Full Terms & Conditions of access and use can be found at
https://www.tandfonline.com/action/journalInformation?journalCode=ibih20
Biotechnic & Histochemistry
ISSN: 1052-0295 (Print) 1473-7760 (Online) Journal homepage: https://www.tandfonline.com/loi/ibih20
Effects of zinc supplementation on
metallothionein levels in ischemic renal tissue
Betul Yazğan, Abdulkerim Kasim Baltaci, Rasim Mogulkoc & Mustafa Cihat
Avunduk
To cite this article: Betul Yazğan, Abdulkerim Kasim Baltaci, Rasim Mogulkoc & Mustafa Cihat Avunduk (2020) Effects of zinc supplementation on metallothionein levels in ischemic renal tissue, Biotechnic & Histochemistry, 95:4, 285-296, DOI: 10.1080/10520295.2019.1691264
To link to this article: https://doi.org/10.1080/10520295.2019.1691264
Published online: 27 Jan 2020.
Submit your article to this journal
Article views: 136
View related articles
View Crossmark data
Effects of zinc supplementation on metallothionein levels in ischemic renal tissue
Betul Yazğana, Abdulkerim Kasim Baltacib, Rasim Mogulkocb, and Mustafa Cihat Avundukc
aFaculty of Medicine, Department of Physiology, Adiyaman University, Adiyaman-Turkey;bFaculty of Medicine, Department of Physiology, Selçuk University, Konya-Turkey;cFaculty of Meram Medicine, Department of Pathology, Necmettin Erbakan University, Konya-Turkey
ABSTRACT
We investigated how zinc (Zn) supplementation affects metallothionein levels in the cortex and medulla of ischemic renal tissue of rats. We used adult male rats divided into four groups: group 1, untreated control; group 2, sham-operated; group 3, reperfusion; group 4, ischemia-reperfusion + 5 g/kg Zn. Renal tissue was analyzed using immunostaining of rat metallothionein. Cells stained with metallothionein were counted and their percentage was calculated. We found that the Zn supplemented ischemia and reperfusion group exhibited a greater percentage of cells stained strongly for metallothionein in the renal cortex than all other groups. In the renal medulla, percentages of weak staining for metallothionein in the control and ischemia and reperfusion groups were greater than those in the sham and Zn-supplemented ischemia/reperfusion groups. Our findings indicate that the main effect of Zn in the renal tissue occurs in the cortex, while metallothionein synthesis in the renal medulla is unaffected.
KEYWORDS
ischemia; kidney; metallothionein; rat; renal tissue; reperfusion; supplementation; zinc
Ischemia is defined as insufficient blood flow to tissue (Malek and Nematbakhsh 2015). Tissue injury results when ischemic tissue suffers hypoxia. Hypoxia decreases cellular activity and toxic metabolites accumulate in the cells. Ischemia initiates a series of biochemical reactions that cause cell dysfunction and ultimately death (Chenet al. 2015; Malek and Nematbakhsh2015). Reperfusion is the resumption of blood flow (Malek and Nematbakhsh2015). During reperfusion, reactive oxygen species (ROS), released by polymorphonuclear leukocytes (PMNL) that had invaded the tissue, intensify the damage already inflicted (Glodowski and Wagener2015; Malek and Nematbakhsh
2015). This situation is described as reperfusion-associated tissue injury (Chenet al. 2015; Malek and Nematbakhsh
2015). The extent of the injury varies depending on the duration of ischemia, temperature of the tissue and tissue-specific factors (Glodowski and Wagener2015; Malek and Nematbakhsh2015). While ROS production is increased after ischemia and reperfusion (I/R), the antioxidant enzymes that detoxify ROS are reduced (Baltaci et al.
2018; Tucci Junior et al. 2008). Imbalance of the antioxidant system during I/R causes ischemic acute renal failure that is characterized by decreased glomerular filtration, tubular necrosis and increased resistance in renal vessels (Chen et al.2015; Malek and Nematbakhsh
2015).
