Microwave Roasting of Pyrite and Pyrite Ash for
Sponge Iron Production
Yıldırım İ. Tosun
Mining Engineering Department, Şırnak University, Şırnak, Turkey
Abstract— In the Southeastern Anatolian Region of
Turkey, in Ergani Elazığ and Siirt Şirvan copper ore concentrators, containing the pyrite and the high pyrite content discarded is received as pyrite concentrate from concentrating copper by flotation swept and waste products. Ergani Concentrator produce the pyrite concentrate by product about 350 thousand tons for sulfuric acid production and about 1,700 thousand tons of pyrite waste sent to dispose, Siirt Şirvan copper pyrite is not also evaluated. These pyrite waste products both should be evaluated by the copper content and the other metals such as, Au, Ag, Co , must be evaluated in terms of high valuable metal contents. In this study, samples are subjected to microwave roasting of pyrite waste and subsequently pelletized and subjected to reductive roasting by coal at 1000C.
Sulfur-containing complexes in Southeast Anatolia pyritic copper ores rich hydrothermal ore deposits is 2-4% Cu constitute the vessels. Ergani, Siirt and Hakkari Sirvan copper, lead, zinc Fe sulfide deposits demonstrates the wide distribution of reserves. Ergani copper concentrate are produced by several million tons of ore concentrate processed at least 100 thousand tons. Every year about 1 million tons of pyrite to go waste. Siirt Shirvan is 400 thousand tons per annum of crude ore concentrate produced 300 thousand tons of waste are produced pyrite. Therefore, it is becoming clearer common waste product of pyrite in the region. Occurs in a particle size of these wastes usually occurs below 100 microns in size may be advantageous for the evaluation. In our country, especially in Siirt and Hakkari region that includes 15 m thick spread over a large area outside the production of this ore and waste of pyrite Fe silicates evaluability it was discussed as chemical production in this study.
In this study; the effect on the physical and chemical parameters and chemical properties making preliminary tests to determine the pelletizing and microvwave roasting conditions, reactivity were investigated. This assay has been determined to be advantageous in the metal results in the production of pyrite solution with the waste.
In this study.the evaluation Ergani copper concentrator and waste in Siirt in this study were identified as potential evaluability in terms of basic qualifications..
Keywords: Pyrite waste, lron production, iron ore, sponge lron,.
1. Introduction
Ergani Elazig and Siirt Shirvan pyrite pyrite copper ore processing in a concentrator concentrating from fotasyo swept the cellulase and taken waste products. Usually Ergani Concentrator 1,700 thousand tons of waste is sent to the pyrite concentrate product sulfuric acid production. chlorine is about 350 thousand tons of waste are disposed of without pyrite in Siirt Sirvan was also evaluated. These waste products in copper and other content from the care of both, Ag, must be evaluated in terms of metal contents as Course. In this study, samples are subjected to roasting pyrite waste sulfuric acid, hypo chloride, and in hydrochloric acid.
The optimum time with dilute acid solutions were leaching. The results obtained are cobalt, copper, is inferred based on the time of acquisition of the roasting of gold and silver. Copper and cobalt have been achieved in 55-73% yield. The reason that the iron content was low as provided an advantageous gain in partial roasting.
The complex pyritic hydrothermal ore deposits of copper sulphide ore in the Southeastern Anatolia Region and partly containing 2-4% Cu is rich vein of form. Ergani, Shirvan Siirt and Hakkari, copper, lead, zinc sulfide deposits Fe shows a large reservoir distribution. Ergani several million tons of copper ore concentrator produced about 100 thousand tons of concentrate processed at least. Approximately 1 million tonnes of waste each year, pyrite and pyrite concentrate exit 300 Binton. Shirvan Siirt 400 thousand tons per year of crude ore concentrator produced 300 thousand tons of waste are produced pyrite. Therefore, it is becoming clearer common waste product of pyrite in the region. Occurs in a particle size of these wastes often occurs under a hyperfine size of 75 microns may be advantageous for the evaluation thereof. In our country, especially in Siirt and Hakkari region of 15 m thickness of 100 km2 that includes in and silikatları other than the production of this ore spread over an area in pyridine Fe production of waste evaluability was discussed as chemicals in this study.
