See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/51922574
Morphometric MRI evaluation of corpus callosum and ventricles in normal
adults
Article in Neurological Research · December 2011
DOI: 10.1179/1743132811Y.0000000030 · Source: PubMed
CITATIONS 10
READS 976
4 authors, including:
Some of the authors of this publication are also working on these related projects:
Myastenia GravisView project
Çukurova Üniversitesi Tıp Fakültesi Organizasyon Şeması- Optimizasyonu ve SimülasyonuView project Pınar Karakaş Cukurova University 14 PUBLICATIONS 332 CITATIONS SEE PROFILE Zafer Koç Baskent University 96 PUBLICATIONS 1,289 CITATIONS SEE PROFILE Filiz Koc Cukurova University 190 PUBLICATIONS 547 CITATIONS SEE PROFILE
All content following this page was uploaded by Zafer Koç on 17 February 2016.
Published by Maney Publishing (c) W. S. Maney & Son Limited
Morphometric MRI evaluation of corpus
callosum and ventricles in normal adults
Pınar Karakas¸
1, Zafer Koc¸
2, Filiz Koc¸
3, M Gu
¨lhal Bozkır
11
Department of Anatomy, Faculty of Medicine, C¸ukurova University,2Department of Radiology, Faculty of
Medicine, Bas¸kent University,3Department of Neurology, Faculty of Medicine, C¸ukurova University, Adana,
Turkey
Objectives: The aim of this study was to determine the normal values of subregions of corpus callosum and ventricles in healthy adult people in our population using magnetic resonance imaging (MRI) and to establish gender differences.
Methods: The MRI of 52 healthy individuals (29 females and 23 males) aged 20–50 years was obtained. The measurements were performed from MRI on a workstation. The midsagittal images were used for measurements of the subregions of corpus callosum and axial images were for lateral and third ventricles. Results: The mean values of the widths of genu, body, splenium, and height of the corpus callosum were 13.28¡2.10, 7.64¡1.07, 12.52¡1.35, and 25.47¡2.20 mm, respectively in females; whereas, the same measurements were 13.23¡2.41, 6.89¡2.12, 11.90¡1.94, and 25.03¡3.38 mm, respectively in males. Moreover, the mean value for the longitudinal dimension of the brain was 150.12¡5.04 mm, while that for the corpus callosum was 71.27¡3.70 mm in females. Additionally, the mean frontal horn width of the lateral ventricle and the transverse inner diameter of the skull were 34.06¡3.05 and 130.76¡6.71 mm in females and 34.03¡2.78 and 129.96¡10.61 mm in males, respectively. Due to these measurements, the values of Evans index which is reflecting the lateral ventricle enlargement were estimated to be 0.25¡1.90 and 0.25¡1.14 in females and males, respectively. According to our last measurement result, the mean values for the third ventricle width were 3.79¡0.85 and 4.12¡0.94 mm in females and males, respectively. These findings show that there are differences between the averages of some indices of corpus callosum of our population and the other populations.
Keywords: Corpus callosum, Lateral ventricle, MRI, Normal adult, Third ventricle
Introduction
The corpus callosum is the major transverse com-missure connecting the cerebral hemispheres and consists of subregions like genu, rostrum, trunk,
and splenium.1,2 It plays a key role in relaying
sensory, motor, and cognitive information between
homologous regions in the hemispheres.3,4Moreover,
its vulnerability to some toxins like alcohol and some white matter diseases like multiple sclerosis is also
important and not to be forgotten.2,5
The corpus callosum develops between 8 and 20 weeks of gestation. Inter-hemispheric crossing fibres begin to transverse the massa commisuralis in the genu region at 11–12 weeks post-conceptional age. Additionally, the rostrum develops at 18– 20 weeks post-conceptional age. Therefore, embry-ologically, the pioneer fibres of the future corpus callosum cross the midline in the 60–80 mm fetus
(12–13 weeks post-conceptional age) and it is effec-tively adult-like in the 140–60 mm fetus (18–20 weeks
post-conceptional age). It has emphasized that
agenesis of corpus callosum or absent rostrum may be rarely seen variations. Absent rostrum is usually found as a chance while evaluating magnetic
reso-nance imaging (MRI) for other purposes.6–8
The anatomy of the corpus callosum has received more interest due to increasing surgical interventions like callosotomies and treatment for some forms of
epilepsy.9,10Thus, some anatomical landmarks could
be found as reference points for microsurgery to this area. Therefore, a detailed morphometric measure-ment of corpus callosum is required. In addition, accurate measurements of the size of this structure in healthy adult people can provide the normal values and thus establish reference values to compare gender, racial differences and evaluate patients’ values.
