Contents lists available atScienceDirect
Environmental and Experimental Botany
journal homepage:www.elsevier.com/locate/envexpbotFerulic acid confers tolerance against excess boron by regulating ROS levels
and inducing antioxidant system in wheat leaves (Triticum aestivum)
☆
Evren Yildiztugay
a, Ceyda Ozfidan-Konakci
b, Huseyin Karahan
a, Mustafa Kucukoduk
c,
Ismail Turkan
d,⁎aSelcuk University, Faculty of Science, Department of Biotechnology, 42250, Konya, Turkey
bNecmettin Erbakan University, Faculty of Science, Department of Molecular Biology and Genetics, 42090, Konya, Turkey cSelcuk University, Faculty of Science, Department of Biology, 42250, Konya, Turkey
dEge University, Faculty of Science, Department of Biology, 35100, Izmir, Turkey
A R T I C L E I N F O Keywords: Antioxidant system Excess boron Ferulic acid Phenolic compounds Water content A B S T R A C T
Ferulic acid (FA; 3-methoxy-4-hydroxycinnamic acid) is a candidate for improving plant tolerance to stress conditions through improving water solubility and antioxidant activity. To our knowledge, no study has thus far explored the potential for exogenous FA application to improve tolerance against excess boron (B) in plants. For this purpose, wheat seedlings grown in hydroponic culture were treated with FA (25 and 75 μM) alone or in combination with B (4 and 8 mM). The results showed that B caused a decrease in water content (RWC), osmotic potential (ΨΠ) and proline content (Pro). FA application prevented decreases of these parameters. 8 mM B in-creased superoxide dismutase (SOD) activity. Superoxide anion radical (O2%―) and hydrogen peroxide (H2O2) increased during B exposure, while catalase (CAT), peroxidase (POX), ascorbate peroxidase (APX) and glu-tathione reductase (GR) activities did not. However, due to increased SOD activity, FA under stress successfully decreased O2%―content. Additionally, exogenously applied FA under 4 mM B stress increased the activities of CAT and POX. While excess B in wheat leaves did not induce activities of APX, GR, monodehydroascorbate reductase (MDHAR) or dehydroascorbate reductase (DHAR) or increase total ascorbate (tAsA) or dehy-droascorbate (DHA) contents, FA with stress did. 25 μM FA with B remarkably maintained regeneration of ascorbate and induced contents of tAsA and GSH (including the ascorbate-glutathione cycle) and induced CAT activity. Taken together, stress-induced H2O2content significantly decreased and the scavenging of OH% in-creased in wheat with FA application through the activation of antioxidant enzymes. Consequently, FA pre-vented lipid peroxidation (TBARS) caused by stress due to increased radical scavenging activity.
1. Introduction
Boron (B) is an essential micronutrient for plant growth and de-velopment (Emebiri et al., 2009) and is widely distributed in nature by natural and anthropogenic sources. B plays a role in many critical biological processes in plants, such as sugars transport, cell wall synthesis, the ascorbate/glutathione cycle, phenolic acid metabolism and several enzymatic activities (Rehman et al., 2006). However, high levels of B cause negative effects by binding to energy-carrying mole-cules which have multiple hydroxyl groups such as adenosine tripho-sphate (ATP) and nicotinamide adenine dinucleotide (NAD) (Reid et al., 2004). Tolerant plants overcome toxic effects of B (2–4 mg L−1B) in
growth media but for sensitive plants, higher than 0.3 mg L-1B are toxic due to negative impacts on metabolism (Nable et al., 1997). Toxic levels of B (5 mM) trigger excessive accumulation of ROS in plants (Emebiri et al., 2009). These free radicals are scavenged by enzymatic and non-enzymatic antioxidant mechanisms in plants (Hossain et al., 2015;Liu et al., 2015, 2018). Phenolic compounds also exhibit free radical scavenging activity, with an efficiency which depends primarily on the number and position of hydroxyl groups in their structure (Liu et al., 2016).
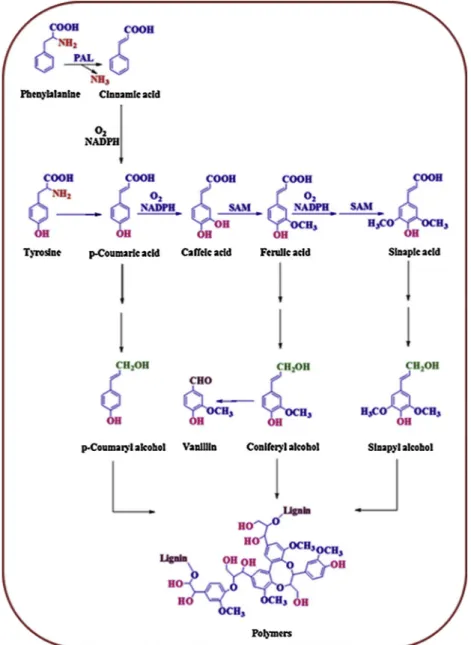
Phenolic compounds have one or more hydroxyl groups bound di-rectly to an aromatic ring (Fig. 1) and range from simple phenolic molecules to highly polymerized compounds (Vermerris and Nicholson,
https://doi.org/10.1016/j.envexpbot.2018.10.029
Received 8 June 2018; Received in revised form 26 October 2018; Accepted 26 October 2018
☆This article is part of a special issue entitled is "Revisiting the role of ROS and RNS in plants under a changing environment" published at the journal Environmental and Experimental Botany 161.
⁎Corresponding author.
E-mail addresses:eytugay@selcuk.edu.tr(E. Yildiztugay),cozfidan@konya.edu.tr(C. Ozfidan-Konakci),sedatkarahan@outlook.com(H. Karahan), mkucukoduk@selcuk.edu.tr(M. Kucukoduk),ismail.turkan@ege.edu.tr(I. Turkan).
Environmental and Experimental Botany 161 (2019) 193–202
Available online 29 October 2018
0098-8472/ © 2018 Elsevier B.V. All rights reserved.
2006). Phenolic compounds, including ferulic acid (FA; 3-methoxy-4-hydroxycinnamic acid), play roles in scavenging of reactive molecules, inhibition of lipid peroxidation and stabilization of cell membranes (Michalak, 2006). FA is especially located in the cell walls of seed coats and in the aleuronic layer in plants (Kumar and Pruthi, 2014). FA is produced from L-tyrosine or cinnamic acid, which is produced by phenylalanine ammonia lyase (PAL) from phenylalanine, through the shikimate pathway (Graf, 1992) in plants (Fig. 1). FA is associated with regulation of plant growth, antioxidant activities, and cytotoxic and antimicrobial properties (Saleh et al., 2014). The antioxidant capacity of FA is due to the ability of the three distinctive structural motifs to scavenge free radicals (Abu El-Soud et al., 2013).