Metallothioneins are low molecular weight metal-binding proteins that help protect against metal toxicity
(Yu et al.2009). Metallothioneins participate in regulating zinc (Zn) levels and distribution in tissues (Kimura and Kambe 2016; Yu et al. 2009). The relation between metallothioneins and Zn is bi-directional; Zn also stimulates metallothionein synthesis (Kimura and Kambe
2016). This relation is supported by evidence that reduced metallothionein levels and increased renal tissue injury in the renal tissues of diabetic rats with Zn deficiency are reversed by Zn supplementation (Ozcelik et al.2012). It also has been reported that metallothioneins could help protect against renal dysfunction caused by hypoxia (Kojima et al. 2009). We investigated how Zn supplementation affects metallothionein levels caused by renal I/R in rats.
Material and methods
Experimental protocol
Our study was conducted at the Experimental Animals Unit of Selcuk University’s Faculty of Veterinary Medicine. Our protocol was approved by the Ethics Committee of Selcuk University Faculty of Veterinary Medicine (20 March 2013, no. 2013/12).
We used 40 12−14-week-old 240−250 g Wistar albino adult male rats. The animals were housed in steel cages that were cleaned daily. Feed was given in steel bowls and normal tap water was available in glass feeding bottles. The feed was supplied by the Experimental Medicine and CONTACTAbdulkerim Kasim Baltaci baltaci61@yahoo.com Faculty of Medicine, Department of Physiology, Selçuk University 42031, Konya, Turkey. BIOTECHNIC & HISTOCHEMISTRY
2020, VOL. 95, NO. 4, 285–296
https://doi.org/10.1080/10520295.2019.1691264
Application Center of Necmettin Erbakan University in the form of normal rat pellets. The animals were given about 10 g of feed/100 g body weight daily. The rats were housed at 21 ºC with a 12 h dark:12 h light cycle. All injections were performed between 9:00 and 10:00 AM. At the end of the 3 week experiment, kidney tissue samples were taken from all animals between 9:00 and 10:00 A.M. under general anesthesia. The samples were stored at -80 °C until the time for analysis.
The rats were divided randomly into four groups of 10. Group 1 was untreated controls. Group 2, sham group, was fed a normal diet. Upon completion of procedures,
animals were anesthetized using 50 mg/kg ketamine HCl (Ketalar, Pfizer, Istanbul, Turkey) and 10 mg/kg xylazine HCl (Rompun 2%, Bayer, Istanbul, Turkey), injected intramuscularly (i.m.) and the left kidney area was surgically opened and closed. Group 3, I/R, left kidney tissues of this group were subjected to ischemia for 45 min (see below) under general anesthesia, then re-perfused for 1 h. Group 4, I/R + Zn, rats were injected intraperitoneally (i.p.) with 5 mg/kg ZnSO4/day starting 21 days prior to the
operation. Upon completion of procedures, left kidney tissues of the rats were subjected to ischemia for 45 min, then to reperfusion for 1 h under general anesthesia.
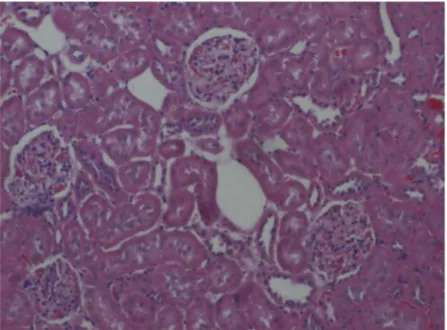
Figure 1.Control group showing weak staining of cortex x 100. H & E.
Figure 2.Control group showing weak staining of medulla. x 100. H & E.