In this study; Low-grade iron ore and metal production by roasting pyrite was carried forward to the waste area. X-ray analysis of the product difraktomet mineralogy and grain size
of the microscopic description ineL was determined the effects of leaching characteristics of physical and chemical parameters. chemical properties making preliminary tests to determine the pelleting and roasting conditions, the reactivity were investigated. As a result of these experiments it was determined to be advantageous in the metal production waste liquors pyridine. Co, Cu, Au, Ag, etc. It can be the gain from the solution by electrolysis, it will provide çöeltil metallic value and benefit to the economy.
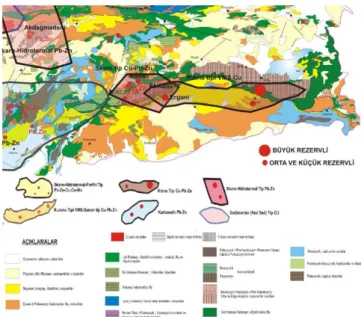
1.1 Siirt, Ergani copper pyrite deposits
Alpine orogeny is located 650 surveys copper deposits in Turkey are seen in lane 4 in the main metallogenic provinces: 1. Coming from Macedonia, the Balkan-Black Sea after passing throughout the Black Sea and Caucasus since the close of Sinop belt extending from Iran to the Himalayas. This generation is common in porphyry copper deposits and Kuroko-type massive sulphide deposits. This generation of on-Derekoy Kırklareli, Bakırçay (Merzifon), Güzelyayla, Macka, Ulutaş-İspir and Ballıca-Yusufeli- (Artvin) are porphyry copper deposits. They are lower than the average copper grade porphyry copper deposits in the Balkans. Also Espie-Lahanos, Cayeli, Blessed are Murgul volcanic massive sulphide deposits and is located on the Cerattepe this generation.
2 came via Cyprus Alexander - Hakkari and then continued between Iran Ophiolite Belt in the Southeastern Anatolia are the Cyprus-type copper deposits. Ergani copper and copper deposits that they mineralization Siirt Madenköy important to this generation.
3. The third is still Globe metallogenic provinces in the Black Sea region of western Cyprus, where copper is the type of bed mattress.
4. plutonizm acidic hydrothermal veins and connected to the kontakmetasomatik copper-lead-zinc deposits found in the northwestern Anatolia Region is the fourth in the metallogenic provinces.
World consumption of copper porphyry meet a large portion of the acidic copper deposits containing calc in igneous rocks are exposed as common in our country. Turkey, however, shows they do not have reserves and grades can be operated under the present circumstances the so far identified porphyry copper deposits. Kırklareli - Derekoy porphyry copper deposits feasibility studies completed in our country is the only bed of this type.
Bakibaba bed with ore reserve in Aşıköy and Cu, Zn, Co, Au, Ag grades are given in Table 1. Table1 shows metal content and economic value. Also, according to this bed of Outokumpu Oy firm developed the flow chart, a yield of 80% to 15% Cu grade is projected chalcopyrite concentrate will be produced, to be obtained from bearing pyrite and chalcopyrite concentrated amounts. When the metal values contained in these beds compared to cobalt copper is seen that three times as much economic value. There is also a copper equivalent gold in bed.
Figure 1. Geological Map Distrubiton of South an Eastern Anatolian Economic Copper Reserves
The general procedure used in evaluating the pyrite solution from the waste is send to the production of sulfuric acid. Chalcopyrite ore from ore by physical methods before concentrators pyrite and copper minerals are enriched by flotation. Then blister with the smelting processes of copper from the copper concentrate obtained after SO2 with the
oxidizing roasting of pyrite concentrate H2SO4 is produced.
During these operations;
gold and silver contained in copper concentrate smelting bilister stay in copper is then recovered during the refining process. But gold and silver will be recovered from the ore ratio is dependent on the ratio of copper concentrates, this rate is around 4% for Ergani ore. Cobalt is going through the converter slag during the smelting and cannot be assessed.
Usually staying in pyrite concentrate Co, Cu, Au and Ag values such as the metal after roasting they remain in pyrite ash waste can only be won when evaluating the ashes.
The general procedure used in evaluating the pyridine solution from the waste is send to the production of sulfuric acid. Chalcopyrite ore from ore by physical methods before concentrators pyridine and copper minerals are enriched by flotation. Then bilister with the smelting processes of copper from the copper concentrate obtained after S02 with the oxidizing roasting of pyrite concentrate H2SO4 is produced. During these operations;
gold and silver contained in copper concentrate smelting bilister stay in copper is then recovered during the refining process. But gold and silver will be recovered from the ore ratio is dependent on the ratio of copper concentrates, this rate is around 4% for Ergani ore. Cobalt is going through the converter slag during the smelting and cannot be assessed.