There is a strong correlation between stress resistance and phenolic compound content in stress-treated plants (Michalak, 2006). Earlier reports show that plants with exogenous or pre-treatment applied phenolics, including salicylic acid, aminolevulinic acid and ferulic acid, can improve stress tolerance under salinity (Idrees et al., 2011) and heat stress (Zhang et al., 2012). For example, cucumber seedlings with 0.5 mM FA (for 2 days) had an alleviation of growth inhibition caused PEG-induced osmotic stress (Li et al., 2013). Also, FA content in plants exposed to stress has been shown to maintain gene expression, reg-ulation of signal transduction pathways and metabolic homeostasis
(metal chelation and modulation of enzyme activity) (Bhardwaj et al., 2017). Based on the aforementioned reports, FA is a candidate for im-proving plant tolerance under stress conditions. On the other hand, Hussain et al. (2017)reported that FA (0.5 and 1 mM for 7 days) had inhibitory effects on growth and biomass of Solanum lycopersicum. There is conflicting information about the exogenous application of FA on growth and metabolism of plants (Blum, 1989). To our knowledge, no study has thus far explored the potential role of exogenous FA ap-plication for improving tolerance against excess B in plants. Therefore, the present study investigated the potential role of FA application to wheat leaves under B toxicity, as quantified by ROS content and anti-oxidant enzyme activities.
2. Material and methods
2.1. Plant material and experimental design
Wheat seeds (Triticum aestivum L.) were obtained from the Bahri Dagdas International Agricultural Research Institute Konya, Turkey. Seeds were surface-sterilized in 5% sodium hypochlorite for 10 min, rinsed five times with sterile distilled water and then allowed to ger-minate on double-layer filter paper wetted with distilled water.
Germinated wheat seedlings were transferred to half strength Hoagland solution and grown under controlled conditions (16/8 h light/dark re-gime at 24 °C, 70% relative humidity and 350 μmol m−2s−1 photo-synthetic photon flux density). The seedlings were grown in hydroponic culture containing this solution for 21 d and the solutions were replaced by fresh half-strength Hoagland solution, twice a week. B and FA were purchased from Sigma Chemical Co. (St. Louis, MO, USA). For FA and B treatments, it was prepared by being dissolved in Hoagland solution and was added to the growth medium at the stage of 21 days old. In a preliminary experiment for determination of ideal experimental B and FA concentrations, 4, 8 and 10 mM B, and 25, 75 μM and 1 mM ferulic acid (FA) were applied to wheat plants. However, in 10 mM B and 1 mM FA-treated groups, the plants could not sustain their viability. Therefore, 25 and 75 μM FA were added alone or in combination with B (4 mM and 8 mM). Plants were harvested after 7 days (d) of treatment and the leaves stored at −86 °C until further analyses.
2.2. Determination of water content, osmotic potential and proline content
After harvest on 7d, six leaves were obtained and their fresh weight (FW) was determined. The leaves were floated on de-ionised water for 6 h and the turgid tissue was blotted dry prior to determining turgid weight (TW). Dry weight (DW) was determined after oven drying at 70 °C. The leaf relative water content (RWC) was calculated by the following formula (Smart and Bingham, 1974):
RWC (%) = [(FW-DW) / (TW-DW)] x 100
Leaves were extracted by crushing the material with a glass rod. Leaf osmotic potential (ΨΠ) was measured by Vapro Vapor pressure Osmometer 5600. ΨΠwas converted to MPa according toSanta-Cruz et al. (2002)by multiplying by a coefficient of 2.408 × 10−3.
Pro content was measured according toBates et al. (1973). The leaves were homogenized in 3% sulphosalicylic acid and homogenate was filtered through filter paper. After addition of acid ninhydrin and glacial acetic acid, the mixture was heated at 100 °C. The mixture was extracted with toluene and the absorbance of the aspired toluene fraction was measured at 520 nm.
2.3. Determination of ROS accumulation
O2%―levels were estimated according toXu et al. (2006)with some modifications. One milliliter of hydroxylammonium chloride (1 mM) was added into 0.5 ml of the supernatant and incubated for 1 h at 25 °C. 1 ml 4-aminobenzenesulfonic acid (17 mM) and 1 ml α-naphthylamine (7 mM) were added and the specific absorption was determined at 530 nm.
Determination of H2O2content was measured according toLiu et al. (2010). Leaves were homogenized in cold acetone and centrifuged. The supernatant was mixed with titanium reagent and then ammonium hydroxide was added to precipitate the titanium-peroxide complex. The reaction mixture was centrifuged. The pellet was washed with cold acetone and dissolved. The absorbance of the solution was measured at 410 nm. H2O2concentrations were calculated using a standard curve prepared with known concentrations of H2O2.
OH%radical scavenging activity was determined according toChung et al. (1997), with minor changes. The reaction mixture contained 20 mM Na-phosphate buffer, 10 mM 2-deoxyribose, 10 mM FeSO4, 10 mM EDTA, 10 mM H2O2, 0.525 mL H2O, and 0.075 mL sample. The mixture was incubated at 37 °C A mixture of 2.8% (w/v) trichloroacetic acid and 1.0% (w/v) thiobarbituric acid in 50 mM NaOH was added to the test tubes and boiled. After the mixture cooled, absorbance was measured at 520 nm against a blank solution. The OH% radical scavenging activity was calculated using the following formula: OH%radical scavenging activity (%) = [(Ao– A1)/Ao] × 100,
where Aowas the absorbance of the blank and A1the absorbance of the sample.
2.4. Determination of lipid peroxidation levels
The level of lipid peroxidation was determined by thiobarbituric acid reactive substances (TBARS) according toRao and Sresty (2000). TBARS concentration was calculated from the absorbance at 532 nm, and measurements were corrected for nonspecific turbidity by sub-tracting the absorbance at 600 nm. The concentration of TBARS was calculated using an extinction coefficient of 155 mM−1cm−1.