Surgery
Rats in groups 3 and 4 were anesthetized by injection i.m. of 50 mg/kg ketamine HCl and 10 mg/kg xylazine HCl. The abdominal area of the rats was shaved and the operation site was cleaned with 10% povidone iodine (Polyad, Drogsan Drug Co., Ankara, Turkey). The area was covered with a sterile cloth leaving only the incision site exposed. A laparotomy was performed using a midline incision on the abdomen using sterile equipment. To induce temporary ischemia in the renal tissue, a vascular clamp was
placed on the hilum and the tissue was subjected to ischemia for 60 min. The clamp then was removed to allow reperfusion for 60 min (Ergur et al. 2008). For the duration of I/R, the abdominal area was covered with a dressing soaked in normal saline for protection. A nephrectomy then was performed to harvest renal tissue samples.
ZnSO4supplementation
We dissolved 0.89 g ZnSO4∙7H2O in 250 ml saline. The
animals in the I/R + Zn group (group 4) were
Figure 3.Sham group cortex showing weak staining. x 100. H & E.
Figure 4.Sham group medulla showing weak staining. x 100. H & E.
supplemented with 5 g/kg intraperitoneal ZnSO4daily
starting 21 days before the operation.
Evaluation of immunoreactivity
Stained specimens were viewed using a Nikon Eclipse E400 light microscope (Nikon Corp., Tokyo, Japan). The same area was photographed for each specimen using a Nikon Coolpix 5000 microscope (Nikon Corp., Tokyo, Japan) photographic attachment (Figures 1–8). The photograph of the Nikon micrometer microscope slide (stage micrometer tyte A MBM11100) also was taken
during the procedure. Separate photographs of cortex and medulla were taken. All photographs then were transferred to a PC and analyzed using Clemex Vision Lite 3.5 Image Analysis program (Clemex Technologies Inc., Quebec, Canada). The length was calibrated by comparing the photograph of the specimen with the photograph of the Nikon micrometer microscope slide (MBM11100; Nikon Corp.), which was taken under the same magnification (Figures 9–11). A 1576097.8 µm2 area then was calculated with the Clemex Vision Lite 3.5 Image Analysis program. The intensity of metallothionein staining was scored as strong,
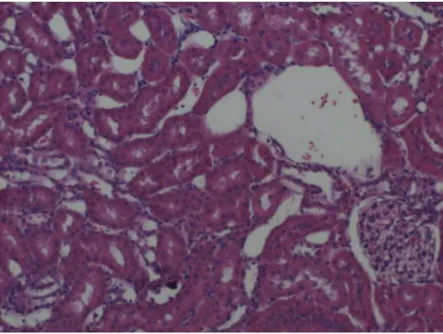
Figure 5.I/R group cortex showing weak staining. x 100. H & E.
Figure 6.I/R group medulla showing weak staining. x 100. H & E.
moderate or weak. The counting process was conducted for each staining feature and for each group separately.
Statistical analysis
The statistical evaluation of data was conducted using Minitab Software Package. After normality analysis and homogeneity testing, the data were analyzed using one-way variance analysis (ANOVA). Tukey’s post hoc test
was used for comparison among groups. Values forp ≤ 0.05 were considered statistically significant.
Results
Metallothionein staining in renal cortex
Metallothionein immunostaining intensity of cortex cells was recorded as weak, moderate or strong in addition to location of staining. The percentage of
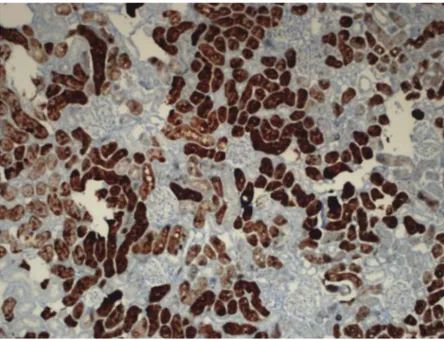
Figure 7.Zn + I/R group. The cortex exhibits strong metallothionein staining. x 100. H & E.
Figure 8.Zn + I/R group. The medulla exhibits weak metallothionein staining. x 100. H & E.
Figure 9.Nikon micrometer microscope slide (Stage Micrometer Type A MBM11100) image for calibration using Clemex Vision Lite 3.5 Image Analysis software.
