ScienceDirect
Materials Today: Proceedings 18 (2019) 1888–1895 www.materialstoday.com/proceedings
2214-7853 © 2019 Elsevier Ltd. All rights reserved.
Selection and/or Peer-review under responsibility of INTERNATIONAL CONGRESS ON SEMICONDUCTOR MATERIALS AND DEVICES.
ICSMD-2017
Inorganic Acid Doped Highly Hydroxylated Polymer Based Thin
Membrane
Morad Abdulwheed RADHA
a,c*, Alpay ŞAHİN
b, Erol PEHLİVAN
c, İrfan AR
b a Department of Fuel and Energy Engineering, Technical College – Kirkuk, 36001 Kirkuk, Iraqb Department of Chemical Engineering, Gazi University, 06570 Ankara, Turkey c Department of Chemical Engineering, Selcuk University, Campus, 42079 Konya, Turkey
Abstract
The effects of phosphoric acid (p) on the polyvinyl alcohol (PVA) as highly hydroxyl–group content polymer have been investigated. Functionalized membranes (p-PVA) have been prepared via sol-gel method with different doped acid ratios. Fourier Transform Infrared (FTIR) spectroscopy is used to confirm the structure of the pure polymer and the synthesized membrane. Water uptake capacity, plane swelling, thickness swelling, proton conductivity by electrochemical impedance spectroscopy (EIS) and thermal stability by thermal gravimetric analysis (TGA) are used to characterize the membranes for their applications in the suitable engineering fields. The results showed that the doped acid has suppressed effects on the hydrophilic properties of the used polymer which leads to decreasing in the water uptake and swelling properties of membranes. Thermally cross-linked 30% p-PVA thin membrane was selected because of its good water uptake capacity (43.78%) and low swelling degree (21.07%). The proton conductivity of all membrane increased with increasing the temperature up to 60 °C for 30% p-PVA thin membrane and it attained to 0.07 S cm-1. The proton conductivity decreased to 0.013 S cm-1 at the same temperature for 40% p-PVA thin
membrane. The thermal stability of doped polymer membranes is significantly higher than pure hydroxylated polymer which indicates the strong bonding between the doped acid and polymer.
© 2019 Elsevier Ltd. All rights reserved.
Selection and/or Peer-review under responsibility of INTERNATIONAL CONGRESS ON SEMICONDUCTOR MATERIALS AND DEVICES.
Keywords: Hydroxylated polymer; inorganic acid; sol-gel; thin membrane; characterization
* Corresponding author. Tel.: +90.535 396 1716
1. Introduction
Polyvinyl alcohol (PVA) based thin membranes have been attracting more attention due to their electrochemical separation task in the fossil oil–independent energy generation technologies, such as fuel cells and hydrogen producing electrolyzers [1-3]. Presence of large quantities of modifiable –OH side groups in PVA made it able to react with active acid groups easily. The feasibility of PVA blending with other engineering polymers leads to use it in the methanol fuel cell technology as a good alternative for Nafion due to its excellent methanol resistance fact [4]. In this experimental study, PVA preferred to form the membrane matrix because of its non-toxic, low cost, good film-forming ability, and water-soluble properties without to need for organic solvents. PVA also has chemical resistance, biocompatibility, and gas barrier properties [5]. However, PVA has undesirable properties, such as poor mechanical durability and low water retention capacity [6].
Besides, extensive swelling of PVA membrane which was affected by unbalanced water content might result in low ion transfer per unit volume; consequently reducing the ionic conductivity property of the synthesized membrane [7]. The low thermal stability of PVA also necessitates the improving steps. To improve the above mentioned negative properties of the PVA, an inorganic filler was doped inside the PVA matrix. For instance, the thermal stability of polyvinyl alcohol, low-density polyethylene and poly (ethylene-co-vinyl alcohol) effectively improved by using a phosphorus-based additive which acts as flame-retardant unlike toxic and corrosive halogen containing additives [8]. Other examples in the literature [9] include the use of phosphoric acid or sulphuric acid doped polyvinyl alcohol to synthesis cross-linked membranes with adding formaldehyde as crosslinking agent. In the related study, they showed that the phosphonated membrane has a lower swelling degree with better proton conductivity than the corresponding sulphonated membrane.