2.5. Enzyme extraction and determination of isozyme and/or enzyme compositions
For protein and enzyme extractions, 0.5 g of each sample was homogenized in 50 mM Tris-HCl (pH 7.8) containing 0.1 mM ethyle-nediaminetetraacetic acid (EDTA), 0.2% Triton X-100, 1 mM phe-nylmethylsulfonyl fluoride and 2 mM dithiothreitol (DTT). For APX activity determination, 5 mM ascorbate (AsA) was added to the homogenization buffer. Samples were centrifuged at 14,000×g for 30 min and supernatant was used for the determination of protein content and enzyme activities. The total soluble protein content of the enzyme extracts was determined (Bradford, 1976) using bovine serum albumin as a standard. All spectrophotometric analyses were conducted on a Shimadzu spectrophotometer (UV 1800).
Samples containing equal amounts of protein (40 μg) were subjected to non-denaturing polyacrylamide gel electrophoresis (PAGE) as de-scribed byLaemmli (1970)with minor modifications. SOD activity was detected by photochemical staining using riboflavin and NBT (Beauchamp and Fridovich, 1971). The units of activity for each SOD isozyme were calculated by running a SOD standard from bovine liver (Sigma Chemical Co., St. Louis, MO, USA). The different types of SOD were discriminated by incubating gels with different types of SOD in-hibitors before staining: Mn-SOD activity was resistant to both inhibitor treatments and SOD activity was sensitive to 2 mM KCN. Cu/Zn-SOD and Fe-Cu/Zn-SOD activities were inhibited by 3 mM H2O2(Vitória et al., 2001). The total SOD (EC 1.15.1.1) activity assay was based on the method of Beauchamp and Fridovich (1971), which uses spectro-photometric analysis at 560 nm to measure the inhibition of the pho-tochemical reduction of nitro blue tetrazolium (NBT). One unit of specific enzyme activity was defined as the quantity of SOD required to produce a 50% inhibition of NBT reduction.
Total CAT (EC 1.11.1.6) activity was estimated according to the method ofBergmeyer (1970), which measured the initial rate of H2O2 disappearance at 240 nm. The decrease in absorption was followed for 3 min, and one unit of CAT was defined as 1 mmol H2O2decomposed min−1mL−1.
POX isozymes were detected according toSeevers et al. (1971). Electrophoretic separation of samples containing 30 μg protein was performed on non-denaturing polyacrylamide. Total POX (EC 1.11.1.7) activity was based on the method described by Herzog and Fahimi (1973). The increase in the absorbance at 465 nm was followed for 3 min. One unit of POX activity was defined as mmol H2O2decomposed min−1mL−1.
Electrophoretic APX separation was performed according toMittler and Zilinskas (1993). Before the samples (40 μg protein) were loaded, gels were equilibrated with running buffer containing 2 mM AsA for 30 min. Total APX (EC 1.11.1.11) activity was measured according to Nakano and Asada (1981). The assay depends on the decrease in ab-sorbance at 290 nm. The concentration of oxidized AsA was calculated by using a 2.8 mM−1cm−1extinction coefficient. One unit of APX was defined as 1 mmol AsA oxidized min−1mL−1.
Total GR (EC 1.6.4.2) activity was measured according toFoyer and Halliwell (1976). Activity was calculated using the extinction coeffi-cient of NADPH (6.2 mM−1cm−1). One unit of GR was defined as
1 mmol GSSG reduced min−1mL−1.
NADPH oxidase (NOX) isozymes were identified by NBT reduction method as described bySagi and Fluhr (2001). The samples containing 40 μg protein was loaded per lane. Total NOX (EC 1.6.3.1) activity was measured according to Jiang and Zhang (2002). The assay medium contained 50 mM Tris-HCl buffer, 0.5 mM XTT, 100 μM NADPH.Na4 and 20 μg of protein sample. After addition of NADPH, XTT reduction was followed at 470 nm. Activity was calculated using the extinction coefficient, 2.16 × 104 M−1cm−1. One unit of NOX was defined as 1 nmol ml−1XTT oxidized min−1.
Gels stained for SOD, POX, APX and NOX activities were photo-graphed with the Gel Doc XR + System and then analyzed with Image Lab software v4.0.1 (Bio-Rad, California, USA). Known standard amounts of enzymes (0.5 units of SOD and 0.2 units of POX) were loaded onto gels. The units of isozyme activity for each group were calculated by comparison with the standard and are given as a graphic below each gel photo. For each isozyme set/group, the average values (shown with the same symbol) were not significantly different at p > 0.05 using Tukey’s post-test.
2.6. Determination of the activity of monodehydroascorbate reductase and dehydroascorbate reductase
Monodehydroascorbate reductase (MDHAR; EC 1.6.5.4) activity was assayed by the method ofMiyake and Asada (1992). The reaction mixture contained 50 mM Hepes–KOH (pH 7.6), 1 mM NADPH, 2.5 mM AsA and 2.5 U AsA oxidase and enzyme extract. The MDHAR activity was measured by decrease in absorbance as the amount of enzyme that oxidizes 1 mM NADPH per minute at 340 nm A molar extinction coef-ficient of 6.2 mM−1cm−1was used for the calculation of enzyme ac-tivity.
Dehydroascorbate reductase (DHAR; EC 1.8.5.1) activity was mea-sured according toDalton et al. (1986). DHAR activity was measured by increase in absorbance at 265 nm due to ascorbate formation. A molar
extinction coefficient of 14.6 mM−1cm−1was used for the calculation of enzyme activity.
2.7. Determination of the contents of dehydroascorbate and ascorbate
Total and reduced ascorbate (AsA) content were determined ac-cording to the method ofDutilleul et al. (2003) with modifications. Total and reduced AsA content were assayed spectrophotometrically at 265 nm in 100 mM K-P buffer with 1.0 U of ascorbate oxidase. To calculate AsA, a specific standard curve of AsA was used. The oxidized form of ascorbate (DHA, dehydroascorbate) was measured using the formula DHA = Total AsA − Reduced AsA.
2.8. Determination of the contents of glutathione and oxidized glutathione
Glutathione (GSH) was assayed according toParadiso et al. (2008), utilizing aliquots of supernatant neutralized with 0.5 M K-P buffer. Based on enzymatic recycling, glutathione is oxidized by DTNB and reduced by NADPH in the presence of GR, and glutathione content is evaluated by the rate of absorption changes at 412 nm. Oxidized glu-tathione (GSSG) was determined after removal of GSH by 2-vinylpyr-idine derivatization. Standard curves with known concentrations of GSH and GSSG were used for the quantification.