Usually staying in pyrite concentrate Co, Cu, Au and Ag values such as the metal after roasting they remain in pyrite ash waste can only be won when evaluating the ashes.
1.2 Copper Concentrator pyrite roasting of Waste In recent years, said the purpose of recovery of metals from sulphide ores hydrometallurgical processes have the option to pyrometallurgical process. The bulk concentrate leaching is produced. Pyrite and gangue other sulphide minerals contained in the ore than the minerals oxidizing agent with the sulfated media Fe3+ or under pressure with O2
(6,7), chlorinated environment Fe3+ or Cu2+ (8-13), nitric acid environment with O2 ( 14) and the ammonia atmosphere under O2 pressure (15 to 17) are dissolved.
1.3. Co, Cu, Au and Ag Recovery from Pyrite Ash Partially tested in pilot scale or industrial applications with past practice (21):Bureau of Mines and Cominco (leaching with ferric chloride), Sherrit Gordon and Pennoro (LIC with cupric chloride),
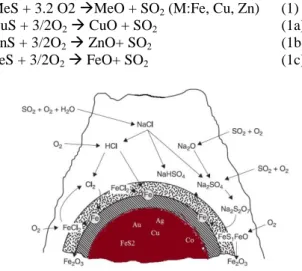
Cymet (ferric chloride + electrolysis), Duval (+ ferric chloride, cupric chloride) and Arbiter (leaching with oxygen in ammonia atmosphere) after roasting pyrite concentrate of metal sulfide are oxidized according to the following reaction:
MeS + 3.2 O2 MeO + SO2 (M:Fe, Cu, Zn) (1)
CuS + 3/2O2 CuO + SO2 (1a)
ZnS + 3/2O2 ZnO+ SO2 (1b)
FeS + 3/2O2 FeO+ SO2 (1c)
Figure 2. The Chemical reactions of the pyrite particles used in Experiment
The roasting was the main application used in the recovery of these metals which is converted into oxide (22, 23).
1.3.1. Leaching with sulfuric acid
Ashes low acid (1% H2SO4) which is the leaching
solution. Water soluble acid-soluble copper sulfate and copper oxide remains in the leach residue by dissolving the other copper compounds in this process. This process, by Amagasaki Dow Seiko order copper to iron pyrite recovery until 1969 from 1953 were applied to the whole (24,25). The main drawbacks by this process are as follows: separated from the insoluble portion of the resulting leach solution is the problem, 30-70% copper with other metals recovery cannot be taken due to efficiency in the desired efficiency is obtained
from leach solution as copper with copper cementation cemented.
1.3.2. Sulfating roasting and leaching
In this process the roasted ash before concentrated sulfuric acid are mixed in specific proportions and, after drying the resulting slurry at 600 to 700 ° C. This product is then roasted in dilute acid leaching (26-29). The objective is sulfated iron oxide without nonferrous metal oxides are soluble in dilute acid to convert to sulfate.
The drawbacks of this process are given below:
The pelletized ash mixed acid to reduce the filtration difficulties must be made of the roasting process.
Copper, zinc and cobalt in after the attainment of high yields, arsenic containing more difficult due to the dissolution of the arsenic leaching from ash no longer be able to be fed into the blast furnace as iron.
The leaching solution with copper cementation of cobalt and zinc occurred by raising the pH.
2. Material and Methods
Experiments in Ergani Elazig and Shirvan the dust waste Siirt concentrator plant that the tumbler as waste is planned to be regarded as construction materials due to the silica content. However, is the average grade% 0,7- 1,2 Cu and contains a total about 270 thousand ton Cu and 30 thousand tons Zn in the waste stock. Also it is expected to contain 255 tons and 63 tons of Ag.
The representing waste samples containing Ergani pyrite waste and Sirvan pyrite waste were taken from the waste stock concentrators and oxidizing conditions for 4 hours at 850° C in 100 g of ash that were roasted and samples were obtained. Analysis of the subjected waste pyrite ash on experimental work is given in Table 1 below. represents the size of the waste sample (90% -325 mesh) over direct hydrometallurgical processes and ash (48% -325 mesh) on the influence of the oxidizing roasting were investigated.