Annealing temperature has a suppressive effect on the volumetric swelling degree of the synthesized thin sheet and consequently the water content properties of the prepared PVA membrane [10, 11]. Therefore, the heat treatment conditions must be taken into account during the PVA based membrane preparation.
Alakanandana et al. [12] recently published a paper concerning about the structural and electrical conductivity studies of pure PVA and succinic acid doped PVA polymer electrolyte system. They found that the ionic conductivity of the succinic acid doped polyvinyl alcohol (PVA) based membrane increases with the increasing succinic acid ratio in PVA polymer matrix synthesized by solution casting technique.
In this work, we report the influence of acid doping ratios in the synthesis of phosphonated polyvinyl alcohol (p-PVA) membrane. As well as, preparing healthy grounds for the modification of p-PVA membrane in the other scientific researches. The characterization tests of the synthesized thin sheets were carried out by using FT-IR, TGA and EIS analysis, and the water content and swelling properties of membranes were calculated as well to find out the difference in their behavior within the completely dried and fully hydrated environment.
2. Experimental
2.1. Materials
98-99% hydrolyzed Polyvinyl alcohol (average MW 85000-124000) purchased from Aldrich Chemistry Company to form the membrane matrix. Aqueous solution of phosphoric acid (85% Merck) was used as a source of protons for protonation of the polymer. All chemicals used were of analytical grade and were used as received without any further purification. In all experiments the used deionized water directly supplied by Millipore
Direct-Q® 3 UV Water Purification System.
2.2. Preparation of Thin Membrane
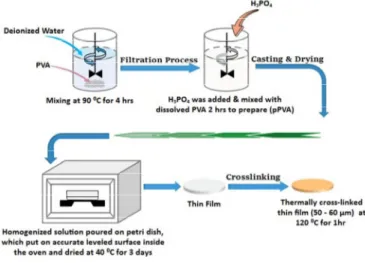
Firstly, 5% (w/w) polyvinyl alcohol solution was prepared by dissolving it in stirred deionized water at 90°C for 4h. Then, phosphoric acid (from 10% to 50%) as functionalization degree was added to resultant solution according to polyvinyl alcohol weight and mixed at 50 °C for 2 h. The obtained mixtures were poured onto sterilized plastic Petri dishes and dried at 40 °C for 3 days. Finally, the dried membrane was peeled off from the petri dish and
annealed at 120 ºC for 1 h to complete the thermal cross-linking process. The details of solution casting synthesis method can be seen in Fig. 1. given below.
Fig. 1. Synthesis steps of thin membrane. Characterization methods
Fourier Transform Infrared (FT-IR) spectroscopy was used to confirm the functional groups are added to membrane structure. For this purpose, a Jasco 480 plus FT-IR instrument was used and absorbance vs. wave length
data was obtained in the range of 4000-400 cm-1 wavelength. Water uptake capacity, swelling, and thermal stability
by thermal gravimetric analysis (TGA) were used to characterize the membranes for their applications in the suitable engineering fields. The TGA experiments of thermally cross-linked membranes were carried out by using METTLER –TOLEDO analyser. The proton conductivity data for each membrane were obtained by measuring the membrane resistance by using Solartron electrochemical impedance system consisting of AC Frequency Response Analyzer 1260 and Electrochemical Interface 1287 combination.
3. Results and Discussion
3.1. Chemical structure confirmation
In order to determine the chemical structure of membranes and especially the addition of acidic active groups to their matrix, synthesized membranes were subjected to the FT-IR analyses as shown in the Fig.2. and Fig.3. below.
Fig. 3. FTIR analysis of functionalized membranes (p-PVA) with different acid ratios.