2.9. Statistical analysis
The experiments were repeated thrice independently and each data point was the mean of six replicates. All data obtained were subjected to a one-way analysis of variance (ANOVA). Statistical analysis of the values was performed by using SPSS 20.0. Tukey’s post-test was used to compare the treatment groups. Comparisons with p < 0.05 were considered significantly different. In all figures, the error bars represent standard errors of the means.
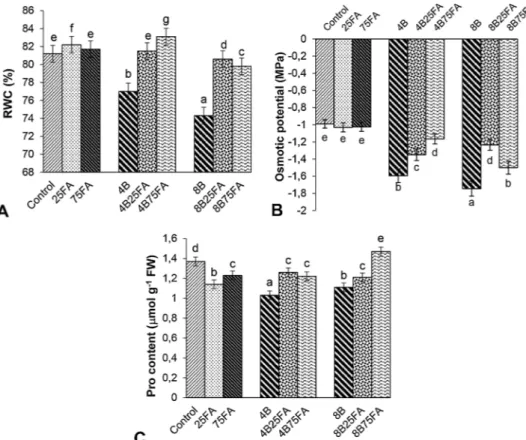
Fig. 2. Effects of exogenous 25 and 75 μM FA treatment on relative water content (RWC, A), osmotic potential (ΨΠ, B) and proline content (Pro, C) in wheat leaves exposed to 4 and 8 mM B stress for 7 days (d).
3. Results
Fig. 2A reveals that the leaf RWC of B-treated plants dropped by 5% and 9% as compared to their controls. However, the plants treated with B and FA were able to maintain 80–85% of their leaf RWC with re-ference to plants that were treated individually with B or FA. 75 μM FA addition alone had no significant effect on RWC. After B treatment, a decrease in osmotic potential (Ψ∏) was observed compared with control (Fig. 2B). FA alone did not affect Ψ∏as compared with control, whereas in combination with B, FA increased Ψ∏compared with B alone. 4 and 8 mM B treatment caused 25% and 23% decrease in proline content (Pro) in compared with control groups, respectively (Fig. 2C). FA treatments showed no significant increase in Pro compared with con-trol, but B + FA significantly increased Pro content compared with B treatment alone.
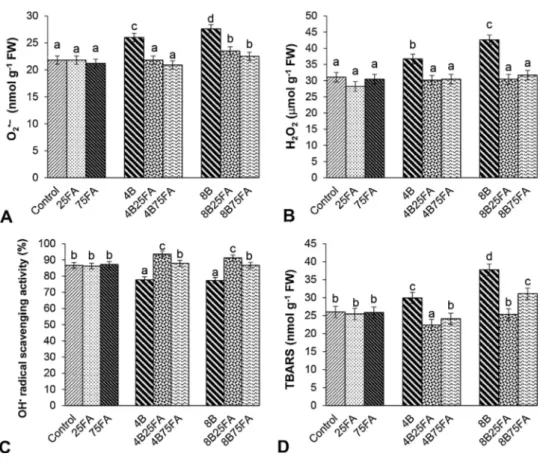
Under B toxicity, O2%―levels increased, reaching maximum levels in plants with 8 mM B (21%) for 7 d (Fig. 3A). While B stress plus FA led to a significant decrease in O2%―content, no changes were observed with only FA treatment. As shown inFig. 3B, both B concentrations led to significant increases in H2O2content. There was a noticeable increase at 8 mM B (by 1.4-fold). On the other hand, H2O2 accumulation in exogenous FA-treated leaves with stress was lower than in B-treated plants. No enhancement was observed in H2O2content of FA-treated leaves under control conditions. While B with FA caused an increase in the scavenging activity of OH%radical compared to the stress groups, FA alone created no remarkable effect on this activity (Fig. 3C). Depending on B treatments, a marked reduction in the scavenging activity of OH% radical was observed. Compared with control groups, wheat leaves treated with excess B demonstrated increased levels of lipid peroxida-tion (Fig. 3D). On the other hand, 25 and 75 μM FA alleviated the in-creases in TBARS content of both B-treated plants.
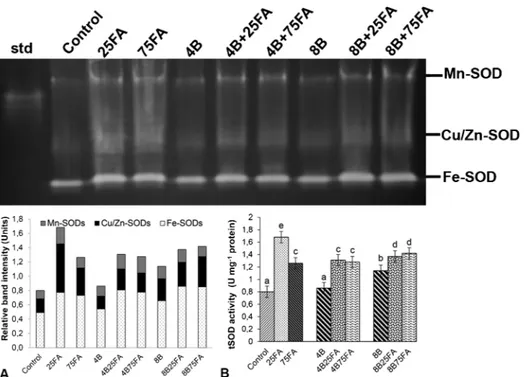
Examination of SOD isoenzyme profiles in the leaves revealed three isoforms (Mn-SOD, Fe-SOD and Cu/Zn-SOD) (Fig. 4A). Quantification of the band intensities showed that there was an enhancement in SOD activity (especially Fe- and Cu/Zn-SOD) at only 8 mM B in the leaves, as
demonstrated by the measurements of total SOD activity (Fig. 4B). Moreover, all FA concentrations had a significant enhancement on SOD activity both alone and in combination with B. In all groups, the Fe-SOD band, which was the most intense, was responsible for this change.
The plants treated with excess B had lower total activity of CAT enzyme than the control groups (Fig. 5). Similarly, FA application alone caused a slight dose-response decrease in total CAT activity in leaves. However, the application of FA along with B resulted in a significant increase in CAT activity compared with the B-treated plants.
Seven isozymes were observed in the evaluation of POX isozyme profiles (Fig. 6A). Total POX activity exhibited a similar isozyme staining pattern in all the treatment groups (Fig. 6B). At 7 d, no en-hancement was observed in total POX activity after exposure to excess B. This result corroborates those observed in the isoenzymes gels, which showed lower or unchanged values in response to B treatment. As shown inFig. 6A-6B, POX activity significantly increased under com-bined treatment (FA plus 4 mM B), whereas POX activity decreased in plants exposed to the high B concentration (8 mM) with FA applica-tions.
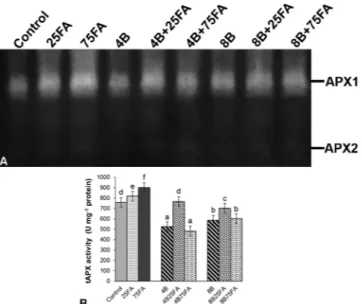
A total of two APX isoenzymes was found and determined as APX1 and APX2 by native PAGE analysis (Fig. 7A). The total APX activities of B-treated plants decreased (Fig. 7B); the decrease was more severe (by 31%) at 4 mM B. Furthermore, the intensities of APX1 and APX2 iso-zymes were induced by only 25 μM FA treatment under B, compared to stress treatment alone. An 8% and 19% increase in APX activity was detected under FA treatment alone (25 and 75 μM) in leaves, respec-tively.
