Localizations of cells that secrete bombesin, glucagon, somatostatin-14
in stomach (proventriculus and gizzard) in chicken (Gallus gallus
domestica L.) during the prenatal and postnatal periods
Kenan ÇINAR, Dilek DİLER
Department of Biology, Faculty of Science and Art, Süleyman Demirel University, 32260 Isparta, Turkey
Summary: In this research, mucosal localizations of bombesin, glucagon and somatostatin-14 immunoreactive in some regions of digestive tract of Gallus gallus domestica during the prenatal and postnatal periods were examined using an immunohistochemical technique. For this purpose 80 embryos (beginning from the 6th day of incubation to end of incubation every days), 5 chicks (a-week-old chick) and 10 adult chicken (45 and 60 days old) were used as material. The sections which taken from proventriculus and gizzard stained immunohistochemically. Bombesin immunoreactive cells were firstly observed in Lamina epithelialis (L. epithelialis) of proventriculus on 17th day of incubation (d.i.). Glucagon immunoreactive cells were firstly seen at few
numbers in glands of proventriculus and L. epithelialis of gizzard at 9 d.i. Somatostatin-14 immunoreactive cells were firstly observed in stratified epithelium of proventriculus and gizzard at 6 d.i. In adult period, bombesin, glucagon and somatostatin-14 immunoreactive cells weren’ t found in glandular epithelium of gizzard.
Keywords: Bombesin, Gallus gallus domestica, glucagon, somatostatin-14, stomach.
Tavuklarda (Gallus gallus domestica L.) prenatal ve postnatal dönemlerde bezli ve taşlı midelerin bombesin, glukagon, somatostatin-14 salgılayan hücrelerinin mukoza yerleşimleri
Özet: Bu araştırmada, prenatal ve postnatal dönemlerde Gallus gallus domestica’ nın sindirim kanalının bazı bölgelerinde bulunan bombesin, glukagon ve somatostatin-14 immunreaktif hücrelerin mukoza yerleşimleri immunohistokimyasal teknik ile incelendi. Bu amaçla 80 embriyo (inkübasyonun 6. gününden itibaren inkübasyonun sonuna kadar her gün), 5 civciv (1 haftalık) ve 10 erişkin tavuk (45 ve 60 günlük) materyal olarak kullanıldı. Bezli ve taşlı midelerden alınan kesitler immunohistokimyasal olarak boyandı. Bombesin immunoreaktif hücreler ilk olarak inkübasyonun 17. gününde bezli midenin Lamina epiteliyalis (L. epiteliyalis)’ inde gözlendi. Glukagon immunoreaktif hücreler ilk kez inkübasyonun 9. gününde bezli mide bezlerinde ve taşlı midenin L. epiteliyalis’inde az sayıda gözlendi. Somatostatin-14 immunoreaktif hücreler ilk kez inkübasyonun 6. gününde bezli ve taşlı mideye ait çok katlı epitelde rastlandı. Erişkin dönemde ise taşlı mide bez epitelinde bombesin, glukagon ve somatostatin-14 immunoreaktif hücreler bulunamadı.
Anahtar sözcükler: Bombesin, Gallus gallus domestica, glukagon, mide, somatostatin-14..
Introduction
Endocrine cells that are located in gastrointestinal tract mucosa, form a part of APUD (Amine Precursor Uptake and Decarboxylation) series which are widely present in organism (4, 13, 27). In recent years, DNES (Diffuse Neuro Endocrine System) has taken place of APUD (14, 19, 20, 35). The systems which are constitued by these cells are called gastroenteropancreatic (GEP) system or gastroenteropancreatic-neuroendocrine system (1, 13, 20, 22, 35). Endocrine cells that include avian pancreatic polypeptide, bombesin, cholecystokinin, chromogranin A and B, gastrin, glucagon, met-enkephalin, motilin, neurotensin, serotonin, somatostatin, substance P and vasoactive intestinal peptid were found in gastrointestinal tract mucosa at the end of the studies done during the prenatal and postnatal periods in birds (2, 10, 16, 24, 26).
