NaNO3, Na2CO3, NaF, Na2SO4, Na2SO3, NaCl, Mg(NO3)2, MgO, Al(NO3)3, AlCl3, C6H10CaO6, CaHPO4, CaSO4, CaF2, Fe2(SO4)3, FeCl3, and FeCl2at 90° scattering angle for 59.5 keV energy␥-rays. The experimental and theoretical values are listed for compounds of some essential elements in
Table 1. Also, the relative deviations (RD) between the experimental and theoretical values (the values of RT and NRT) are presented in the last two columns inTable 1at ±, ( is the standard deviation). They have been calculated using the following equation:
RD⫽[s(E, q)theo⫺s(E, q)exp]
± (5)
As seenTable 1, the molecular differential scattering cross sec-tions increase as the effective atomic number increases for
com-Fig. 1. Experimental geometry.
Fig. 2. An exemplary spectrum of 59.5 keV␥-rays scattered by FeCl2.
498 Can. J. Phys. Vol. 94, 2016
pounds of Mg or Al elements. But these results have not been observed for compounds of Na, Ca, and Fe. We observe the evident effects of changing molecular weight in the molecular differential scattering cross sections for compounds of Mg and Al elements. Such effects have been observed by earlier investigators [21]. These investigators studied molecular differential scattering cross sections for Zeff≥ 42.94, but we determined molecular differential scattering cross sections for Zeff ≤ 25.99. Although our experimental results agree generally with both RT and NRT, there tends to be an overall better agreement with the RT theory for compounds of Na, Al, Ca, and Fe. For compounds of Mg there is an overall better agreement with the NRT theory. Generally, previous researchers have studied scattering cross sections of Z ≥ 26 elements and their compounds [21–29], but we studied compounds of Z ≤ 26 elements. The ratios of experimental and theoretical molecular scattering differential cross section are graphically presented inFig. 3.
As examined inFig. 3, the ratios of experimental and theoretical molecular scattering differential cross sections are between 0.5 and 1.5. According to relative differences, there is an overall better agreement between experimental and theoretical values. It is ob-served that relative differences in experimental results may not be directly explained by the number of atoms changing in a com-pound. We have not observed that when the number of atoms in a compound changes, significant differences between experimen-tal and theoretical values are not seen for these compounds. These differences can connect from below cases. The independent atomic model is a good approach that has been employed to calculate successfully the molecular scattering differential cross sections. The independent atomic model presumes that each atom scatters independently, neglects multiple scattering within the molecule, and presumes that any redistribution of atomic electrons due to molecular binding is unimportant [30]. In the independent atomic model the mixture or sum rule is used to obtain molecular form factors and incoherent scattering functions for a chemical com-pound [8]. At low energies such as this, the main effects in photon absorption are the photoelectric effect and Compton scattering. Additionally, at photon energies below 100 keV, scattering inter-actions of␥-rays with matter are of considerable importance [20,
21]. Therefore, we have selected 59.5 keV gamma-photon energy. To the best of our knowledge, there are no experimental data on molecular differential scattering cross sections reported in the literature for these compounds at this photon energy and scatter-ing angle. The present results constitute the first experimental measurements. Consequently, we believe that these values will be
most useful to further work and create a strong basis for other works. In the future, the experiment can be repeated for different values of energy and scattering angles, with different samples.
Acknowledgements
This work was supported by the Atatürk University Scientific Research Projects Fund, project No. 2012/174.
References
1. R.D. Luggar and W.B. Gilboy. Radiat. Phys. Chem. 56, 213 (1999). doi:10.1016/
S0969-806X(99)00278-9.
2. P. Latha, K.K. Abdullah, M.P. Unnikrishnan, K.M. Varier, and B.R.S. Babu. Phys. Scr. 85, 1 (2012). doi:10.1088/0031-8949/85/03/035303.