In our study, three GR isoenzymes (GR1-2-3) were detected in all groups (Fig. 8A). A slight decrease or unchanged activity in GR activity was observed under B stress. Similar to the APX activity, upon applying only low FA concentration to B-treated plants, a clear increase of the total GR activity was observed (Fig. 8B).
Three NOX isozymes were defined by electrophoretic separation throughout the experiment (Fig. 9A). Each of the NOX isozymes (NOX1-2-3) was reduced after exposure to excess B, whereas NOX isozymes
Fig. 3. Effects of exogenous 25 and 75 μM FA treatment on superoxide anion radical content (O2%―, A), hydrogen peroxide (H2O2, B), the scavenging activity of hydroxyl radical (OH%
, C) and lipid peroxidation (TBARS, D) in wheat leaves exposed to 4 and 8 mM B stress for 7 days (d).
exhibited a slight increase with B plus FA application. Isozyme analysis indicated that the observed increase in total NOX activity was due to intensities of NOX2 and NOX3 with the FA treatment without B (Fig. 9B).
B-induced MDHAR activity was similar to the control groups (Fig. 10A). However, addition of FA to stress-treated leaves increased this activity. Enhancement of this activity was maintained by FA treatment alone. In wheat leaves grown under B toxicity, DHAR activity decreased or was similar to the control groups (Fig. 10B). However, when B and FA were applied together, DHAR activity was more induced in comparison with the stress alone. On the other hand, the both FA treatments alone caused an increase in DHAR activity. The notable decreases in DHA content caused by 4 and 8 mM B were by 1.4- and 1.2-fold, respectively (Fig. 10C). However, except for 8 mM B plus 75 μM FA, addition of FA to B-treated plants significantly increased DHA content when compared with the stress treatment alone. Similarly, DHA contents in plants with FA treatment alone (25 and 75 μM) were in-creased by 18% and 10% as compared to the control, respectively. 4 and 8 mM B stress treatments led to a 36% and 23% decrease in tAsA content, respectively (Fig. 10D). Only 25 μM FA addition had an in-duction on tAsA contents of wheat leaves subjected to B. FA treatments in the absence of stress caused no enhancement in tAsA content. GSH
content decreased or did not change under stress conditions (Fig. 10E). However, unlike FA alone, a notable increase in GSH content occurred with the FA application under stress. The results summarized in Fig. 10F show that B caused no change in GSSG levels throughout the experiment. On the other hand, FA treatment with/without stress caused a remarkable increase in GSSG content.
4. Discussion
The effects of excess B on physiology, biochemistry and cellular structure of plants have been well documented (Shah et al., 2017). One of the indicators of B toxicity in plants is decreased water content (Simon et al., 2013). In this study, this reduction of RWC was more severe at 8 mM B. Similar results have been reported by Shi et al. Fig. 4. Effects of exogenous 25 and 75 μM FA treatment on relative band intensity of different types of superoxide dismutase isoenzymes (SOD, A) and total SOD activity (B) in wheat leaves exposed to 4 and 8 mM B stress for 7 days (d).
Fig. 5. Effects of exogenous 25 and 75 μM FA treatment on total catalase ac-tivity (CAT) in wheat leaves exposed to 4 and 8 mM B stress for 7 days (d).
Fig. 6. Effects of exogenous 25 and 75 μM FA treatment on relative band in-tensity of different types of peroxidase isoenzymes (POX, A) and total POX activity (B) in wheat leaves exposed to 4 and 8 mM B stress for 7 days (d).
(2005), who demonstrated that B-induced disruption of RWC seems to be associated with impaired plasma membrane permeability and ex-cessive water loss. On the other hand, exogenously applied FA can promote RWC of wheat leaves by increasing water absorption, as ob-served by Li et al. (2013). In accordance with the RWC results, ΨΠ decreased under B exposure. However, when FA was applied to B-treated plants, FA prevented this decline in ΨΠ. Pro, an osmoprotectant, plays a role in elimination of ROS and protection of membranes against lipid peroxidation (Hong et al., 2000). B toxicity increased Pro content in tomato and pepper (Eraslan et al., 2007). In contrast, in our study, under excess B, a decline in Pro levels was detected in wheat leaves. Thus, there was an inverse relationship between the levels of Pro and TBARS in stress-treated wheat plants. Judging by the increased Pro content in wheat exposed to B with FA, FA may play a role in protection of membrane stability (decreased TBARS content) and water status (increased RWC) in wheat. Also,Abu El-Soud et al. (2013)showed that there is a relationship between FA-induced Pro accumulation and
activated antioxidant enzymes.
Excess B causes production of reactive oxygen species (ROS) in plants through decreased CO2assimilation and chlorophyll efficiency (Han et al., 2009). The first step in ROS stress alleviation is activation of SOD, which converts superoxide anion radical to H2O2(Hossain et al., 2015). Under B toxicity, a general stimulation of SOD was previously reported byHamurcu et al. (2013). In contrast, in this study, while SOD activity was similar to the control group at 4 mM B, an increase ob-served in SOD activity was obob-served only at 8 mM B. In wheat leaves, O2%―and H2O2content increased throughout the experimental period. In spite of the unchanged SOD activity at 4 mM B, the induction of H2O2 content at this B concentration might be connected with another source of H2O2accumulation.Asada (1999)reported that H2O2is also pro-duced by the activation of other enzymes such as glycolate oxidases, glucose oxidases, amino-acid oxidases and sulfite oxidases as well as SOD. FA application to B-treated wheat leaves led to an induction of SOD activity, resulting a decrease in O2%― content. The antioxidant activity of FA is related to its extended side chain conjugation (Buanafina, 2009).Graf (1992) previously reported that FA shows a scavenging activity against ROS which is similar to that of SOD. Therefore, in wheat leaves, FA might modulate the amount superoxide anion radical via a SOD-like activity. On the other hand, H2O2can be converted to non-toxic molecules such as H2O and oxygen by activities of CAT, POX, APX and GR (Hossain et al., 2015). However, in the current study, no increase was detected in the activities of CAT, POX, APX and GR in stress-treated wheat. The non-activation of these anti-oxidants in wheat might also be related to the H2O2accumulation. Our results are in accord with the findings obtained byShah et al. (2017), who observed a decrease in antioxidant enzymes such as SOD, POX and APX activity under excess B in citrange plants. In the current study, under excess B, there was a positive correlation between H2O2content and TBARS content. It was suggested byFarag et al. (2017)that higher B accumulation in leaves could be one of the major causes of lipid membrane peroxidation (as observed TBARS content). When FA was applied in combination with 4 mM B, the activities of CAT and POX increased. POX is directly involved in lignin biosynthesis, wherein the precursors of lignin are enzymatically dehydrogenated to phenoxy ra-dicals in the cell wall (Lee et al., 2007). In wheat leaves exposed to low B concentrations, FA might be connected with lignin synthesis via FA-induced POX activity. Also, B markedly increases contents of phenolic compounds that are ester-bound to cell wall polysaccharides, by which FA has a role in organizing cell growth (Lindsay and Fry, 2008).