Table 1. Chemical composition of the sample used in Experiment Waste Kümülati f Miktar Stok Cu % S% Co % Au,pp m Ag,pp m Ergan i 2,200,00 0 0,7 37, 4 0,33 3 16 Şirva n 1,300,00 0 1,2 33, 2 0,22 2 22
3. Results and Discussion 3.1 Effective Microwave Roasting
Ergani and Shirvan pyrite copper concentrator waste of 100g health represents 75-micron powder samples between 1 yl 5 min time in oxidizing conditions prior to oxidative leaching is subjected to microwave roasting. Test results were determined as after 30 min boiling with 5M HCI acid Cu and Co recovery yields.
Figure 3. Microwave Roasting in order to burn pyrite, and direct effect on waste metal extracts
Figure 4. Effects of Microwave Roasting in Ergani pyrite, Direct Effect on waste metal extracts
Figure 5. Effects of Microwave Roasting in Sirvan pyrite, Direct Effect on waste metal extracts
3.2. Leaching with sulfuric acid and Hydrochloric Acid
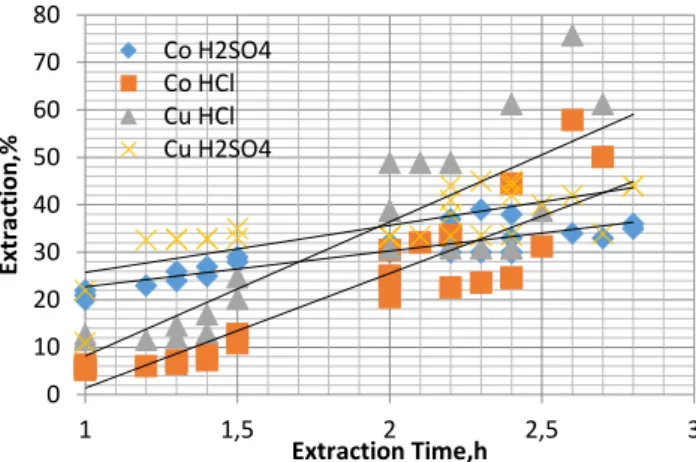
After Ergani pyrite wastes directly from Microwave acted Acid Leaching be effective in certain time 2.5 min to 100 ° C.test leaching, the results are shown in Figure 4.
Figure 5. Effect of Sirvan pyrite Microwave Firing of waste sulfuric acid and 3M and 3M HCl acid leach affect the metal recovery by oxidizing the copper waste Şirvan pyrite leaching at 100°C, 3 hours, 1.5 solid / liquid ratio, 3M H2SO4
solution is carried out at 100g waste samples. ICP analysis of the filtered product solution Cu and Co was examined for. In the hydrochloric acid leach 100 ° C, 3 hours, 1.5 solid / liquid ratio, 100g are performed in solving waste samples of 3M HCl solution. The findings obtained in optimum conditions is shown in Figure 5.
3.2.1 Pyrite Type
The advantages of such a procedure are that coarse coal particles have a lower heating rate than fines, thus, temperature control during pyrolysis would be enhanced. Additionally, there will be improved coal dust control during pyrolysis and the specific energy consumptions required for complete pyrolysis by microwave heating and conventional pyrolysis were about 0,18 and 4,4 kW h/kg, respectively. Typically, the energy consumption in ball milling is between about 4,5 and 9 kWh/kg. Therefore, the combined energy consumption for microwave roasting plus regrinding would still be lower than conventional.
The two major causes of pyrite and iron ores are the presence of ferrous ions and magnetic attenuation of waves in carbonaceous matter and ultra-fine sulphide particles, pyrite and pyhrotite in coal matrix. When less permitivity is due to the presence of both sulphides in coal matter, the shale, silicate clay matrix is called transparent and reflects through under the microwaves. The carbonaceous matter in the ore adsorbs heat. The most important matter are the organic carbon and coal carbon. The constituents of the organic carbon are amorphous.
Such ores require pretreatment to break down the matrix of the sulphides and oxidize or passivate the carbonaceous matter before pyrolysis. The microwave treatment methods include roasting, chlorination, pressure oxidation, drying, torrefaction, pyrolysis and also digestion and gasification of waste. Microwaves could be utilized as an alternative source of energy for the treatment of ores in some of the unit operations such as drying, calcining, roasting and smelting. Carbon and metal sulphides are known to be very good microwave absorbers and they can be rapidly and selectively heated. Some researhers may improve heat it was indirectly heated by microwaves, therefore using magnetite as a susceptor.
In the present study, the microwave roasting of sample with coal pyrite and copper pyrite was investigated. The concentrate was very responsive to microwave heating and this resulted in almost complete roasting and in some cases sintering of the material as seen in Figure 5.