Bombesin is a tetradecapeptide which was isolated from the skin of the frog Bombina bombina and has been shown to have a wide variety of actions in mammals including stimulation of gastrin, cholecystokinin and pancreatic polypeptide release, gastrointestinal motility, pancreatic enzyme secretion, hypothermia, hypoglycaemia and reduction of glomerular filtration (11, 28, 29).
Glucagon the other peptide to be studied by means of localization in proventriculus and gizzard producedin the gut, pancreas, and the central and peripheral nervous systems and exhibit a wide variety of biological actions inwhich several act as neurotransmitters (15).
Somatostatin is a cylic fourteen amino acid peptide isolated first from the hypothalamus and found to block the secretion of growth hormone and was subsequently shown brain, pancreas and gastrointestinal tract (3, 7, 18, 31). It is capable of inhibiting the release of glucagon,
gastrin, motilin, pancreatic polypeptide, GIP, secretin, prolactin and TSH and gastric acid and pancreatic exocrine secretion (17, 28, 30).
The aim of this study was to determine mucosal localizations of cells that secrete bombesin, glucagon and somatostatin-14 in proventriculus and gizzard by the peroxidase-antiperoxidase (PAP) method in Gallus
gallus domestica during the prenatal and postnatal
periods and to provide data for the other researches.
Materials and Methods
Materials were obtained from 80 embryos (beginning from the 6th day of incubation to the time of hatching every days), 5 young chicks (a-week-old chick) and 10 adult chicken (45 and 60 days old). The samples were taken from proventriculus and gizzard were fixed in Bouin’ s fluid for 24h. After dehydration by passing tissues through a series of alcohol solutions, the samples were vacuum-embedded in parafin. After trimmed 10μm at every turn, serial sections were cut 6-7μm. Sections were stained immunohistochemically using the peroxidase-antiperoxidase (PAP) method (25). After dewaxing, the sections were hydrated through a graded series of alcohol solutions and treated with 0.3% H2O2 in methanol for 20 min. The sections were rinsed with 0.01M PBS (pH 7.4) containing 0.2% Triton X–100 and 0.1% Bovine serum albumine. Subsequently, the sections were incubated for 20–30 min at room temperature in normal goat serum (01–6201, Zymed), then they were incubated with specific antisera for 16–20 h at 4°C. Details of antisera used in this study are listed in Table I. After rinsing in TRIS bufferded saline (TBS), the sections were incubated in goat anti rabbit IgG (1/50; G– 5268, Sigma) for 20–30 min. Following a secondary rinse in TBS, the sections were incubated with PAP complex (1/100; P1291, Sigma) for 30 min at room temperature. The immunoreactions were visualized using a solution of 0.06% DAB (3.3’-diaminobenzidine tetrahydrochloride (0430-5G, Amresco) containing 0.03% H2O2 in 0.5M TBS pH 7.6 for 10–30 min. The sections passed through the series of alcohol and xylene series were investigated under light microscopy after having been covered with entellan.
Table 1. Antisera used.
Tablo 1. Kullanılan antiserumlar.
Antisera Source Code Dilution
Rabbit Anti-bombesin Novocastra 400803 1/200 Rabbit Anti-glucagon Novocastra 402303 1/200 Rabbit Anti- somatostatin-14 Novocastra 404407 1/200
The method of Masson's trichrome was practised on the sections in order to identify the histological structure (5). The counting of endocrine cells was made in proventriculus and gizzard having an average of 25–80 µm epithelium’ s thickness and 1000–7000 µm epithelium’ s length at the early stages of development (6th -11th days of incubation). At the next stages of development, since 12 d.i. approximately 15000 plica gastrica (at the begining their width was 30 µm, length was 55 µm; at the next stages their width was 10 µm, their length was 125 µm) belonging to each period had been found when the plica gastrica was shaped. And also immunoreactive cells were counted in these plica gastrica. All the periods that immunoreactive cells in glands of proventriculus and gizzard having nearly diameter of 55–700 µm were also counted in all periods that were studied. The number of immunoreactive cells in each area were measured by the method of One-Way ANOVA.