Figure 10.Calculation of area for renal samples at 20 x magnification using Clemex Vision Lite 3.5 image analysis system.
cells stained strongly for metallothionein in I/R + Zn group was significantly higher than the levels in the other groups (p < 0.05). The percentage of cells stained strongly for metallothionein in the control, sham and I/R groups did not differ significantly (Table 1, Figures 1,3, 5, 7, 12, 16, 14, 16, 18).
In the moderate metallothionein staining category, we found no significant difference among groups. By contrast, we found a significant difference in the weak staining category (p < 0.05). The percentage of cells stained weakly with MT in the I/R + Zn group was significantly less than the levels in all other groups (Table 1,Figures 1,3,5,7,12,14,16,18).
Metallothionein staining in renal medulla
Metallothionein immunostaining of medulla cells was categorized as weak, moderate or strong. The percentage of cells stained for metallothionein in the
strong and moderate categories did not differ significantly (Table 2, Figures 2, 4, 6, 8, 13, 15, 17,
19). The percentage of cells stained for metallothionein in the control group was significantly greater than for the sham, I/R and I/R + Zn groups, however, in the weak metallothionein staining category (p < 0.05) (Table 2,Figures 2,4,6,8,13,15,17,19).
Discussion
We found the highest metallothionein levels in the cortex of the Zn supplemented I/R group. Under physiological conditions, Zn stimulates metallothionein (Ozcelik et al.
2012). Metallothioneins are important for maintaining intracellular zinc homeostasis (Mocchegiani et al.2008). Metallothioneins are free radical scavengers (Rutkay-Nedecky et al. 2013) that inhibit oxidative reactions (Hozumi 2013). Metallothioneins protect renal tissue against heavy metals and oxidative stress (Ozcelik et al.
2012; Szczurek et al.2009).
Both metallothioneins and Zn are both located in the renal cortex (Szczurek et al.2009). These investigators also reported that a Zn-deficient diet reduced both metallothionein and Zn levels in renal tissue, whereas Zn supplementation increased metallothionein levels, especially in the proximal tubules of the renal cortex. Our findings indicate that Zn supplementation of metallothionein can prevent cell injury from (ROS) in the proximal tubule cells of the renal cortex. Ozcelik et al. (2012) reported that Zn-supplemented diabetic rats
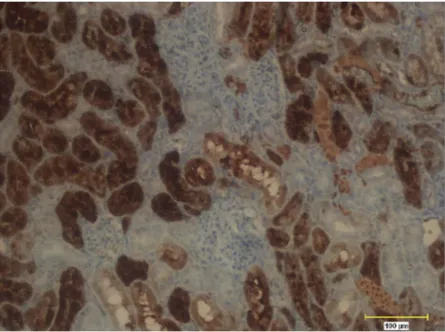
Figure 11.Labeling and counting based on staining intensity of MT stained renal tubule cells using Clemex Vision Lite 3.5 Image Analysis software. Black arrow, strongly stained; blue arrow, markedly stained; white arrow, weakly stained. x 20.
Table 1.Metallothionein staining intensity of renal cortex
Strong staining with MT Moderate staining with MT Weak staining with MT Control 0.29 ± 0.07b Control 0.41 ± 0.09 Control 0.31 ± 0.10a
Sham 0.26 ± 0.04b Sham 0.35 ± 0.08 Sham 0.39 ± 0.09a I/R 0.29 ± 0.08b I/R 0.39 ± 0.08 I/R 0.32 ± 0,04a I/R + Zn 0.42 ± 0.05a I/R + Zn 0.40 ± 0.03 I/R + Zn 0.18 ± 0.03b
Total 0.32 ± 0.09 Total 0.39 ± 0.07 Total 0.30 ± 0.10 Data are percentage of stained cells ± SD. Means with different superscripts
in the same column are significantly different (p < 0.05). Groups: Control, sham, ischaemia-reperfusion (I/R), ischaemia-reperfusion + zinc (I/R+ Zn) Metallothionein: MT
exhibited increased levels of Zn and metallothionein in renal tissue, particularly in the proximal convoluted tubules, compared to diabetic controls. Consequently, Zn could inhibit oxidative stress in diabetic rats by stimulating metallothionein synthesis.