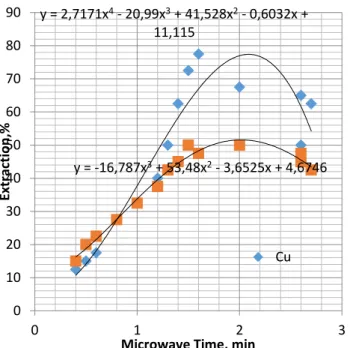
y = -10,701x3 + 30,218x2 + 18,125x + 3,6269 y = 13,236x4 - 82,427x3 + 160,68x2 - 90,806x + 30,052 0 10 20 30 40 50 60 70 80 0 0,5 1 1,5 2 2,5 3 Extr ac tion ,%
Microwave Time, min Cu y = -16,787x3 + 53,48x2 - 3,6525x + 4,6746 y = 2,7171x4 - 20,99x3 + 41,528x2 - 0,6032x + 11,115 0 10 20 30 40 50 60 70 80 90 0 1 2 3 Extr ac tion ,%
Microwave Time, min Cu
The changes in the heat absorbing behaviour of the copper pyrite were monitored and the optimum conditions for leaching the roasting rate were established as seen in Figure 6.
Figure 6. Time effect on temperature in Microwave Roasting
Figure 7. Porosity effect on permitivity in Microwave Roasting
800 W, the 15 g sample reached a temperature of about 500
oC while the 25 g sample reached 600 oC. When 50 g of
sample was used, the temperature rose to about 700 oC.
Generally, in laboratory scale microwave processing, the sample temperature increases with sample mass. In contrast to conventional heating, in microwave systems the heat is generated internally and thus the heat loss from the sample is a major factor that controls the heating behaviour. For samples with a relatively low mass, the high surface area to volume ratio restricts the rate of temperature rise and also the maximum attainable temperature. As a result, the permittivity values are relatively low and the sample cannot effectively couple with the microwave field. On the other hand, for the same cross-sectional area of the crucible, as the sample mass is increased, there is a reduction in the surface area to volume ratio and this reduces the heat loss from the interior, leading to a higher bulk sample temperature. Additionally, as the sample mass increases, there is more material to interact with the electric field.
For comparison purposes, oxidation of the sulphides and the carbonaceous matter in the refractory concentrate was carried out in both the microwave system and a conventional resistance furnace. At an incident microwave power of 800 W, the heat generated was enough to cause melting of the sample and thus further tests were conducted at a lower
incident power of 600 W. At 600 W, appreciable oxidation was achieved, without excessive rise in temperature. However, with some samples, sintering was observed. As shown by the results in Figure 3 the roasting process is almost complete at about 600 oC and therefore the conventional roasting tests were performed at 600 oC. Since the permittivity
decreased above 700 oC, additional roasting was carried out
above 700 oC. Figure 4 shows the effect of roasting time on
the carbon content of the concentrate as a function of processing time for both conventional and microwave roasting. It can be seen that the carbon content decreases significantly faster in the microwave tests than in conventional roasting. More than 75% of the carbon was removed within minutes with microwave roasting, while in conventional roasting the same degree of carbon removal would require hours. The behaviour of the coal pyrite is presented in Figure 5. For both microwave and conventional processing, the rate of temperature at the end was higher than that of coal pyrite, which likely reflects the larger amount of iron in the sample. After 30 min, 85% of the sulphur was removed by conventional roasting, while for microwave heating about 65% of the pyrite sulphur was removed at the end of 3 mins. In microwave processing the pyrolysis of coal and pyrite content of coal particles (Figure 6), which are excellent microwave absorbers, are at higher temperatures than in conventional pyrolysis and this leads to higher pyrolysis rates.