Results
The tables II, III and IV show the distribution and frequency of bombesin, glucagon and somatostatin immunoreactive cells in proventriculus and gizzard respectively.
Table 2. Distribution of bombesin immunoreactive cells in the proventriculus and gizzard of Gallus gallus domestica in prenatal and postnatal periods.
Tablo 2. Prenatal ve postnatal dönemlerde Gallus gallus domestica’nın bezli ve taşlı midelerdeki bombesin immunoreaktif hücrelerin dağılımları.
Bombesin Proventriculus Gizzard
L.
epithelialis epithelium Glandular L. epithelialis epitheliumGlandular
6 (d.i.) 0 0 0 0 7 (d.i.) 0 0 0 0 8 (d.i.) 0 0 0 0 9 (d.i.) 0 0 0 0 10 (d.i.) 0 0 0 0 11 (d.i.) 0 0 0 0 12 (d.i.) 0 0 0 0 13 (d.i.) 0 0 0 0 14 (d.i.) 0 0 0 0 15 (d.i.) 0 0 0 0 16 (d.i.) 0 0 0 0 17 (d.i.) 0.750±0.500 0 0 0 18 (d.i.) 0.500±0.577 0.500±0.577 0 0 19 (d.i.) 1.000±0.000 1.000±0.000 0 0 20 (d.i.) 1.250±0.500 1.000±0.000 0 0 21 (d.i.) 1.000±0.000 0.750±0.957 0 0 a-week-old 1.000±0.000 0 0.500±0.577 0 chick 45 days 1.000±0.000 0 0.500±0.577 0 60 days 1.000±0.000 0 0.500±0.577 0 Anova (F) 22.367 7.622 2.667 0 Sig. 0.000 0.000 0.003 0
Table 3. Distribution of glucagon immunoreactive cells in the proventriculus and gizzard of Gallus gallus domestica in prenatal and postnatal periods.
Tablo 3 Prenatal ve postnatal dönemlerde Gallus gallus domestica’nın bezli ve taşlı midesindeki glukagon immunoreaktif hücrelerin dağılımları.
Bombesin Proventriculus Gizzard L.
epithelialis epithelium Glandular L. epithelialis epitheliumGlandular
6 (d.i.) 0 0 0 0 7 (d.i.) 0 0 0 0 8 (d.i.) 0 0 0 0 9 (d.i.) 0 0.750±0.500 1.250±0.500 0 10 (d.i.) 1.000±0.000 0 1.500±0.577 0 11 (d.i.) 2.250±0.500 0.500±0.577 1.500±0.577 0 12 (d.i.) 2.250±0.500 0.750±0.500 1.500±1.000 0 13 (d.i.) 2.000±0.816 0.500±0.577 1.000±0.000 0 14 (d.i.) 2.500±1.290 1.000±0.000 0.750±0.500 0 15 (d.i.) 2.000±0.816 0.500±0.577 1.250±0.957 0 16 (d.i.) 2.000±0.816 0.500±0.577 1.250±0.957 0 17 (d.i.) 2.000±0.816 0.750±0.500 1.500±0.577 0 18 (d.i.) 1.500±0.577 0.500±0.577 1.000±0.816 0 19 (d.i.) 1.500±0.577 0.500±0.577 1.000±0.000 0 20 (d.i.) 0.500±0.577 0 0 0 21 (d.i.) 0.500±0.577 0.500±0.577 0.750±0.957 0 a-week-old 0.500±0.577 0 0 0 chick 45 days 1.000±0.000 0.500±0.577 0 0 60 days 0.750±0.500 0.500±0.577 0 0 Anova (F) 8.840 2.000 5.761 0 Sig. 0.000 0.025 0.000 0
Table 4. Distribution of somatostatin-14 immunoreactive cells in the proventriculus and gizzard of Gallus gallus domestica in prenatal and postnatal periods.