It can be seen obviously in the blue colored FT-IR spectrum of pure PVA in the Fig. 2. peaks at 3302, 2938,
1427 and 1080 cm-1 are the characteristic peaks of OH, CH, CH
2, and C−O−H bonds respectively in the pure PVA
[8,13,14]. After addition of phosphoric acid to the polymer matrix, the strong absorbance at 1080 cm-1 in the
spectrum of pure PVA, arising from the C−Ostreach and –OH bend is decreased in intensity in the spectra of the p-PVA samples, indicating the replacement some of the C−O−H bonds by C−O−P bonds which can be seen in the Fig.
3.clearly [8]. The peak at 1094 cm-1 attributed to the C−O−P bond while the organic phosphate (P=O) peak appear at
1250−1350 cm-1 [15].
Furthermore, appearing the peaks at 1329 and 485 cm-1 attributed to P=O and O−P−O bonds respectively [13].
It can be seen obviously in the Fig.3., that increasing the doped acid ratios inside the polymer backbone led to increase the peak intensity of OH, P=O and C−O−P bands up to a point, while excessive acid doping caused disappearing OH group in the PVA and overlapping peaks of PO-H and C−O−P groups as it is in the 40%p-PVA and 50%p-PVA spectrum [13]. We observed that the PO−H band for p-PVA membrane shifted to a lower wavenumber with increasing the functionalization degree by the doped acid ratio, which is attributed to the higher number of the unreacted free acid molecules in the film.
3.2. Water uptake capacity
The balanced water present in the membrane structure plays an imperative role in terms of the proton transport mechanism to obtain on the optimum proton conductivity [7]. To find the water uptake (%) of thin membranes, the
dried thermally cross-linked membranes with same sizes were weighted and noted as their dry weight (Wd). After
that they were immersed in deionized water for 1 day at 25 oC. Then the wet membrane was taken from water and
excess water on the surface of membrane was removed by using water-resistant wipe and instantly weighted to
obtain the wet membrane weight (Ww). In Fig. 4. decreasing in the water uptake capacity of the membranes up to
40% functionalization degree by phosphoric acid doping to the PVA matrix may be explained by two phenomena. The first one consumption of the hydrophilic OH groups in polymer chain via acidic active groups and the second reason may be the decrease in the empty space that is available for water molecule entrance into the membrane matrix, due to the cross-linking between acid and the polymer chain [9]. We found that the water uptake capacity of pure PVA film under the same experimental conditions equals to 210%. Water uptake capacity values were calculated by using the following equation:
Fig. 4. Effect of protonic acid on the water uptake of PVA. 3.3. Swelling ratio
The swelling properties of membranes are an undesirable feature in many engineering fields because of their adverse affect on diffusion of molecules or ions. The membranes swelling in the dry and aqueous environment were studied by water immersing method. Fig.5. shows the effect of the protonic acid on the total swelling behaviours of PVA. In order to find the total or volumetric swelling ratio of the protonated thin membrane, plane swelling and thickness swelling of dried and wet thermally cross-linked membrane calculated together. The thickness of the membranes was measured by using highly accurate Electro physic, Mini-Test 650 model instrument at 15 points and recorded by taking average of these values. The swelling measurements were made for 3 separate samples for each membrane and the result values deviated by 1-8% from the mean values. In this study, the swelling ratio of all synthesized membranes behaves parallel to their water uptake capacity. But, it is desirable that the membrane has adequate water retention capacity without swelling.
The addition of phosphoric acid up to 40% resulted reduction in the swelling properties of the membranes, which can be explained by the reaction of the hydrophilic OH groups present in the polyvinyl structure with the added acid and thus reducing their ability to retain water and affecting swelling in the same tendency.
On the other hand further increase in phosphoric acid addition beyond 40%, membrane began to gain swelling due to the superiority of hydrophilic nature of phosphoric acid. In another word, the 50% p-PVA membrane became more sticky and wet despite heat treatment at 120 °C due to higher interaction between PVA and PA which leads to dehydration and phase separation of the produced film which makes the film absorbs moisture which causes increase in the intensity of the OH peak of the higher protonated PVA film which can be confirmed clearly in the FT-IR spectrum [13].