To remove H2O2 content, APX catalyzes oxidation of AsA. The
Fig. 7. Effects of exogenous 25 and 75 μM FA treatment on relative band in-tensity of different types of ascorbate peroxidase isoenzymes (APX, A) and total APX activity (B) in wheat leaves exposed to 4 and 8 mM B stress for 7 days (d).
Fig. 8. Effects of exogenous 25 and 75 μM FA treatment on relative band in-tensity of different types of glutathione reductase isoenzymes (GR, A) and total GR activity (B) in wheat leaves exposed to 4 and 8 mM B stress for 7 days (d).
Fig. 9. Effects of exogenous 25 and 75 μM FA treatment on relative band in-tensity of different types of NADPH oxidase isoenzymes (NOX, A) and total NOX activity (B) in wheat leaves exposed to 4 and 8 mM B stress for 7 days (d).
regeneration of AsA is catalyzed by the activities of GSH-dependent DHAR and GR (in the GSH regeneration cycle) or NADH-dependent MDHAR (Asada, 1999) via DHA or MDHA contents. In the current study, although B did not trigger the induction of APX, GR, MDHAR and DHAR, or AsA and DHA contents, in B-treated wheat leaves, FA (especially 25 μM FA) did. AsA and GSH could both be sufficiently regenerated through the increased activities of MDHAR, DHAR and GR in the B + FA treatment. These results are compatible with the findings ofLi et al. (2013), who observed that exogenously applied FA stimu-lated activities of these enzymes in cucumber seedlings. AsA and GSH, as a redox buffer in the apoplast, play role in alleviating the injury caused by ROS (Schonhof et al., 2007). Thus, FA could maintain the pool of ascorbate and glutathione under excess B in wheat leaves. The findings obtained byLi et al. (2011)are consistent with our results, who indicated that 5-aminolevulinic acid led to increased contents of GSH and AsA in cucumber leaves as compared to PEG stress treatment.
OH%, known as the most reactive radical, attacks the fatty acid side chains of membrane phospholipids and stimulates lipid peroxidation
caused by stress conditions (Liu et al., 2014). B enters plant cells by passive diffusion through the lipid bilayer of the plasma membrane and through proteinaceous water channels (Emebiri et al., 2009). Because of the loss of OH%radical scavenging activity under B exposure, TBARS content was increased in wheat. Our results are consistent with the findings ofArdic et al. (2009)andLiu et al. (2017), who observed high levels of lipid peroxidation in chickpea and wheat plants when exposed to excess B, respectively. On the other hand, in wheat leaves with B + FA, lower contents of TBARS were observed in comparison to stress alone, consistent with the findings ofLi et al. (2013).
B inhibited RWC, ΨΠand Pro content in wheat leaves. However, FA application prevented inhibition on these parameters. Under stress conditions, the activation of SOD enzyme observed only at 8 mM B. In wheat leaves, O2%―content was induced throughout the experimental period. The activities of CAT, POX, APX and GR did not increase in wheat exposed to B toxicity. This led to accumulation of H2O2. However, due to increased SOD activity, FA under stress successfully decreased O2%―content. The exogenously applied FA under 4 mM B
Fig. 10. Effects of exogenous 25 and 75 μM FA treatment on monodehydroascorbate reductase activity (MDHAR, A), dehydroascorbate reductase activity (DHAR, B) and dehydroascorbate content (DHA, C), total ascorbate content (tAsA, D), reduced glutathione content (GSH, E) and oxidized glutathione content (GSSG, F) in wheat leaves exposed to 4 and 8 mM B stress for 7 days (d).
increased the activities of CAT and POX. Although B did not induce APX, GR, MDHAR and DHAR, or AsA and DHA contents, FA in wheat leaves with stress did. 25 μM FA under B remarkably maintained the regeneration and induced contents of tAsA and GSH including ascor-bate-glutathione cycle as well as induced CAT activity. Therefore, due to this activation of antioxidant enzymes, stress-induced H2O2content and the activity of OH%significantly decreased in wheat with FA ap-plication. FA prevented the lipid peroxidation (by reduced TBARS content) caused by B exposure.
5. Conclusion
Results of the recent studies indicate that FA has the ability to confer tolerance in plants against stress conditions. However, the effects of FA on biological pathways and the mechanisms by which FA induces stress tolerance are expected to be examined in detail. Especially, the future molecular analyses will aim at elucidating the precise molecular link between FA and the gene expressions for enzymes including ascorbate glutathione cycle.
Author statement
Evren Yildiztugay: Conceptualization, Methodology, Software. Ceyda Ozfidan-Konakci: Visualization, Data curation, Writing- Original draft preparation. Huseyin Karahan: Formal Analysis, Investigation. Mustafa Kucukoduk: Supervision, Resources. Ismail Turkan: Writing-Reviewing and Editing, Supervision.
Acknowledgments
We are thankful to Bahri Dagdas International Agricultural Research Institute for providing the seeds of wheat. Financial support for this work was provided by Selcuk University Scientific Research Projects Coordinating Office (project number: 16201088). The authors are also grateful to Assist. Prof. Dr. Robert Waller Murdoch and Dr. Abdullah Akdam for helpful comments to improve the manuscript.
References
Abu El-Soud, W., Hegab, M.M., AbdElgawad, H., Zinta, G., Asard, H., 2013. Ability of ellagic acid to alleviate osmotic stress on chickpea seedlings. Plant Physiol. Biochem. 71, 173–183.