3. P.J. Parsons and F. Barbosa. Spectrochim. Acta B: Atom. Spect. 62, 992 (2007).
doi:10.1016/j.sab.2007.03.007.
4. L. Gillian, D.F. Jack, G. Benjamin, E.N. David, J.P. Patrick, and S. John. Clin. Lab. Stand. Inst. 17 (1997).
5. J.E. Andrews, P. Brimblecombe, T.D. Jickells, P.S. Liss, and B.J. Reid. An intro-duction to environmental chemistry. Oxford: Blackwell Science. 2004. 6. D.A. Bradley, S.C. Roy, L. Kissel, and R.H. Pratt. Radiat. Phys. Chem. 56, 175
(1999). doi:10.1016/S0969-806X(99)00280-7.
Table 1. Experimental and theoretical molecular differential scattering cross sections for compounds, 10−24(cm sr)−1.
Samples Zeff (Exp)[20] Zeff (WinXCom)[20] Zeff (FFAST)[20] s(E, q)Exp s(E, q)NRT s(E, q)RT RDNRT RDRT
NaO2C2H3 5.808 5.640 5.642 0.01080 ±0.00523 0.015281 0.015270 0.856790 0.854620 NaNO3 8.086 8.556 — 0.02346±0.00469 0.021800 0.021781 −0.353940 −0.358060 Na2CO3 9.450 8.889 8.862 0.01615±0.02546 0.024275 0.024250 0.319130 0.318150 NaF 9.897 10.038 10.109 0.02162±0.03166 0.025970 0.025935 0.137400 0.136300 Na2SO4 11.648 10.795 10.834 0.02226±0.04167 0.025952 0.025942 0.088600 0.088360 Na2SO3 12.364 11.244 11.215 0.03193±0.01366 0.025450 0.025439 −0.474380 −0.475150 NaCl 14.230 14.976 14.969 0.01384±0.01187 0.020634 0.020657 0.572370 0.574330 Mg(NO3)2 7.921 8.336 8.342 0.01595±0.00415 0.014215 0.014202 −0.418070 −0.421170 MgO 10.042 10.143 10.285 0.03644±0.06248 0.034892 0.034857 −0.024780 −0.025340 Al(NO3)3 8.535 8.288 8.348 0.01362±0.00457 0.016700 0.016687 0.673960 0.671200 AlCl3 14.832 16.335 16.332 0.03092±0.01802 0.023340 0.023389 −0.420640 −0.417920 C6H10CaO6 6.474 6.813 6.731 0.01227±0.00770 0.015210 0.015212 0.381820 0.382030 CaHPO4 12.290 12.880 12.747 0.02728±0.03657 0.028880 0.028899 0.043750 0.044270 CaSO4 15.698 14.052 14.067 0.02655±0.04552 0.029187 0.029207 0.057920 0.058370 CaF2 14.569 15.913 15.811 0.03635±0.06448 0.030500 0.030623 −0.090730 −0.088820 Fe2(SO4)3 15.661 17.276 17.257 0.02721±0.06234 0.030010 0.029963 0.044910 0.044160 FeCl3 20.537 21.371 21.378 0.03230±0.03601 0.027630 0.027627 −0.129690 −0.129760 FeCl2 22.126 22.324 22.270 0.02735±0.06094 0.030070 0.030043 0.044630 0.044190
Fig. 3. exp/theoversus experimental effective atomic numbers of
7. W.M. Elshemey, A.A. Elsayed, and A. El-Lakkani. Phys. Med. Biol. 44, 2907 (1999). doi:10.1088/0031-9155/44/12/304. PMID:10616144.
8. O. I˙çelli and S. Erzeneog˘lu. Nucl. Instrum. Methods Phys. Res. B, 215, 9 (2004). 9. M.E. Poletti, O.D. Gonçalves, and I. Mazzaro. X-Ray Spectrom. 31, 57 (2002).
doi:10.1002/xrs.538.