Tang et al. (2010) reported that Zn supplementation begun three months after the onset of diabetes significantly reduced renal oxidative stress. Zn supplementation also increased metallothionein synthesis in renal proximal tubule cells. These investigators
Figure 12.Control group showing weak staining of cortex. x 20. IHC.
Table 2.Metallothionein staining intensity of renal medulla
Strong staining with MT
Moderate staining with MT
Weak staining with MT Control 0.03 ± 0.03 Control 0.15 ± 0.09 Control 0.79 ± 0.09a
Sham 0.12 ± 0.07 Sham 0.25 ± 0.10 Sham 0.59 ± 0.10b
I/R 0.11 ± 0.12 I/R 0.24 ± 0.12 I/R 0.65 ± 0.15b I/R + Zn 0.15 ± 0.10 I/R + Zn 0.25 ± 0.09 I/R + Zn 0.60 ± 0.14b Total 0.10 ± 0.09 Total 0.22 ± 0.10 Total 0.66 ± 0.14 Data are percentage of stained cells ± SD. Means with different superscripts
in the same column are significantly different (p < 0.05). Groups: control, sham, ischaemia-reperfusion (I/R), ischaemia-reperfusion + zinc (I/R+ Zn) Metallothionein: MT
Figure 13.Control group showing weak staining of medulla. x 20. IHC.
concluded that Zn might reduce pathological changes in the kidneys of diabetic animals by stimulating metallothionein synthesis (Tang et al.2010). Similarly, Zn supplementation to counter acute toxicity caused by diluted uranium (DU) in rats inhibited lipid oxidation in renal tissue by activating metallothionein synthesis (Hao et al.
2012). Metallothioneins inhibit lipid oxidation caused by cadmium (Sabolić et al.2010).
Indomethacin, a nonsteroidal anti-inflammatory drug (NSAD) causes oxidative stress and mitochondrial
dysfunction in the renal cortex (Varghese et al. 2009). Although Varghese et al. (2009) reported that Zn supplementation prevented renal lipid oxidation caused by indomethacin; metallothioneins did not produce this effect. The role of metallothioneins in detoxification and regulation of metal homeostasis in the case of mercury toxicity have been documented (Peixoto et al. 2007). Because metallothionein levels in the liver and kidneys of Zn-supplemented rats increased, it was reported that Zn supplementation reduced renal cortex toxicity by
Figure 14.Sham group cortex. The number of cells stained strongly for metallothionein in the control, sham and I/R groups was similar. x 20. IHC.
Figure 15.Control group weaker metallothionen staining compared to other groups. x 20. IHC.
activating metallothionein synthesis in cases of renal toxicity caused by mercury (Peixoto et al.2007).
Zn has been shown to protect kidneys from damage in rats exposed to petrol vapor by stimulating metallothionein synthesis (Grebić et al. 2007). Similarly, Zn supplementation in cases of renal failure and proximal tubule necrosis caused by gentamicin injection increased metallothionein content in the cortex of renal tissue and inhibited both renal failure and tubule necrosis (Yang et al.
1994); our findings are consistent with earlier reports.
Supplementation with 5 mg/kg ZnSO4 to rats with
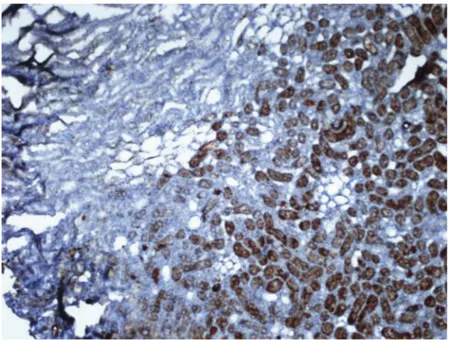
induced diabetes prevented the impairment of renal functions caused by diabetes. In addition, Zn supplementation increased metallothionein synthesis in the tubule cells of the renal tissue of both diabetic and normal rats. Zn deficiency, on the other hand, caused increased oxidative stress and reduced metallothionein synthesis in animals (Li et al. 2013). Our finding of elevated metallothionein levels in the cortex after supplementation with 5 mg/kg ZnSO4is consistent with earlier reports. Figure 16.Cortex of I/R group showed weaker metallothionen staining compared to I/R + Zn group.Control, sham and I/R groups show similar strong staining for metallothionein. x 20. IHC.