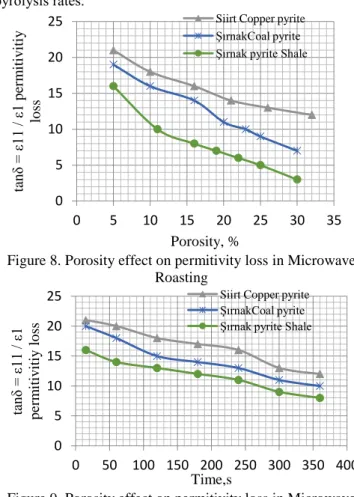
Figure 8. Porosity effect on permitivity loss in Microwave Roasting
Figure 9. Porosity effect on permitivity loss in Microwave Roasting 0 100 200 300 400 500 600 700 800 0 100 200 300 400 T em p era tu re ,C Time,s Siirt Copper pyrite ŞırnakCoal pyrite Şırnak pyrite Shale
0 100 200 300 400 500 600 0 5 10 15 20 25 30 35 T em p era tu re , C Porosity, %
Siirt Copper pyrite ŞırnakCoal pyrite Şırnak pyrite Shale
0 5 10 15 20 25 0 5 10 15 20 25 30 35 tan δ = ε1 1 / ε 1 p er m itiv iti y lo ss Porosity, %
Siirt Copper pyrite ŞırnakCoal pyrite Şırnak pyrite Shale
0 5 10 15 20 25 0 50 100 150 200 250 300 350 400 tan δ = ε1 1 / ε 1 p er m itiv itiy lo ss Time,s
Siirt Copper pyrite ŞırnakCoal pyrite Şırnak pyrite Shale
All figures and tables must be consecutively numbered with Arabic numerals (1, 2, 3 etc.) and inserted as close as possible to the corresponding text. In the text, refer to the figure by its number, not its relative position.
4. Conclusions
In this study; The low-grade ore with pyrite waste Fe in physical, chemical, giving place to the results of tests carried out were determined by examining the difference between the textural properties of pyrite waste. Evaluation of copper kosantrat waste in Ergani and Siirt in this study were identified as potential evaluability in terms of basic qualifications. pyrite ash from the roasting process with this metallic values ??obtained after acquisition of iron is determined as iron raw materials rich product can be evaluated. Especially with microwave roasting process it is very economical.
Figure 6 is obtained from ore according to the flow chart bulk concentrate 700 C oxidizing acidic oxidizing the ash obtained after roasting and microwave leaching Co, Cu, Au and Ag higher in RA is economically recovered. Also iron content will be higher.
Figure 10 The prompted evaluation for the Pyrite Ash of Ergani and Sirvan.
The specific energy consumptions required to achieve
oxidation of the refractory concentrate by microwave
heating and conventional roasting were about 0,128 and
4,58 kW h/kg, respectively. In conventional roasting, the
specific energy values are higher since the surroundings
also have to be heated, while in the microwave process
only the sample and the sample carrier are heated.
The oxidation reactions of sulphides are exothermic and
if heating is not controlled, there is the tendency for
sintering and/or melting to take place. In microwave
heating it is more difficult to control the temperature,
than in conventional roasting. As a result, some local
melting occurred and there was some sintering and
formation of some glassy material.
The roasting of copper pyrite and iron ores, the pyrites
of coal and shale using microwave radiation has been
investigated. The test results showed that there was a
continuous mass loss from room temperature to 700
oC
with a total mass loss of 12%. The major mass loss of
over 10% occurred between 400
oC and 600
oC in coal
pyrolysis. This occurred due to the sulphur combustion
of and reaction of exothermic heat release from pyrite
from the sample. The real and relative permittivities
were very high and increased significantly with
decreasing frequency. Beyond 400
oC the permittivity
decreased and this was attributed to the removal of most
of the combustion of pyrite. The copper pyrite could be
heated rapidly and temperatures of over 600
oC were
attained with a 50 g sample after microwave heating for
3 min.
For microwave pyrolysis, both the heating rate and the
pyrolysis rates were higher with depending on porosity
and the specific energy consumptions were lower than
the corresponding values for conventional pyrolysis. In
roasting and pyrolysis processes, the pyrite content was
readily effective in accomplished amount of the reacted
matter. Due to the high temperatures generated in
microwave heating, sections of the carrier had sintered
in which pyrite oxidation of about 65% complished in
microwave were roasting.
The microwave heating behavior studies showed that the
sample temperature increased with increasing incident
microwave power, processing time and sample mass.
Due to the hyperactive response of the iron ore to the
microwaves, a low incident power of 600W was found
to be suitable for roasting, as higher powers resulted in
sintering and melting of the concentrate. The copper
pyrite values after combustion in roasting were over
25% and these were similar to those obtained by
conventional roasting. The main advantages of
microwave roasting were that both the total pyrolysis
rates and the heating rates were higher and the specific
energy consumptions were lower than in coal pyrite.
Acknowledgement
The author woul like to respectfully thank to ALFA MAKİNA VE KAZAN AŞ, in their efforts.