10. S. Krishnaveni and R. Gowda. Nucl. Instrum. Methods Phys. Res. B: Beam Interact. Mater. Atoms, 229, 333 (2005). doi:10.1016/j.nimb.2004.12.134. 11. A. Tartari, A. Taibi, C. Bonifazzi, M. Gambaccini, and M. de Felici. X-Ray
Spectrom. 34, 421 (2005). doi:10.1002/xrs.847.
12. M.O. Pereira, C.C. de Claudio, J.d.A. Marcelino, and T.L. Ricardo. Nucl. In-strum. Methods Phys. Res. B, 280, 39 (2012).
13. M.P. Singh, A. Sharma, B. Singh, and B.S. Sandhu. Radiat. Meas. 59, 30 (2013).
doi:10.1016/j.radmeas.2013.09.003.
14. J.H. Hubbell, W.J. Veigele, E.A. Briggs, R.T. Brown, and R.J. Howeron. J. Phys. Chem. Ref. Data, 4, 471 (1975). doi:10.1063/1.555523.
15. J.H. Hubbell and I. Overbo. J. Phys. Chem. Ref. Data, 8, 69 (1979).
16. S. Kahane. At. Data. Nucl. Data Tables, 68, 323 (1998). doi:10.1006/adnd.1998.
0770.
17. A. Tartari. Radiat. Phys. Chem. 56, 205 (1999). doi:10.1016/S0969-806X(99)
00281-9.
18. M. Donativi, S. Quarta, R. Cesareo, and A. Castellano. Nucl. Instrum. Methods Phys. Res. B: Beam Interact. Mater. Atoms, 264, 189 (2007). doi:10.1016/j.nimb.
2007.09.017.
19. K. Singh, H. Singh, V. Sharma, R. Nathuram, A. Khanna, R. Kumar, S.S. Bhatti, and H.S. Sahota. Nucl. Inst. Meth. B: Beam Interact. Mater. Atoms, 194, 1 (2002). doi:10.1016/S0168-583X(02)00498-6.
20. B. Akça and S.Z. Erzeneog˘lu. Sci. Technol. Nucl. Ins. 2014, 1 (2014). doi:10.
1155/2014/901465.
21. O. I˙çelli and S.Z. Erzeneog˘lu. Phys. Scr. 71, 344 (2005). doi:10.1238/Physica.
Regular.071a00344.
22. L. Kissel, R.H. Pratt, and S.C. Roy. Phys. Rev. A, 22, 1970 (1980). doi:10.1103/
PhysRevA.22.1970.
23. S.C. Roy, L. Kissel, and R.H. Pratt. Phys. Rev. A, 27, 285 (1983). doi:10.1103/
PhysRevA.27.285.
24. J. Eichler, S. de Barros, O. Goncalves, and M. Gaspar. Phys. Rev. A, 28, 3656 (1983). doi:10.1103/PhysRevA.28.3656.
25. K. Siddappa, N.G. Nayak, K.M. Balakrishna, N. Lingappa, and Shivaramu. Phys. Rev. A, 39, 5106 (1989). doi:10.1103/PhysRevA.39.5106. PMID:9901075. 26. N.G. Nayak, K. Siddappa, K.M. Balakrishna, and N. Lingappa. Phys. Rev. A, 45,
4490 (1992). doi:10.1103/PhysRevA.45.4490.
27. S. Erzeneog˘lu and Y. S¸ahin. Spectrosc. Lett. 31, 595 (1998). doi:10.1080/
00387019808002752.
28. O. Içelli and S. Erzeneog˘lu. Spectrochim. Acta B, 57, 1317 (2002). 29. O. Içelli and S. Erzeneog˘lu. J. Quant. Spect. Radiat. Trans. 74, 531 (2002). 30. H.S. Massey. Electronic and ionic impact phenomena. Vol. 2. Clamndon,
Oxford. 1969.
500 Can. J. Phys. Vol. 94, 2016