Figure 17.I/R group exhibits weaker metallothionen staining compared to control group. 20 x. IHC.
Metallothionein levels in I/R + Zn group were higher than for the control, sham and I/R groups. We found similar metallothionein levels in the renal cortex of the control and I/R group and higher metallothionein levels in the I/R + Zn group compared to the I/R group, which is consistent with earlier studies (Baltaci et al.2018; Hu et al.2017; Li et al.2013).
We found no significant difference in metallothionein content in the renal medulla among the groups. This finding was unexpected, because we found elevated
metallothionein levels in the cortex of the I/R + Zn group. Approximately 80% of nephrons are located in the renal cortex (Kojima et al. 2009; Tucci Junior et al. 2008); therefore, our findings for the medulla may be attributed to the presence of fewer nephrons in the medulla. Also, Zn, which stimulates metallothioneins and their synthesis, is located particularly in the cortex (Szczurek et al. 2009). Yang et al. (1994) reported that in rats with induced renal failure and proximal tubule necrosis, Zn supplementation increased the metallothionein content of the renal cortex.
Figure 18.Zn + I/R group cortex exhibits strong metallothionein staining. x 20. IHC.
Figure 19.Zn + I/R group the medulla exhibits weak metallothionein staining. x 20. IHC.
Our findings are consistent with the literature. We found that Zn supplementation affected the cortex, but not the medulla.
Ours is the first report of evaluating metallothionein levels in the cortex and medulla of the ischemic kidneys separately. We found that the highest metallothionein levels in the renal cortex were in the I/R + Zn group. To the contrary, metallothionein levels in the renal medulla were unaffected by Zn supplementation.
Acknowledgments
This study was supported by the Scientific Research Projects Coordinatorship of Selcuk University (SUBAPK; project no. 13102019).
Declaration of interest
Authors declare no conflict of interest.
References
Baltaci AK, Yuce K, Mogulkoc R (2018). Zinc metabolism and metallothioneins. Biol Trace Elem Res. 183:22–31. Baltaci SB, Mogulkoc R, Baltaci AK, Emsen A, Artac H (2018) The
effect of zinc and melatonin supplementation on immunity parameters in breast cancer induced by DMBA in rats. Arch Physiol Biochem. 124:247–252.
Chen CC, Chapman WC, Hanto DW (2015) Ischemia-reperfusion injury in kidney transplantation. Front Biosci. 1:117–134. Ergur BU, Kiray M,Pekcetin C, Bagriyanik HA, Erbil G (2008)
Protective effect of erythropoietin pretreatment in testicular ischemia-reperfusion injury in rats. J Pediatr Surg. 43:722–728. Glodowski SD, Wagener G (2015) New insights into the
mechanisms of acute kidney injury in the intensive care unit. J Clin Anesth. 27:175–180.
Grebić D, Jakovac H, Mrakovcic-Sutic I, Tomac J, Bulog A, Micovic V, Radosević-Stasić B 2007 Short-term exposure of mice to gasoline vapor increases the metallothionein expression in the brain, lungs and kidney. Histol Histopathol. 22:593–601.
Hao Y, Ren J, Liu J, Luo S, Ma T, Li R, Su Y (2012) The protective role of zinc against acute toxicity of depleted uranium in rats. Basic Clin Pharmacol Toxicol. 111:402–410.
Hozumi I (2013) Roles and therapeutic potential of metallothioneins in neurodegenerative diseases. Curr Pharm Biotechnol. 14:408–413.