Ventricular size is important for determination of many neurological diseases and ventricular enlarge-ment shows loss of brain parenchyma. Furthermore, Correspondence to: Pınar Karakas¸, Department of Anatomy, Faculty of
Medicine, C¸ ukurova University, Adana 01330, Turkey. Email: pkarakas@ cu.edu.tr
Published by Maney Publishing (c) W. S. Maney & Son Limited
measurements of the ventricular size are used in studies
of hydrocephalus following third ventriculostomy.11–15
Due to the literature findings, the majority of the reports about normal values for ventricular size involve
children and adolescents younger than 20 years.11,16,17
In this respect, the values of ventricular size in adults could be necessary for evaluating the pathologies from normal brains.
The aim of this study was to document the normal values of subregions of corpus callosum and lateral and third ventricles using MRI in healthy adult females and males in our population and to identify the gender differences, if any.
Materials and Methods
The research protocol of this study was approved by our institutional review board, and ethics committee approval also was obtained. Written informed con-sent was obtained from all individuals before MRI examination. This study is based on retrospective evaluation of cerebral MRI in consecutive 52 (29 females and 23 males) individuals aged 20–50 years (mean¡standard deviation of females: 35.64¡8.74 years; mean¡standard deviation of males: 36.11¡ 13.10 years) over a period of 2 years between March 2007 and March 2010. Inclusion criteria were no neurological signs, no intracranial lesions on MRI, and no history of neurological illness, current or past psychiatric illness, alcoholism, or drug use problems.
MRI was performed using a 1.5-T MRI system (Siemens; Avanto, Erlangen, Germany). A circularly polarized matrix coil was used for radiofrequency reception of the MRI signal. Routine brain MRI protocol including axial T2-weighted turbo spin echo (TSE) (TR/TE, 4500/118 ms; slice thickness, 6 mm; gap, 1.5 mm) and sagittal T1-weighted spin echo (SE)
(TR/TE, 450/11 ms; slice thickness, 5 mm; gap, 1.5 mm) was used.
Image analyses were performed randomly by two observers [observer 1, a radiologist (ZK) and observer 2, an anatomist (PK)]. The two observers reviewed MR images and performed all measure-ments in consensus. For intraobserver variability, all measurements were randomly performed by consen-sus in different sessions separated from the first evaluations by at least 3 weeks to minimize recall bias. Averages of the two measurements were used for final value of all measured region. The measure-ments were performed from digital MR images on a workstation (Advantage 4.4; GE Heathcare, Milwaukee, WI, USA) using caliper function with 62 magnification. Six measurements from subre-gions of the corpus callosum were determined using the midsagittal T1-weighted SE image as follows (Fig. 1):
(A) the width of the genu;
(B) the width of the body;
(C) the width of the splenium;
(D) the length of the corpus callosum from anterior
to posterior;
(E) the maximum height of the corpus callosum. A
line was taken between the inferior points of the rostrum and splenium. Another line parallel to this was taken the top point of corpus callosum. Distance between these lines was recorded; (F–G) the distance from the frontal to occipital pole
(the longitudinal dimension of the brain). Using axial T2-weighted TSE images, the following parameters were measured (Figs. 2 and 3):
Figure 1 Midsagittal T1-weighted SE MRI (TR/TE, 450/ 11 ms) of measurement sites of a normal adult. (A) The width of the genu. (B) The width of the body. (C) The width of the splenium. (D) The length of the corpus callosum from anterior to posterior. (E) The maximum height of the corpus callosum. (F–G) The distance from the frontal to occipital pole.