Tablo 4. Prenatal ve postnatal dönemlerde Gallus gallus domestica’nın bezli ve taşlı midelerindeki somatostatin-14 immunoreaktif hücrelerin dağılımları.
Bombesin Proventriculus Gizzard
L. epithelialis Glandular
epithelium L. epithelialis epitheliumGlandular
6 (d.i.) 2.500±0.577 0 2.500±1.290 0 7 (d.i.) 2.750±0.957 0 2.500±1.290 0 8 (d.i.) 2.500±0577 0 2.250±1.258 0 9 (d.i.) 2.250±0.957 2.500±0.577 3.000±0.816 0 10 (d.i.) 1.000±0.000 0 3.250±0.500 0 11 (d.i.) 1.250±0.500 3.000±0.816 3.250±0.957 0 12 (d.i.) 1.250±0.500 2.750±1.500 3.000±0.816 0 13 (d.i.) 1.250±0.500 3.000±0.816 3.250±0.500 0 14 (d.i.) 1.750±0.957 3.250±0.500 3.750±0.500 0 15 (d.i.) 1.750±0.500 4.000±0.000 4.250±0.500 0 16 (d.i.) 1.500±0.577 3.750±0.957 3.750±0.500 0 17 (d.i.) 1.500±0.577 3.500±1.290 3.750±0.500 0 18 (d.i.) 2.000±0.816 1.750±0.957 3.500±0.577 0 19 (d.i.) 1.750±0.957 2.000±0.816 3.750±0.500 0 20 (d.i.) 1.500±0,577 0 1.000±0.816 0 21 (d.i.) 1,250±0.500 0 0 0 a-week-old 1.500±0.577 3.750±0.500 0 chick 45 days 1.500±0.577 0 0 0 60 days 1.250±0.500 0 0 0 Anova (F) 2.316 22.641 16.938 0 Sig. 0.008 0.000 0.000 0 Bombesin
Proventriculus: Few bombesin immunoreactive
cells were firstly seen at 17 d.i. in L. epithelialis. Few cells that indicate this immunoreactivity were observed in glandular epithelium at 18 d.i. Very little increase was detected in the number of bombesin immunoreactive cells in L. epithelialis and glands at 19d.i. While it was identified that bombesin immunoreactive cells were not present in a-week-old chick and adult chicken’s glandular epithelium, in L. epithelialis it was noticed that this immunoreactive cell had similar qualities with the previous period.
Gizzard: No bombesin cells were observed in
prenatal period. In a week and adult period, although the number of cells was few in L. epithelialis of gizzard, they were not seen glandular epithelium.
Glucagon
Proventriculus: Few cells were firstly detected in
proventricular glands at 9 d.i. Glucagon immunoreactive cells were firstly observed in L. epithelialis at 10 d.i. At 11 d.i. glucagon immunoreactive cells were dense in L. epithelialis but they were few in glands. At 18 d.i. there was very little decrease in the number of cells as compared to the previous periods. At 20 d.i. while no glucagon cells were seen in glands, their number was few in L. epithelialis of proventriculus. Some increase in the number of glucagon immunoreactive cells was attained as opposed to the previous period at 21 d.i. In a-week-old chick, no cells were observed in glandular epithelium. In adult period it was seen that glucagon immunoreactive cells were at few numbers in proventricular glands and L. epithelialis (Fig.1).