3.4. Proton conductivity
The proton conductivity data for each membrane were obtained by measuring the membrane resistance. The four
electrodes technique and ZView software were used at 10 mV in 100 Hz-105 Hz frequency range measurement
conditions. The measurements at different temperatures (20 – 80 ºC) were carried out in fully hydrated environment
of 0.1 M H2SO4 solution to enable proton release by creating a protic medium. Pure PVA membrane does not
possess any protonic conductivity [6]. The ionic conductivity of the 20% p-PVA membrane exhibits the maximum conductivity value. Higher functionalization degree of the doped acid groups caused low conductivity because of reduction of the water uptake capacity of the membranes. High acid loading will advance the crosslinking effect, which is, in turn; bring few interconnected channels and low free space in the membrane structure. The proton conductivity of all membrane increased with increasing the temperature up to 60 °C but, started to decline after 60 °C due to water retention reduction inside the membranes, which acts as proton carrier. According to the Arrhenius method, the proton conductivity increases with increasing temperature. Proton mobility increased with increasing temperature. Therefore, proton diffusion in the membrane has been increased. The decrease in proton conductivity at 80 °C is due to loss of proton carrier water. The harmonic rise in the proton conductivity by increasing the temperature from 25 – 60 °C implying (Fig. 6), that the proton conductivity is a thermally effected process (or “implying that proton conductivity is a highly temperature dependent process”). For more explanation, the proton mobility increases with increasing temperature. However, at temperatures above 80 °C not only membrane dehydration occurs as with Nafion membrane, but also it increases the tortuousness of proton – conduction pathways and hence causes the difficulty in the proton mobility [16,17]. The decrease in proton conductivity at 80 °C is due to ruining the continuous proton conductive pathways within the synthesized membrane structure. Thus, losing the water content which acts as proton carrier, damages these pathways at high temperatures.
The phosphoric acid doped membrane (has a better capacity of retaining water under low-humidity conditions
and that the dynamics of the hydrogen bonding is more constrained with˗PO3H2 than with˗SO3H), was used as a
proton conductor and preferred on the water dependent sulfonic acid doped membranes [6]. J. Lobato et al. [18]
observed that the proton conductivity of membrane can be significantly increased after doping with H3PO4.
However, leaching of active groups from the membrane matrix by inorganic acid is a big problem, especially when membranes contacted with liquid fuels, results a fast decrease in their conductivity [6]. Therefore, during the usage of membranes, the humidity condition should be taken into account to minimize the extra contact between the membrane and liquid fuels.
3.5. Thermogravimetric analysis
TGA measurements were carried out at a heating rate of 10 °C/min and 25-600 °C temperature range under N2
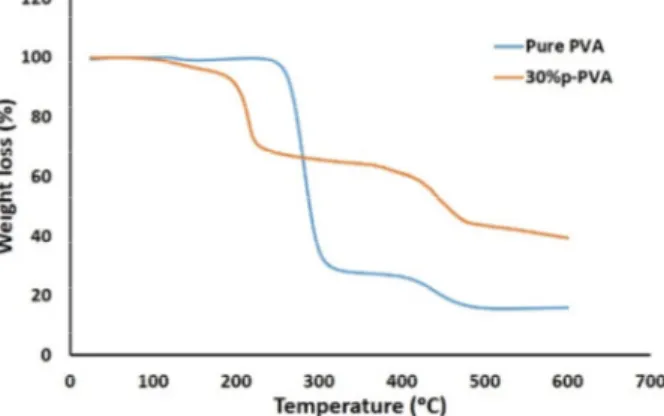
environment. The TGA spectrums of pure PVA and 30%p-PVA membranes are shown in Fig. 7. The slope of the TGA curve is much higher for pure PVA than for acid doped PVA membrane. This shows a lack of thermal stability and rupture of pure PVA chains [19]. The 30% p-PVA membrane has very small initial mass loss at low temperatures because of the evaporation of the free water. However, it seems that the thermal stability is improved in the acid doped membrane due to the effect of phosphoric acid and thermal crosslinking process.