Ardic, M., Sekmen, A., Turkan, I., Tokur, S., Ozdemir, F., 2009. The effects of boron toxicity on root antioxidant systems of two chickpea (Cicer arietinum L.) cultivars. Plant Soil 314, 99–108.
Asada, K., 1999. The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Phys. 50, 601–639.
Bates, L., Waldren, R., Teare, I., 1973. Rapid determination of free proline for water-stress studies. Plant Soil 39, 205–207.
Beauchamp, C., Fridovich, I., 1971. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 44, 276–287.
Bergmeyer, H.U., 1970. Methoden der enzymatischen Analyse. 2. Verlag Chemie.
Bhardwaj, R.D., Kaur, L., Srivastava, P., 2017. Comparative evaluation of different phe-nolic acids as priming agents for mitigating drought stress in wheat seedlings. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 87, 1133–1142.
Blum, A., 1989. Osmotic adjustment and growth of barley genotypes under drought stress. Crop Sci. 29, 230–233.
Bradford, M.M., 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254.
Buanafina, M.M.D., 2009. Feruloylation in grasses: current and future perspectives. Mol. Plant 2, 861–872.
Chung, S.-K., Osawa, T., Kawakishi, S., 1997. Hydroxyl Radical-scavenging Effects of Spices and Scavengers from Brown Mustard (Brassica nigra). Biosci. Biotechnol. Biochem. 61, 118–123.
Dalton, D.A., Russell, S.A., Hanus, F.J., Pascoe, G.A., Evans, H.J., 1986. Enzymatic-re-actions of ascorbate and glutathione that prevent peroxide damage in soybean root-nodules. Proc. Natl. Acad. Sci. U. S. A. 83, 3811–3815.
Dutilleul, C., Driscoll, S., Cornic, G., De Paepe, R., Foyer, C.H., Noctor, G., 2003. Functional mitochondrial complex I is required by tobacco leaves for optimal pho-tosynthetic performance in photorespiratory conditions and during transients. Plant Physiol. 131, 264–275.
Emebiri, L., Michael, P., Moody, D., 2009. Enhanced tolerance to boron toxicity in two-rowed barley by marker-assisted introgression of favourable alleles derived from
Sahara 3771. Plant Soil 314, 77–85.
Eraslan, F., Inal, A., Gunes, A., Alpaslan, M., 2007. Boron toxicity alters nitrate reductase activity, proline accumulation, membrane permeability, and mineral constituents of tomato and pepper plants. J. Plant Nutr. 30, 981–994.
Farag, M., Najeeb, U., Yang, J.H., Hu, Z.Y., Fang, Z.M., 2017. Nitric oxide protects carbon assimilation process of watermelon from boron-induced oxidative injury. Plant Physiol. Biochem. 111, 166–173.
Foyer, C.H., Halliwell, B., 1976. The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133, 21–25.
Graf, E., 1992. Antioxidant potential of ferulic acid. Free Radic. Biol. Med. 13, 435–448.
Hamurcu, M., Sekmen, A.H., Turkan, I., Gezgin, S., Demiral, T., Bell, R.W., 2013. Induced anti-oxidant activity in soybean alleviates oxidative stress under moderate boron toxicity. Plant Growth Regul. 70, 217–226.
Han, S., Tang, N., Jiang, H.X., Yang, L.T., Li, Y., Chen, L.S., 2009. CO2 assimilation, photosystem II photochemistry, carbohydrate metabolism and antioxidant system of citrus leaves in response to boron stress. Plant Sci. 176, 143–153.
Herzog, V., Fahimi, H., 1973. Determination of the activity of peroxidase. Anal. Biochem. 55, e62.
Hong, Z., Lakkineni, K., Zhang, Z., Verma, D.P.S., 2000. Removal of feedback inhibition of Δ1-pyrroline-5-carboxylate synthetase results in increased proline accumulation and protection of plants from osmotic stress. Plant Physiol. 122, 1129–1136.
Hossain, M.A., Bhattacharjee, S., Armin, S.M., Qian, P.P., Xin, W., Li, H.Y., Burritt, D.J., Fujita, M., Tran, L.S.P., 2015. Hydrogen peroxide priming modulates abiotic oxida-tive stress tolerance: insights from ROS detoxification and scavenging. Front. Plant Sci. 6.
Hussain, I., Singh, N.B., Singh, A., Singh, H., Singh, S.C., Yadav, V., 2017. Exogenous application of phytosynthesized nanoceria to alleviate ferulic acid stress in Solanum lycopersicum. Sci. Hortic-Amsterdam 214, 158–164.
Idrees, M., Naeem, M., Aftab, T., Khan, M.M.A., 2011. Salicylic acid mitigates salinity stress by improving antioxidant defence system and enhances vincristine and vin-blastine alkaloids production in periwinkle [Catharanthus roseus (L.) G. Don]. Acta Physiol. Plant. 33, 987–999.
Jiang, M., Zhang, J., 2002. Involvement of plasma-membrane NADPH oxidase in abscisic acid- and water stress-induced antioxidant defense in leaves of maize seedlings. Planta 215, 1022–1030.
Kumar, N., Pruthi, V., 2014. Potential applications of ferulic acid from natural sources. Biotechnol. Rep. 4, 86–93.
Laemmli, U.K., 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685.
Lee, B.R., Kim, K.Y., Jung, W.J., Avice, J.C., Ourry, A., Kim, T.H., 2007. Peroxidases and lignification in relation to the intensity of water-deficit stress in white clover (Trifolium repens L.). J. Exp. Bot. 58, 1271–1279.
Li, D.M., Nie, Y.X., Zhang, J., Yin, J.S., Li, Q., Wang, X.J., Bai, J.G., 2013. Ferulic acid pretreatment enhances dehydration-stress tolerance of cucumber seedlings. Biol. Plant. 57, 711–717.
Li, J.T., Qiu, Z.B., Zhang, X.W., Wang, L.S., 2011. Exogenous hydrogen peroxide can enhance tolerance of wheat seedlings to salt stress. Acta Physiol. Plant. 33, 835–842.
Lindsay, S.E., Fry, S.C., 2008. Control of diferulate formation in dicotyledonous and gramineous cell-suspension cultures. Planta 227, 439–452.
Liu, L.L., He, J.H., Xie, H.B., Yang, Y.S., Li, J.C., Zou, Y., 2014. Resveratrol induces an-tioxidant and heat shock protein mRNA expression in response to heat stress in black-boned chickens. Poultry Sci. 93, 54–62.