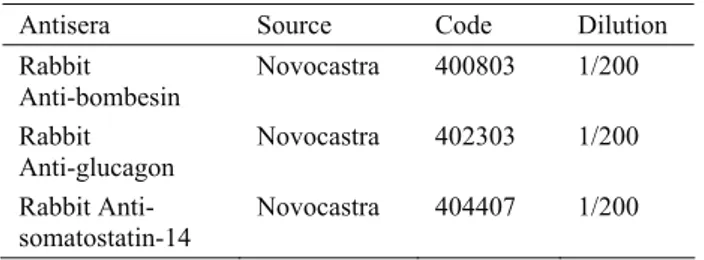
Figure 1. Adult chicken (60 days). Glucagon immunoreactive cell, L. epithelialis, proventriculus. PAP method. X 550. Şekil 1. Erişkin dönem (60 günlük). Glukagon immunoreaktif hücre, L. epiteliyalis, bezli mide. PAP metod. X 550.
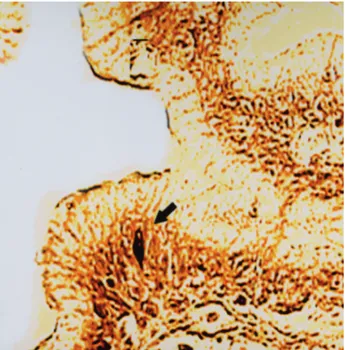
Figure 2. Somatostatin-14 immunoreactive cells, glandular epithelium, proventriculus a-week-old chick. PAP method. X 400.
Şekil 2. 1 haftalık dönem. Somatostatin-14 immunoreaktif hücreler, L. epiteliyalis, glandular epitel, bezli mide. PAP metod. X 400.
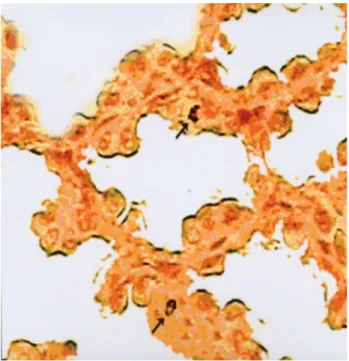
Figure 3. Prenatal period (9 d.i.). Somatostatin-14 immunoreactive cell, L. epithelialis, gizzard. PAP method. X 630.
Şekil 3. Prenatal dönem (9 günlük). Somatostatin-14 immunoreaktif hücre, L. epiteliyalis, L. epiteliyalis, taşlı mide. PAP metod. X 630.
Gizzard: The first cells appeared in L. epithelialis of
gizzard at 9 d.i.. Whereas there was little decrease in the density of glucagon immunoreactive cells in L. epithelialis at 13 d.i., very little increase was observed at 14 d.i. At 15 and 16 d.i., cells density decreased. At 18 d.i. very little decrease was observed in the cells of glucagon immunoreactive in gizzard, these cells were not
noticed at 20 d.i. It was that there was increase in the cells’ numbers when we compared it with the previous day. In this period glucagon immunoreactive cells were not detected.
Somatostatin-14
Proventriculus: Somatostatin-14 immunoreactive
cells were firstly observed at few numbers in stratified epithelium of proventriculus at 6 d.i. At 9 d.i. their number was few in glands of proventriculus. It was seen that the number of immunoreactive cells in glands increased and in L. epithelialis were few at 11 d.i. Although the number of immunoreactive cells in glands increased at 15 d.i. it was found that it was few at 18 d.i. Though in a-week-old chick’s glands of proventriculus the cells’ density increased (Fig. 2), in adult period immunoreactive cells were not observed. But they were seen in L. epithelialis in this period.
Gizzard: Somatostatin-14 immunoreactive cells
were firstly observed at few numbers in stratified epithelium of gizzard at 6 d.i. Very little increase was observed in L. epithelialis at 9 d.i. (Fig.3). It was detected that the number of immunoreactive cells increased in L. epithelialis at 15 d.i. While they decreased in L. epithelialis at 20 d.i., they were not seen at 21 d.i. In this period somatostatin-14 immunoreactive cells were not observed in gizzard.