Hu B, Wu Y, Tong F, Liu J, Shen X, Shen R, Xu G (2017) Apocynin alleviates renal ischemia/reperfusion injury through regulating the level of zinc and metallothionen. Biol Trace Elem Res. 178:71–78. Kimura T, Kambe T (2016) The functions of metallothionein
and ZIP and ZnT transporters: an overview and perspective. Int Mol Sci. 17:336.
Kojima I, Tanaka T, Inagi R, Nishi H, Aburatani H, Kato H, Miyata T, Fujita T, Nangaku M (2009) Metallothionein is upregulated by hypoxia and stabilizes hypoxia-inducible factor in the kidney. Kid Int. 75:268–277.
Li B, Tan Y, Sun W, Fu Y, Miao Cai L (2013) The role of zinc in the prevention of diabetic cardiomyopathy and nephropathy. Toxicol Mech Methods. 23:27–33.
Malek M, Nematbakhsh M (2015) Renal ischemia/
reperfusion injury; from pathophysiology to treatment. J Ren Inj Prev. 4:20–27.
Mocchegiani E, Malavolta M, Muti E, Costarelli L, Cipriano C, Piacenza F, Tesei S, Giacconi R, Lattanzio F
(2008) Zinc, metallothioneins and longevity:
interrelationships with niacin and selenium. Curr Pharm Des. 14:2719–2732.
Ozcelik D, Naziroglu M, Tuncdemir M, Celik O, Ozturk M, Flores-Arce MF (2012) Zinc supplementation attenuates metallothionein and oxidative stress changes in kidney of streptozotocin-induced diabetic rats. Biol Trace Elem Res. 150:342–349.
Peixoto NC, Serafim MA, Flores EM, Bebianno MJ, Pereira ME (2007) Metallothionein, zinc, and mercury levels in tissues of young rats exposed to zinc and subsequently to mercury. Life Sci. 81:1264–1271. Ruttkay-Nedecky B, Nejdl L, Gumulec J, Zitka O, Masarik M,
Eckschlager T, Stiborova M, Adam R, Kizek R. (2013) The role of metallothionein in oxidative stress. Int J Mol Sci. 14:6044–6066.
Sabolić I, Breljak D, Skarica M, Herak-Kramberger CM (2010) Role of metallothionein in cadmium traffic and toxicity in kidneys and other mammalian organs. Biometals. 23:897–926.
Szczurek EI, Bjornsson CS, Noto AD, Taylor CG (2009)
Renal metallothionein responds rapidly and site
specifically to zinc repletion in growing rats. J Trace Elem Med Biol. 23:176–182.
Tang Y, Yang Q, Lu J, Zhang X, Suen D, Tan Y, Jin L, Xiao J, Xie R, Rane M, Li X, Cai L (2010) Zinc supplementation partially prevents renal pathological changes in diabetic rats. J Nutr Biochem. 21:237–246.
Tucci Junior S, Carvalho RM, F.M. Celini FM, Cologna AJ, Suaid HJ, Tirapelli LF, Martins AC (2008) Renal
ischemia and reperfusion injury: influence of
chorpromazine on renal function and lipid peroxidation. Acta Cir Bras. 23 Suppl:142–46.
Varghese J, Faith M, Jacob M (2009) Zinc prevents
indomethacin-induced renal damage in rats by
ameliorating oxidative stress and mitochondrial
dysfunction. Eur Pharmacol. 614:114–121.
Yang CL, Du XH, Zhao JH, Chen W, Han YX (1994) Zinc-induced metallothionein synthesis could protect from gentamicin nephrotoxicity in suspended proximal tubules of rats. Ren Fail. 16:61–69.
Yu J, Fujishiro H, Miyataka H, Oyama TM, Hasegawa T, Seko Y, Miura N, Himeno S (2009) Dichotomous effects of lead acetate on the expression of metallothionein in the liver and kidney of mice. Biol Pharm Bull. 32:1037–1042.