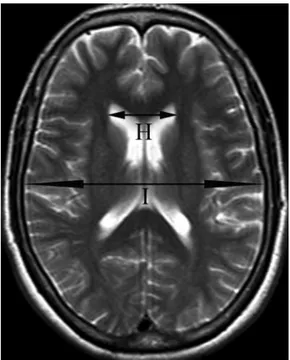
Figure 2 Axial T2-weighted TSE MRI (TR/TE, 4500/118 ms) of measurement sites of a normal adult. (H) The maximum width between the frontal horns of the lateral ventricles. (I) The maximum transverse inner diameter of the skull at the same level.
Karakas¸ et al. Morphology of corpus callosum and ventricles
Published by Maney Publishing (c) W. S. Maney & Son Limited
(H) the maximum width between the frontal horns of
the lateral ventricles (frontal horn width);
(I) the maximum transverse inner diameter of the
skull at the same level. After these measurements, Evans ratio (H/I) was calculated. It is assumed to reflect frontal horns ventricular enlargement;
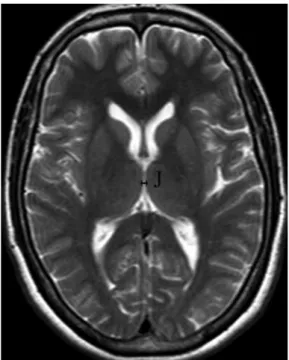
(J) the maximum width of the third ventricle
mea-sured at the level of the section where Monro’s foramen was visible.
The data were divided into two groups due to gender and statistical analysis was performed with SPSS 10.0. From these measurements, means, standard deviations, minimum, and maximum values were
evaluated. The intraobserver variability agreement of measurements was calculated using Spearman’s rank correlation test.
Results
The dimensions of corpus callosum and ventricular size of healthy adults are shown in Table 1.
From the 52 MRI scans, various dimensions of corpus callosum and ventricular size were measured (Figs. 1–3). The mean values of the widths of genu, body, splenium, and height of the corpus callosum were 13.28¡2.10, 7.64¡1.07, 12.52¡1.35, and 25.47¡ 2.20 mm in females, respectively; whereas, the same measurements were 13.23¡2.41, 6.89¡2.12, 11.90¡ 1.94, and 25.03¡3.38 mm in males, respectively. Moreover, the mean value for the longitudinal dimension of the brain was 150.12¡5.04 mm, while that for the corpus callosum was 71.27¡3.70 mm in females, a ratio of .2 : 1. This ratio was also similar in males (the same longitudinal dimensions were 152.53¡5.43 and 73.05¡ 5.28 mm in males, respec-tively) (Fig. 1). Additionally, the mean frontal horn width of the lateral ventricle and transverse inner diameter of the skull were 34.06¡3.05 and 130.76¡ 6.71 mm in females and 34.03¡2.78 and 129.96¡ 10.61 mm in males, respectively (Fig. 2). Due to these measurements, Evans ratios were 0.25¡1.90 and 0.25¡1.14 in females and males, respectively. Accord-ing to our last measurement result, the mean values for the third ventricle width were 3.79¡0.85 mm in females and 4.12¡0.94 mm in males (Fig. 3). The intraobserver variability agreement of the measure-ments was found to be good (0.89–0.96) between the evaluation in different sessions by two observers in Figure 3 Axial T2-weighted TSE MRI (TR/TE, 4500/118 ms)
of measurement site of the third ventricle of a normal adult. (J) The maximum width of the third ventricle.