Discussion and Conclusion
It was reported that the first bombesin cells in proventriculus were seen in chicken at 11 and 18 d.i. (10, 16), in duck at 17 d.i. (9). We determined the first cells in L. epithelialis of proventriculus at 17 d.i. while previous authors (10, 16) found them at 11 and 18 d.i.
Though (6, 8) reported that few number of bombesin immunoreactive cells were present in glandular epithelium of proventriculus in adult ostrich and domestic duck, this study found no bombesin cells that localize in glandular epithelium in adult period.
Castaldo and Lucini (1994) demonstrated the first, few bombesin immunoreactive cells in duck gizzard at 23 d.i. In present study few number of immunoreactive cells was firstly detected in a-week-old chick’ s L. epithelialis.
It was reported that bombesin immunoreactive cells in adult ostrich gizzard were numerous (6) whereas the number of cells was few in adult domestic duck gizzard (8). Contrary to these results no cells detected in postnatal period.
Though it was indicated that glucagon immunoreactive cells in L. epithelialis of proventriculus were appeared at 12 d.i. in quail (32), at 13 d.i. in chicken (2), we determined the first cells in L. epithelialis at 10 d.i.
Bezeuidenhout and Van Aswegen (1990) reported that glucagon immunoreactive cells located in proventricular glands of adult ostrich. Few number of cells in glands were identified in this study.
Yamaguchi et al. (1987) demonstrated glucagon immunoreactive cells in quail gizzard at 13 d.i. whereas we firstly these cells in L. epithelialis of gizzard at 9 d.i.
Though Bezeuidenhout and Van Aswegen (1990) identified endocrine cells showing immunoreactivity to glucagon in adult ostrich gizzard, we did not observe them in adult gizzard.
Yamaguchi et al. (1987) reported that in quail embryos, somatostatin immunoreactive cells were firstly found in glands and L. epithelialis of proventriculus at 12 d.i. Martinez et al. (1993) firstly detected these cells in proventriculus of chicken embryos at 16 d.i. Castaldo and Lucini (1994) reported that in duck proventriculus this immunoreactive cells were first appeared among the epithelial cells of the glands at 19 d.i. but in this study somatostatin immunoreactive cells were firstly detected in stratified epithelium of proventriculus at 6 d.i.
Various investigators (6, 8, 12, 21, 34) detected somatostatin immunoreactive cells in vast numbers in proventricular glands of different adult birds. Saito et al. (1989) showed that moderate number of somatostatin immunoreactive cells were found in the proventricular glands of adult domestic pigeon. Contrary to these investigators’ (6, 8, 12, 21, 34) results we did not identify somatostatin immunoreactive cells in proventricular glands. Bezeuidenhout and Van Aswegen (1990) is study on ostrich reported these cells were seen in large numbers in L. epithelialis. In this study, it was observed that few number of somatostatin immunoreactive cells in L. epithelialis of proventriculus is in agreement with the findings of Richardson et al. (1988).
Castaldo and Lucini (1994) firstly detected a few somatostatin immunoreactive cells in duck gizzard at 25 d.i. We identified this immunoreactive cells in stratified epithelium of gizzard at 6 d.i.
Although various investigators (6, 8, 12, 21, 34) detected somatostatin immunoreactive cells in gizzard of different species in adult period, no somatostatin immunoreactive cells were identified in this study.
In conclusion, the time of first appearance of bombesin, glucagon, somatostatin immunoreactive cells in the prenatal period may be related to time of hatching in different avian species. Also the findings obtained in the adult period showed both some differences and similiarities from those of other avian species, concerning the relative frequency and distribution of these immunoreactive cells in the gastrointestinal tract seem to be affected by feding habits of different avian species.