References
[1] Siirt Madenköy Fizibilite Etudu, MTA, Ankara, 1981 [1].YU, H., HANSEN, K., WADSWORTH, M., A Kinetic
Study of The Leaching of Chalcopyrite at Eleveted Temperatures, Metall. Trans., 4, 1973, s. 2138-2144 [2].GERLACH, J.K , GOCK, E, D., GHOSH, S, K.,
Activation and Leaching of Chalcopyrite Concentrates with Dilute Sulfuric Acid, Int. Symposium on Hydrometallurgy, Chicago, AIME, Ed. Evans, D, J, I., Shoemaker, R, S., New York 1973, s. 403-416.
[3].HOVER, E, P., WONG, M, M., Recovery of Elemental Sulfur from Non Ferrous Minerals Ferric Chloride Leaching of Chalcopyrite Concentrate, U.S. Bur. of Mines, Rl 7474, J. of Metals, vol 23, no 2, 1971, s. 25-29
0 10 20 30 40 50 60 70 80 1 1,5 2 2,5 3 Ex tr ac ti o n ,% Extraction Time,h Co H2SO4 Co HCl Cu HCl Cu H2SO4
[4].HOVER, E, P., BAKER, R, D., WONG, M, M., Improvement in the Ferric Chloride Leaching of Chalcopyrite Concentrates, U S Bur. of Mines Rl 8007, 1975
[5].KUNDA, W., HİTESMAN, R., VELTMAN, H., Treatement of Sulphıdıc Copper Concentrate in Chloride Systems, Extractive Metallurgy of Copper, Las Vegas, AIME New York, 1976 s 738-813
[6].ARBITER, N., KUHN, M, C, KLİNG, H., Anaconda s Arbiter Process for Copper, CİM Bulletin,vol. 67, no 742, February, 1974, s. 62-71
[7].TOZAWA, K., UMETSU, Y., SATO, K., On Chemistry of Ammonia Leaching of Copper Concentrate, Extractive Metallurgy of Copper, Las Vegas, AIME New York, vol. II, chap. 36, 1976, s. 706-721
[8].SCOTT, T, R., DYSON, N, F., The Benefication of Chalcopynte Concentrates by Leaching with HCI-CaCI2 and HCI-MgCI2 Solutions, CSIRO IR 827, Trans. Inst. Mm. Met., 85 c, 1976, s. 40
[9].CANBAZOĞLU, M., Sülfürlü Cevherlerin Hıdrometalurjik Yöntemlerle Değerlendirilmesi, Madencilik. Aralık, 1979, s. 9-20.
[10]. KUYUCAK, S., Türkiye'nin Pirit Külü Kaynaklarının Demir ve Demir Dışı Metaller Yönünden Değerlendirilmesi, MTA Teknoloji Dairesi, Araştırma Raporu 1, Ankara, Nisan 1977
[11]. OKUBO, Y., Kowa Seiko Pelletizing Chlorination Process-Integral Utilization of Iron Pyrites, Journal of Metals, March, 1968, s. 63-67
[12]. Chen TT, Dutrizac JE, Haque KE, Wyslouzil W, Kashyap S. 1984, The relative transparency of minerals to microwave radiation. Canadian Metallurgical Quarterly, 123, 3, s. 349–51.
[13]. Amankwah, R.K., Pickles, C.A., 2005. Microwave calcination and sintering of manganese carbonate ore.
Canadian Metallurgical Quarterly 44 (2), 239–248.
[14]. Amankwah, R.K., Pickles, C.A., Yen, W.T., 2005b. Gold recovery by microwave augmented ashing of waste activated carbon. Minerals Engineering 18 (2), 517–526. [15]. Gabriel C., Gabriel S., Grant E.H., Halstead B.S.J.,
Mingos D.M.P., 1998, Dielectric parameters relevant to microwave dielectric heating. Chemical Society Reviews, 27, s.213–23.
[16]. Datta A K; Sun E; Solis A (1995). Food dielectric property data and their composition-based prediction. In:
Engineering Properties of Foods (Rao M A; Rizvi S S,
eds), Chapter 9, 457–494. Marcel Dekker, Inc., New York [17]. Datta A K; Nelson S O (2000). Fundamental Physical
Aspects of Microwave Absorption and Heating in
Handbook of Microwave Technology for Food Applications. CHIPS Publications, USA
[18]. Decareau R V (1985). Microwaves in the Food
Processing Industry. Academic Press, Orlando, FL, USA
[19]. El-Shami S M; Selim I Z; El-Anwar I M; Hassan M M (1992). Dielectric properties for monitoring the quality of heated oils. Journal of the American Oil Chemists’ Society
(JAOCS), 69(9), 872–875
[20]. Walkiewicz JW, Kazonich G, McGill SL., 1988, Microwave heating characteristics of selected minerals and compounds. Minerals and Metallurgical Processing , 5, 1, s.39–42.