Table 1 Dimensions of corpus callosum and ventricular size (mm)
Dimensions Sex Mean¡standard deviation Minimum–maximum
A Female 13.28¡2.10 9.75–19.33 Male 13.23¡2.41 10.80–20.13 B Female 7.64¡1.07 5.86–9.40 Male 6.89¡2.12 3.90–12.63 C Female 12.52¡1.35 10.21–16.42 Male 11.90¡1.94 8.30–15.04 D Female 71.27¡3.70 64.50–79.10 Male 73.05¡5.28 65.71–84.90 E Female 25.47¡2.20 21.30–29.05 Male 25.03¡3.38 18.83–31.72 F–G Female 150.12¡5.04 143.80–159.80 Male 152.53¡5.43 141.02–160.82 H Female 34.06¡3.05 26.70–40.00 Male 34.03¡2.78 28.45–39.60 I Female 130.76¡6.71 110.10–145.10 Male 129.96¡10.61 107.30–143.87
Evans index Female 0.25¡1.90 0.21–0.29
Male 0.25¡1.14 0.24–0.28
J Female 3.79¡0.85 2.50–5.70
Male 4.12¡0.94 3.00–5.60
Note: A: the width of the genu; B: the width of the body; C: the width of the splenium; D: the length of the corpus callosum from anterior to posterior; E: the maximum height of the corpus callosum; F–G: the distance from the frontal to occipital pole; H: the maximum width between the frontal horns of the lateral ventricles; I: the maximum transverse inner diameter of the skull at the same level; Evans ratio: H/I; J: the maximum width of the third ventricle.
Published by Maney Publishing (c) W. S. Maney & Son Limited
consensus (minimum correlation coefficient50.89 for ‘maximum width of the third ventricle’, and max-imum50.96 for ‘distance from the frontal to occipital pole’).
Discussion
This study included various parameters of the corpus callosum and ventricular size in healthy adults and compared them with other populations. The radi-ologically created values of corpus callosum and ventricles in both genders are valid for morphologi-cally normal brains and malformations should there-fore be carefully determined.
The corpus callosum is a topographically orga-nized neural structure that is composed of the majority of the commisural fibres connecting the
two cerebral hemispheres.18,19Morphological
varia-bility in the corpus callosum is often found in diseases such as Alzheimer’s disease, depression,
autism, and schizophrenia.4,18,20 Furthermore,
stu-dies using postmortem material or MRI obtained some sex differences in the size of subregions of corpus
callosum.18,21,22 When we analyse our images, the
averages of the widths of genu, mid-body, and splenium were 13.28, 7.64, and 12.52 mm in females and 13.23, 6.89, and 11.90 mm in males, respectively. Females’ body and splenium values are found higher than males’. It is in concordance with most literature findings that suggested the splenium size difference
between genders.18,21,23–25 However, it was generally
declared that males have larger bodies than females.5
But we found the opposite. In a study consisting of Chinese adults, these values were 11.68, 6.33, and
11.53 mm, respectively.22Same dimensions were 9.91,
5.58, and 9.94 mm in Japanese individuals while they
were 11.2, 8, and 11.5 mm in Indian, respectively.10,26
Furthermore, Mourgela et al. reported the widths of genu and splenium to be 21.3 and 7.4 mm,
respectively.27According to these data, we found
di-fferences in all average values of Chinese, Japanese, and Indian compared with our results: they have lower values than us. Mourgela’s averages were significantly different from ours. The width of genu
was y8 mm greater than ours; however, the width
of splenium was 5 mm lower than us.