References
1. Abad ME, Binkhorst FM, Elbal MT, Rombout JH (1987): A comparative immunocytochemical study of the gastro-entero-pancreatic (GEP) endocrine system in a stomachless and a stomach-containing teleost. Gen Comp Endocr, 66, 123-136.
2. Alison BC (1989): The distribution and ontogeny of gastrin / CCK-, somatostatin- and neurotensin-immunoreactive cells in the gastrointestinal tract of the chicken. Histol Histopathol, 4, 55-62.
3. Alumets J, Sundler F, Håkanson R (1977): Distribution, ontogeny and ultrastructure of somatostatin immunoreactive cells in the pancreas and gut. Cell Tissue Res, 185, 465-479.
4. Andrew A, Kramer B, Rawdon BB (1983): Gut and pancreatic amine precursor uptake and decarboxylation cells are not neural crest derivates. Gastroenterology, 84, 429-430.
5. Bancroft JD, Steven A, Turner DR (1996): Theory and Practice of Histological Techniques. Churchill Livingstone, New York, London, Edinburg, Madrid, Melbourne, San Francisco, Tokyo.
6. Bezuidenhout AJ, Van Aswegen G (1990): A light microscopic and immunocytochemical study of the gastrointestinal tract of the ostrich (Struthio camelus L., Onderstepoort J Vet, 57, 37-48.
7. Brazeau P, Vale W, Burgus R, Ling N, Butcher M, Rivier J, Guillemin R (1973): Hypothalamic polypeptide
that inhibits the secretion of immunoreactive pituitary growth hormone. Science, 179, 77-79.
8. Castaldo L, Lucini C (1991): An immunohistochemical study on the endocrine cells in the gastrointestinal tract of the domestic duck. Eur J Basic Appl Histochem, 35, 131-143.
9. Castaldo L, Lucini C (1994): Ontogenesis of some endocrine cells in the duck gastrointestinal tract. Eur J Histochem, 38, 319-26.
10. D'este L, Campo S, Salvi E, Renda T (1984): Ontogenesis of bombesin-like immunoreactive cells in the chicken proventriculus. Basic App Histochem, 28, 143-150. 11. Erspamer V, Melchiorri P, Falconieri Erspamer V,
Negri L (1978): Polypeptides of the amphibian skin active on the gut and their mammalian counterparts. 51-64. In: MI Grossman, V Speranza, N Basso, E Lezoche (Eds), Gastrointestinal Hormones and Pathology of the Digestive System. Plenum Press, New York.
12. Hashimoto N, Yamada J, Richardson KC, Kitamura N, Yamashita T (1993): An immunohistochemical study on the gastrointestinal endocrine cells of three honeyeaters: singing honeyeater (Meliphaga virescens), spiny-cheeked honeyeater (Acanthogenys rufogularis) and brown honeyeater (Lichmera indistincta). Eur J Basic Appl Histochem, 37, 233-240.
13. Junqeria LC, Carnerio J, Kelley RD (1992): Basic Histology. 8th edition, Appleton and Lange, USA.
14. Karaöz E (2002): Özel Histoloji. Süleyman Demirel Üniversitesi Tıp Fakültesi Yayınları No:29, Isparta. 15. Kieffer TJ, Habener JF (1999): The glucagon-like
peptides. Endocr Rev, 20, 876-913.
16. Martinez A, López J, Sesma P (1993): Development of the diffuse endocrine system in the chicken proventriculus. Cell Tissue Res, 271, 107-113.
17. Menteş NK (1983): Klinik Gastroenteroloji. Ege Üniversitesi Tıp Fakültesi, Cilt 2, 4. baskı, İzmir.
18. Müller EE, Locatelli V, Cocchi D (1999): Neuroendocrine control of growth hormone secretion. Physiol Rev, 79, 511-607.
19. Naruse H, Gomi T, Kimura A, Adriaensen D, Timmermans JP (2005): Structure of the respiratory tract of the red-bellied newt Cynops pyrrhogaster, with reference to serotonin-positive neuroepithelial endocrine cells. Anat Sci Int, 80, 97-104.