[21]. Walkiewicz J.W., Clark A.E., McGill S.L., 1991, Microwave assisted grinding. IEEE Transactions on
IndustryApplications ,27, 2, s.239–43.
[22]. Haque KE. Microwave energy for mineral treatment processes—a brief review, 1999, International Journal of
Mineral Processing, 57, 1, s.1–24.
[23]. Jacob J., Chia L.H.L., Boey F.Y.C.,.1995, Review— thermal and non-thermal interaction of microwave radiation with materials. Journal of Materials Science, 30, 21, s.5321–7.
[24]. Kelly RM, Rowson NA., 1995, Microwave reduction of oxidised ilmenite concentrates. Minerals Engineering, 8, 11, s.1427–38.
[25]. Metaxas, A.C., Meredith, R.J., 1983. Industrial
Microwave Heating. Chapter 10, Peter Peregrinus,
London, UK.
[26]. Hutcheon, R.M., De Jong, M.S., Adams, F.P., 1992. A system for rapid measurement of RF and microwave properties up to 1400 _C. Journal of Microwave Power and Electromagnetic Energy 27 (2), 87–92.
[27]. Hutcheon, R.M., De Jong, M.S., Adams, F.P., Lucuta, P.G., McGregor, J.E., Bahen, L., 1992a. RF and microwave dielectric measurements to 1400 _C and dielectric loss mechanisms. In: Materials Research Society
Symposium Proceedings (Microwave Processing of Materials III), vol. 269, pp. 541–551.
[28]. Hutcheon, R.M., Hayward, P., Smith, B.H., Alexander, S.B., 1995. High-temperature dielectric constant measurement – another analytical tool for ceramic studies. Microwaves: Theory and Application in Materials Processing III, vol. 59. Ceramic Transactions, American Ceramic Society, pp. 235–241.
[29]. Karmazsin, E., 1987. Use of low – and high-power microwave energy for thermal analysis. Thermochimica
Acta , 110, 289–295.
[30]. Lu, T., Pickles, C.A., Kelebek, S., 2007. Microwave heating behaviour of a gibbsite type bauxite ore. In: Bekguleryuz, M.O., Paray, F., Wells, M. (Eds.),
Proceedings of Symposium on Light Metals in Transport Applications. MetSoc (CIM), Toronto, Ont. Canada, pp.
421–449 (August 25–30).
[31]. Ma, J., Pickles, C.A., 2003. Microwave segregation process for nickeliferous silicate laterites. Canadian
Metallurgical Quarterly 42 (3), 313–326.
[32]. Veasey TJ, Fitzgibbon KE., 1990, Thermally assisted liberation—a review. Minerals Engineering , 3, 1/2, s.181–5.
[33]. Xia D.K., Pickles C.A., 2000, Microwave caustic leaching of electric arc furnace dust, Minerals Engineering, 13, 1, s.79–94.
[34]. Kılıç Ö., 2009, Mikrodalga ile Isıl İşlem Uygulamanın Kireçtaşı Kalsinasyonuna Etkisi, Madencilik, 48, 3, s 45-53.
[35]. Kingman S.W., Vorster W., Rowson N.A., 1999, The influence of mineralogy on microwave assisted grinding.
Minerals Engineering, 3,3, s.313–27.
[36]. Marland S, Han B, Merchant A, Rowson N., 2000, The effect of microwave radiation on coal grindability. Fuel, 79, 11, s.1283–8.
[37]. Salsman J.B., Williamson R.L., Tolley W.K., Rice D.A., 1996, Short-pulse microwave treatment of disseminated sulphide ores. Minerals Engineering, 9, 1, s.43–54.
[38]. Standish, N., Worner, H.K., Gupta, G., 1990. Temperature distribution in microwave heated iron ore–
carbon composites. J. Microwave Power Electromagnet
Energy 25 _2., 75–80.
[39]. Standish, N., Worner, H.K., Obuchowski, D.Y., 1991. Particle size effect in microwave heating of granular materials. Powder Technology 66, 225–230.
[40]. VanWyk EJ, Bradshaw SM, de Swardt JB., 1998 The dependence of microwave regeneration of activated carbonon time and temperature, Journal of Microwave
Power and Electromagnetic Energy ,33, 3, s.151–7. .