Some direct identifiable landmarks for corpus callosum could be useful for surgery; however, the variability of its morphology makes it difficult. During the callosotomy, rostral majority of the corpus callosum must be split to increase the clinical
success.9,28,29 Moreover, it was shown that during
microsurgery, there was distortion of the corpus callosum due to the flattening of the brain. This
situation resulted in increasing horizontal and
decreasing vertical dimensions of corpus callosum, by 15% of its total length and 20% of its maximum
height, respectively.9 Gonc¸alves-Ferreira et al.
eval-uated an average total length of 74.9 mm and a total height of 25.5 mm; whereas, they were 75.7 and
32.7 mm respectively in Indian.9,10 Moreover, they
were identified to be 70.7 and 24.5 mm respectively in Chinese population and 69.7 and 25.8 mm
respec-tively in Japanese.22,26From our study, these
dimen-sions were 71.2 and 25.4 mm in females whereas 73 and 25 mm in males, respectively. Due to these reports, we found some differences between other populations and our values: especially Indian have higher values than us. Conversely, the average total lengths of corpus callosum of Chinese and Japanese people were lower than ours. However, in this investigation, the average total length of corpus callosum was closer to the values given in classical
publications.9According to this paper, the
measure-ment of the maximum height of the corpus callosum shows that the depth of the surgical incision to have a complete sectioning of the rostral corpus callosum must be 25 mm.
The lengths of the brain were established to be 150.1 mm in females and 152.5 mm in males in our study group. In addition, when we investigated the ratio of the length of the brain to that of the corpus callosum, it was 2.1. This dimension result showed
similarity with other researches.10,27,30
Moreover, ventricular enlargement indicates loss of volume of brain parenchyma and this atrophy is associated with many neurological diseases like
dementia, stroke, Huntington’s disease, etc.12 It was
also notified that the most consistent structural neu-roimaging finding is enlargement of lateral and third
ventricles in schizophrenic patients.31,32Additionally,
it was informed that patients with multiple sclerosis had significantly larger ventricular sizes than healthy controls and showed an increase in size with
pro-gression of the disease.12,33,34 The linear
measure-ments of ventricles were made for evaluating the normative ventricles’ values in this paper. Lateral ventricles enlargement was evaluated by averages of Evans ratio and this ratio was found to be 0.25 in both genders in our investigation. These values were similar with those in previous reports from Norway
and England.13,33However, Evans ratio was reported
to be 0.26 and 0.27 in females and males, respectively
in Japanese.26From several studies about the average
of third ventricle width, they were reported to be 3 mm in females and 4 mm in males from Norway; whereas, Turner and colleagues obtained the same
dimension as 5.14 mm.13,33 Meanwhile, this width
was found to be 5.7 mm in Japanese people.26 Our
values for this distance are closer to that in the report from Norway.
In this study, we observe that there are differences between the averages of some indices of corpus
Karakas¸ et al. Morphology of corpus callosum and ventricles
Published by Maney Publishing (c) W. S. Maney & Son Limited
callosum of Indian, Japanese, Chinese, and our population. However, in a study from Greece, we identify that there are also different averages from ours. Moreover, it is seen that ventricular dimen-sions are similar with those of European population. We think that these diversities may be a result of some factors such as race, genetic variables, indivi-dual constitution, and age. New, sex-specific norma-tive standards for radiological indices of corpus callosum and ventricular size in our population are presented. These linear measurements should be applicable for routine MRI reading without any need of an extra programme in a computer. Callosotomy is often performed using a restricted technique to access to intraventricular pathology and rostral subcallosal areas and to treat some
epilepsy forms.9Therefore, the precise knowledge of
subregions of corpus callosum could be helpful for the surgeon to have a good result. Furthermore, the assessment of ventricular size is generally obtained from subjective evaluation without determination of the normative standards and the effect of brain volume change during ageing in healthy people has to be taken into account in studies of different brain
pathologies.13It therefore becomes necessary to have
normal values for various racial groups, to compare with normal as well as pathological conditions. As a result, we believe that these normal reference values of corpus callosum and ventricles obtained from MRI are essential baseline data for interpreting pathological changes, planning surgery, and deter-mining presence and progress of some neurological diseases.