20. Pan QS, Fang ZP, Zhao YX (2000): Identification, localization and morphology of APUD cells in gastroenteropancreatic system of stomach-containing teleosts. World J Gastroentero, 6, 842-847.
21. Richardson KC, Yamada J, Wooller RD (1989): An immunohistochemical study of the New Holland honeyeater, Phylidonyris novaehollandiae. Aust J Zool, 36, 483-496.
22. Rodrigues A, Pena L, Flores JM, Gonzales M, Castano M (1992): Immunocytochemical study of diffuse neuroendocrine system cells in equine lungs. Anat Histol Embryol, 21, 138-145.
23. Saito T, Yamada J, Kitamura N, Yamashita T (1989): An immunohistochemical study on the distribution of endocrine cells in the gastrointestinal tract of domestic pigeon, (Columba livia var domestica). Z Mikrosk Anat Forsc, 103, 237-246.
24. Salvi E, Buffa R, Renda TG (1995): Ontogeny, distribution and amine/peptide colocalization of chromogranin A- and B-immunoreactive cells in the chicken gizzard and antrum. Anat Embryol (Berl), 192, 547-55.
25. Sternberger LA (1986): Immunocytochemistry. 3rd
edition, John Wiley and Sons, New York.
26. Sundler F, Alumets J, Fahrenkrug J, Håkanson R, Schaffalitzky de OB (1979): Cellular localization and ontogeny of immunoreactive vasoactive intestinal polypeptide (VIP) in the chicken gut. Cell Tissue Res, 196, 193-201.
27. Tanyolaç A (1999): Özel Histoloji. Ankara Üniversitesi Veteriner Fakültesi, Ankara.
28. Telatar H, Şimşek H (1993): Gastroenteroloji. Hacettepe Üniversitesi Tıp Fakültesi İç Hastalıklar Anabilim Dalı. Gastroenteroloji Ünitesi, Hekimler Yayın Birliği, Ankara. 29. Vaillant C, Dockray GJ, Walsh JH (1979): The avian
proventriculus is an abundant source of endocrine cells with bombesin-like ımmunoreactivity. Histochemistry, 64, 307-314.
30. Vale W, Rivier C, Brazeau P, Guillemin R (1974): Effects of somatostatin on the secretion of thyrotropin and prolactin. Endocrinology, 95, 968-977.
31. Weir GC, Goltsos PC, Sternberg EP, Patel YC (1976): High concentration of somatostatin immunoreactivity in chicken pancreas. Diabetologia, 12, 129-132.
32. Yamaguchi S, Yamada J, Kitamura N, Yamashita T (1986): Ontogeny of the endocrine cells in the quail proventriculus. Z Mikrosk Anat Forsc, 100, 981-989. 33. Yamaguchi S, Yamada J, Kitamura N, Yamashita T
(1987): Histological and immunohistochemical study on ontogeny of the endocrine cells in the quail gizzard. Gegenbaurs Morpho Jahrb, 133, 71-78.
34. Yamanaka Y, Yamada J, Kitamura N, Yamashita T (1989): An immunohistochemical study on the distribution of endocrine cells in the chicken gastrointestinal tract. Z Mikrosk Anat Forsc, 103, 437-446.
35. Youson JH, Al-Hahrouki AA, Naumovski D, Conlon JM (2001): The endocrine cells in the gastroenteropancreatic system of the Bowfin, Amia calva L: Immunohistochemical, ultrastructural and immunohistochemical analysis. J Morphol, 250, 208-224.
Geliş tarihi: 20.09.2007 / Kabul tarihi: 23.02.2008 Address for correspondance
Yrd. Doç.Dr. Kenan Çınar SDÜ Fen Edebiyat Fakültesi Biyoloji Bölümü
32260 Isparta kcinar@fef.sdu.edu.tr