Fig. 7. Thermal stability for pure PVA and 30%p-PVA membranes.
3. Conclusions
It was observed as a result of this study that, protonation process up to 40% led to lower water uptake capacity and consequently lower swelling ratios.The FT-IR spectrums show that the desired active groups are successfully adopted into the structure of pure PVA. Thermally cross-linked 30% p-PVA thin membrane, because of its good water content and low swelling degree is seen to be a good candidate for using in the engineering fields if it reinforced with other inorganic additives. Inserting the phosphoric acid active groups to the polymer matrix enhanced the thermal stability of the pure PVA. These can be attributed to the phosphate groups in the phosphoric acid which have more potential to behave as a crosslinking agent and contribute to bringing significant thermal stability to thin membrane. Decreasing the proton conductivity with increasing temperature consider a concerning issue should be addressed.
Acknowledgements
We thank Gazi University-Department of Chemical Engineering for providing laboratory devices and Selcuk University Scientific Research Projects (BAP- Grant number 16201108) for the financial support of this work.
References
[1] U. Thanganathan, M. Nogami, International J. Hydrogen Energy, 40 (2015) 1935–1944. [2] Y. Li, H. Wang, Q. Wu, X. Xu, S. Lu*, Y. Xiang, Electrochimica Acta 224 (2017) 369–377.
[3] S. Seetharaman, S. Ravichandran, D. J. Davidson, S. Vasudevan, and G. Sozhan, Separation Science and Technology, 46 (2011) 1563–1570. [4] T. Yang, International J. Hydrogen Energy, 33 (2008) 6772–6779.
[5] S. Virtanen, J. Vartianen, H. Setälä, T. Tammelin, S. Vuoti, RSC Adv. 2014, 4, 11343−11350.
[6] J. Maiti, N. Kakati, S. H. Lee, S. H. Jee, B. Viswanathan, Y. S. Yoon, J. Power Sources 216 (2012) 48–66. [7] N. Kakati, J. Maiti, G. Das, S. H. Lee,Y. S. Yoon, International J. Hydrogen Energy, 40 (2015) 7114–7123. [8] M. Banks, J. R. Ebdon, M. Johnson, Polymer, 1993, Volume 34, Number 21.
[10] J. S. Gonzalez, V. A. Alvarez, Thermochimica Acta 521 (2011) 184–190.
[11] J. S. Park, H. AKim, J. B. Choi, H. J. Gwon, Y. M. Shin, Y. M. Lim, M. S. Khil, Y. C. Nho, Radiat. Phys. Chem. 81 (2012) 857–860. [12] A. Alakanandanaa, A. R. Subrahmanyamb, J. Siva Kumar, Materials Today: Proceedings, 3 (2016) 3680–3688.
[13] A. M. Saat, M. R. Johan, Scientific World Journal, Article ID 439839 (2014) 1–7.
[14] A. Iribarren, A. L. Marzo, and H. Lemmetyinen, Revista Cubana de Quimica, 21 (2009) 3–9. [15] V. Pupkevich, V. Glibin, D. Karamanev, J. Power Sources 228 (2013) 300–307.
[16] S. Gao, H. Xu, T. Luo, Y. Guo, Z. Li, A. Ouadah,Y. Zhang, Z. Zhang, C. Zhu, J. Membr. Sci. 536 (2017) 1–10. [17] K. Feng, L. Liu, B. Tang, N. Li, P. Wu, ACS Appl. Mater. Interfaces 8 (2016) 11516–11525.
[18] J. Lobato, P. Cãnizares, M.A. Rodrigo, J.J. Linares, J.A. Aguilar, J. Membr. Sci. 306 (2007) 47–55. [19] C.C.Yang, W. C. Chien,Y. J. Li, J. Power Sources, 11 (2010) 3407−3415.