In conclusion, there are differences between the averages of some indices of corpus callosum of our population and the other populations. Ventricular dimensions of our population are similar with those of European population. New, sex-specific normative standards for radiological indices of corpus callosum and ventricular size in our population are applicable for routine MRI reading.
References
1 Williams PL, Bannister LH, Berry MM, Collins P, Dyson M, Dussek JE, et al. Gray’s anatomy. 38th ed. Edinburgh: Churchill Livingstone; 1995. p. 901–1397.
2 Georgy BA, Hesselink JR, Jernigan TL. MR imaging of the corpus callosum. AJR 1993;160: 949–55.
3 Ota M, Obata T, Akine Y, Ito H, Ikehira H, Asada T, et al. Age-related degeneration of corpus callosum measured with diffusion tensor imaging. NeuroImage 2006;31:1445– 52.
4 Hofer S, Frahm J. Topography of the human corpus callosum revisited — comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. NeuroImage 2006;32:989– 94.
5 Sullivan EV, Rosenbloom MJ, Desmond JE, Pfefferbaum A. Sex differences in corpus callosum size: relationship to age and intracranial size. Neurobiol Aging 2001;22:603–11.
6 Griffiths PD, Batty R, Reeves MJ, Connolly DJA. Imaging the corpus callosum, septum pellucidum and fornix in children:
normal anatomy and variations of normality. Neuroradiology 2009;51:337–45.
7 Warren DJ, Connolly DJA, Griffiths PD. Assessment of sulcation of the fetal brain in cases of isolated agenesis of the corpus callosum using inutero MR imaging. Am J Neuroradiol 2010;31:1085–90.
8 Glenn OA, Goldstein RB, Li KC, Young SJ, Norton ME, Busse RF, et al. Fetal magnetic resonance imaging in the evaluation of fetuses referred for sonographically suspected abnormalities of the corpus callosum. J Ultrasound Med 2005;24:791–804.
9 Gonc¸alves-Ferreira AJ, Herculano-Carvalho M, Melancia JP, Farias JP, Gomes L. Corpus callosum: microsurgical anatomy and MRI. Surg Radiol Anat 2001;23:409–14.
10 Gupta T, Singh B, Kapoor K, Gupta M, Kochhar S. Normative data of corpus callosal morphology in a North-West Indian population — an autopsy and MRI study. J Nepal Med Assoc 2009;48:46–51.
11 Kulkarni AV, Drake JM, Armstrong DC, Dirks PB. Measurement of ventricular size: reliability of the frontal and occipital horn ratio compared to subjective assessment. Pediatr Neurosurg 1999;31:65–70.
12 Martola J, Stawiarz L, Fredrikson S, Hillert J, Bergstrom J, Flodmark O, et al. Rate of ventricular enlargement in multiple sclerosis: a nine year MRI follow-up study. Acta Radiol 2008;5:570–9.
13 Aukland SM, Odberg MD, Gunny R, Chong WK, Eide GE, Rosendahl K. Assessing ventricular size: is subjective evalua-tion accurate enough? New MRI-based normative standards for 19-year-olds. Neuroradiology 2008;50:1005–11.
14 O’Hayon BB, Drake JM, Ossip MG, Tuli S, Clarke M. Frontal and occipital horn ratio: a linear estimate of ventricular size for multiple imaging modalities in pediatric hydrocephalus. Pediatr Neurosurg 1998;29:245–9.
15 Jamous M, Sood S, Kumar R, Ham S. Frontal and occipital horn width ratio for the evaluation of small and asymmetrical ventricles. Pediatr Neurosurg 2003;39:17–21.
16 Xenos C, Sgouros S, Natarajan K. Ventricular volume change in childhood. J Neurosurg 2002;97:584–90.
17 Davies MW, Swaminathan M, Chuang SL, Betheras F. Reference ranges for the linear dimensions of the intracranial ventricles in preterm neonates. Arch Dis Child 2000;82:F218– F23.