Liu, S., Yang, R., Tripathi, D.K., Li, X., Jiang, M., Lv, B., Ma, M., Chen, Q., 2018. Signalling cross-talk between nitric oxide and active oxygen in Trifolium repens L. plants re-sponses to cadmium stress. Environ. Pollut. 239, 53–68.
Liu, S., Yang, R., Pan, Y., Ma, M., Pan, J., Zhao, Y., Cheng, Q., Wu, M., Wang, M., Zhang, L., 2015. Nitric oxide contributes to minerals absorption, proton pumps and hormone equilibrium under cadmium excess in Trifolium repens L. Plants. Ecotox. Environ. Saf. 119, 35–46.
Liu, S., Yang, R., Pan, Y., Ren, B., Chen, Q., Li, X., Xiong, X., Tao, J., Cheng, Q., Mingdong, M., 2016. Beneficial behavior of nitric oxide in copper-treated medicinal plants. J. Hazard. Mater. 314, 140–154.
Liu, Z., Wang, L., Wang, C.Y., Sun, S.J., Qin, L., An, Y., Xing, Y.S., Sun, H.W., 2017. Effect of corrosion inhibitor benzotriazole on the uptake and translocation of Cd in rice (Oryza sativa L.) under different exposure conditions. Chemosphere 186, 24–30.
Liu, Z.J., Guo, Y.K., Bai, J.G., 2010. Exogenous hydrogen peroxide changes antioxidant enzyme activity and protects ultrastructure in leaves of two Cucumber ecotypes under osmotic stress. J. Plant Growth Regul. 29, 171–183.
Michalak, A., 2006. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Polish J. Environ. Stud. 15.
Mittler, R., Zilinskas, B.A., 1993. Detection of ascorbate peroxidase-activity in native gels by inhibition of the ascorbate-dependent reduction of nitroblue tetrazolium. Anal. Biochem. 212, 540–546.
Miyake, C., Asada, K., 1992. Thylakoid-bound ascorbate peroxidase in spinach-chlor-oplasts and photoreduction of its primary oxidation-product monodehydroascorbate radicals in thylakoids. Plant Cell Physiol. 33, 541–553.
Nable, R.O., Banuelos, G.S., Paull, J.G., 1997. Boron toxicity. Plant Soil 193, 181–198.
Nakano, Y., Asada, K., 1981. Hydrogen peroxide is scavenged by ascorbate-specific per-oxidase in spinach chloroplasts. Plant Cell Physiol. 22, 867–880.
Paradiso, A., Berardino, R., de Pinto, M.C., Sanita di Toppi, L., Storelli, M.M., Tommasi, F., De Gara, L., 2008. Increase in ascorbate-glutathione metabolism as local and precocious systemic responses induced by cadmium in durum wheat plants. Plant Cell Physiol. 49, 362–374.
Rao, K.M., Sresty, T., 2000. Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci. 157, 113–128.
Rehman, S., Park, T.I., Kim, Y.J., Seo, Y.W., Yun, S.J., 2006. Inverse relationship between boron toxicity tolerance and boron contents of barley seed and root. J. Plant Nutr. 29, 1779–1789.
Reid, R.J., Hayes, J.E., Post, A., Stangoulis, J.C.R., Graham, R.D., 2004. A critical analysis of the causes of boron toxicity in plants. Plant Cell Environ. 27, 1405–1414.
Sagi, M., Fluhr, R., 2001. Superoxide production by plant homologues of the gp91phox NADPH oxidase. Modulation of activity by calcium and by tobacco mosaic virus in-fection. Plant Physiol. 126, 1281–1290.
Saleh, A.M., Madany, M.M.Y., Gonzalez, L., 2014. The effect of coumarin application on early growth and some physiological parameters in Faba bean (Vicia faba L.). J. Plant Growth Regul. 34, 233–241.
Santa-Cruz, A., Martinez-Rodriguez, M.M., Perez-Alfocea, F., Romero-Aranda, R., Bolarin, M.C., 2002. The rootstock effect on the tomato salinity response depends on the shoot genotype. Plant Sci. 162, 825–831.
Schonhof, I., Klaring, H.P., Krumbein, A., Claussen, W., Schreiner, M., 2007. Effect of temperature increase under low radiation conditions on phytochemicals and ascorbic acid in greenhouse grown broccoli. Agric. Ecosyst. Environ. 119, 103–111.
Seevers, P., Daly, J., Catedral, F., 1971. The role of peroxidase isozymes in resistance to wheat stem rust disease. Plant Physiol. 48, 353–360.
Shah, A., Wu, X., Ullah, A., Fahad, S., Muhammad, R., Yan, L., Jiang, C., 2017. Deficiency and toxicity of boron: alterations in growth, oxidative damage and uptake by citrange
orange plant. Ecotox. Environ. Saf. 145, 575–582.
Shi, Q., Bao, Z., Zhu, Z., He, Y., Qian, Q., Yu, J., 2005. Silicon-mediated alleviation of Mn toxicity in Cucumis sativus in relation to activities of superoxide dismutase and as-corbate peroxidase. Phytochemistry 66, 1551–1559.
Simon, I., Diaz-Lopez, L., Gimeno, V., Nieves, M., Pereira, W.E., Martinez, V., Lidon, V., Garcia-Sanchez, F., 2013. Effects of boron excess in nutrient solution on growth, mineral nutrition, and physiological parameters of Jatropha curcas seedlings. J. Plant Nutr. Soil Sci. 176, 165–174.
Smart, R.E., Bingham, G.E., 1974. Rapid estimates of relative water content. Plant Physiol. 53, 258–260.
Vermerris, W., Nicholson, R., 2006. Phenolic Compound Biochemistry. Springer, Dordrecht ISBN 13, 978-971.
Vitória, A.P., Lea, P.J., Azevedo, R.A., 2001. Antioxidant enzymes responses to cadmium in radish tissues. Phytochemistry 57, 701–710.
Xu, J.Y., Liu, Y., Cui, S.R., Miao, X.W., 2006. Behavioral responses of tilapia (Oreochromis
niloticus) to acute fluctuations in dissolved oxygen levels as monitored by computer
vision. Aquacult. Eng. 35, 207–217.
Zhang, W.B., Jiang, H.W., Qiu, P.C., Liu, C.Y., Chen, F.L., Xin, D.W., Li, C.D., Hu, G.H., Chen, Q.S., 2012. Genetic overlap of QTL associated with low-temperature tolerance at germination and seedling stage using BILs in soybean. Can. J. Plant Sci. 92, 1381–1388.