18 Salat D, Ward A, Kaye JA, Janowsky JS. Sex differences in the corpus callosum with aging. Neurobiol Aging 1997;18:191–7. 19 Pandya DN, Seltzer B. The topography of commisural fibers.
In Lepore F, Ptito M, Jasper HH, editors. Two hemispheres – one brain: function of the corpus callosum. New York: Alan R Liss; 1986. p. 47–73.
20 Piven J, Bailey J, Ranson BJ, Arndt S. An MRI study of the corpus callosum in autism. Am J Psychiatry 1997;154:1051–6. 21 Dubb A, Gur R, Avants B, Gee J. Characterization of sexual
dimorphism in the human corpus callosum. NeuroImage 2003;20:512–9.
22 Yang JL, Guo YM, Gao YJ, Ma MY, Zhang QJ, Xu M. A MRI quantitative study of corpus callosum in normal adults. J Med Coll PLA 2008;23:346–51.
23 Allen LS, Richey MF, Chai YM, Gorski RA. Sex differences in the corpus callosum of the living human being. J Neurosci 1991;11:933–42.
24 Holloway RL, deLacoste MS. Sexual dimorphism in the human corpus callosum: an extension and replication study. Hum Neurobiol 1986;5:87–91.
25 Yoshii F, Barker W, Apicella A, Chang J, Sheldon J, Duara R. Measurements of the corpus callosum on magnetic resonance scans: effects of age, sex, handedness and disease. Neurology 1986;36:133.
26 Takeda S, Hirashima Y, Ikeda H, Yamamoto H, Sugino M, Endo S. Determination of indices of the corpus callosum associated with normal aging in Japanese individuals. Neuroradiology 2003;45:513–8.
27 Mourgela S, Anagnostopoulou S, Sakellaropoulos A, Gouliamos A. An MRI study of sex and age related differences in the dimensions of the corpus callosum and brain. Neuroanatomy 2007;6:63–5.
28 Gonc¸alves-Ferreira AJ, Farias JP, Carvalho MH, Melancia J, Migue´ns J. Corpus callosotomy: some aspects of its micro-surgical anatomy. Stereotact Funct Neurosurg 1995;65:90–6. 29 Lehman RM, Olivier A, Moreau JJ, Tampieri D, Henri C. Use
of the callosal grid system for the preoperative identification of the central sulcus. Stereotact Funct Neurosurg 1992;59:178–88.
Published by Maney Publishing (c) W. S. Maney & Son Limited
30 Suganthy J, Raghuram L, Antonisamy B, Vettivel S, Madhavi C, Koshi R. Gender and age-related differences in the morphology of the corpus callosum. Clin Anat 2003;16:396– 403.
31 Bersani G, Paolemili M, Quartini A, Clemente R, Gherardelli S, Iannitelli A, et al. Neurological soft signs and cerebral measurements investigated by means of MRI in schizophrenic patients. Neurosci Lett 2007;413:82–7.
32 Lawrie SM, Whalley H, Kestelman JN, Abukmeil SS, Byrne M, Hodges A, et al. Magnetic resonance imaging of brain in people
at high risk of developing schizophrenia. Lancet 1999;353:30– 3.
33 Turner B, Ramli N, Blumhardt LD, Jaspan T. Ventricular enlargement in multiple sclerosis: a comparison of three-dimensional and linear MRI estimates. Neuroradiology 2001; 43:608–14.
34 Liu C, Edwards S, Gong Q, Roberts N, Blumhardt LD. 3-D estimates of brain and spinal cord atrophy in multiple sclerosis. Clinical correlates of spinal cord and cerebral atrophy in multiple sclerosis. J Neurol Neurosurg Pyschiatry 1999;66:323–30.
Karakas¸ et al. Morphology of corpus callosum and ventricles
Neurological Research 2011 VOL. 33 NO. 10 1049
View publication stats View publication stats