Full Terms & Conditions of access and use can be found at
https://www.tandfonline.com/action/journalInformation?journalCode=wsfr20
International Journal of Fruit Science
ISSN: 1553-8362 (Print) 1553-8621 (Online) Journal homepage: https://www.tandfonline.com/loi/wsfr20
Postharvest Quality Extension of Minimally
Processed Table Grapes by Chitosan Coating
Ferhan K. Sabir, Ali Sabir, Sevil Unal, Merve Taytak, Alper Kucukbasmaci & Omer Faruk Bilgin
To cite this article: Ferhan K. Sabir, Ali Sabir, Sevil Unal, Merve Taytak, Alper Kucukbasmaci & Omer Faruk Bilgin (2019) Postharvest Quality Extension of Minimally Processed Table Grapes by Chitosan Coating, International Journal of Fruit Science, 19:4, 347-358, DOI: 10.1080/15538362.2018.1506961
To link to this article: https://doi.org/10.1080/15538362.2018.1506961
Published online: 07 Aug 2018.
Submit your article to this journal
Article views: 396
View related articles
View Crossmark data
Postharvest Quality Extension of Minimally Processed
Table Grapes by Chitosan Coating
Ferhan K. Sabira, Ali Sabira, Sevil Unalb, Merve Taytakb, Alper Kucukbasmacib, and Omer Faruk Bilginb
aDepartment of Horticulture, Selcuk University Agriculture Faculty, Konya, Turkey;bDepartment of
Horticulture, Selcuk University Graduate School of Natural and Applied Science, Konya, Turkey
ABSTRACT
Demand for fresh grapes is increasing globally due to their rich composition in phenolic compounds, which have a strong anti-oxidant capacity. However, fresh table grapes deteriorate rapidly due to berry water loss and pathogen growth, which make it difficult to preserve without treatment. Chitosan coating, as a healthy, simple and innovative technology against to common SO2fumigation, was tested at various concentrations (0%, 0.5%,
1.0% and 2.0%) for the effectiveness on postharvest quality extension of detached grapes of ‘Alphonse Lavallée’ cultivar. Chitosan coating at all doses significantly retarded the loss in berry weight, extended the skin rupture force and total phenol content. Visual quality was higher due to coating the berries with chitosan. Chitosan at all concentrations was effective on delaying maturity index (used to express postharvest senescence) and changes in berry colour values such as L*, C and Hue angle. Among the applied doses, 1% chitosan solution can be recom-mended to apply since higher doses were more effective with similar results on overall quality features of berries. Overall find-ings demonstrated that chitosan as an edible coating with their unique barrier can be utilized as a natural preservative of detached grapes to extend the postharvest quality up to 28 days.
KEYWORDS
Fresh fruits; cold storage; fruit processing; product coating; antioxidant
Introduction
Grapes are increasingly appreciated for their rich composition in phenolic compounds, giving them a great nutritional value. Anthocyanins are natural pigments, largely distributed in nature and generally present in grapes. Grape berries demonstrate to have a great antioxidant activity, due to their high content in phenolics (Solari-Godiño et al., 2017). In addition, resveratrol (3,5,4′-trihydroxy-trans-stilbene), a stilbenoid, is found at high concentra-tions in grapes. Resveratrol has antioxidant, anti-inflammation, antibacterial and anticancer properties (Jang et al.,1997). Demand for organic fresh grape is therefore increasing in accordance with the enhancement in living stan-dard and increasing awareness about the harmful effects of chemicals. However, fresh berries easily deteriorate due to water loss and mould growth
CONTACTAli Sabir asabir@selcuk.edu.tr https://doi.org/10.1080/15538362.2018.1506961
(Karabulut et al., 2004; Sabir and Sabir, 2013) resulting in difficulties for postharvest quality extension without treatment (Min et al., 2001). Nevertheless, interdisciplinary studies gained great advances in postharvest technology for long-term storage of table grapes to achieve a better balance between supply and demand in table grape industry. SO2fumigation is still a
commercially widespread method globally used for long-term storage of table grapes. In spite of its excellent effects on decay control, SO2 fumigation is
becoming very restrictive because its residues are dangerous to people and may give a sulphurous flavour to the fruit. Also, SO2 fumigation may cause
sunken burning patches around the pedicel end. Thus, present interest focuses on the use of healthy materials with safe, simple and innovative technology. Recently, there has been an increasing concern in marketing ready-to-eat (stem-detached) grapes rather than the conventional packages of intact clusters (Sabir et al., 2011). Berry detachment is generally advanta-geous because the berries are conventionally sized to suit a single consumer purchase and the hard packages protect the commodity. However, detaching process can induce the pathogen inoculation, and pedicel removal may accelerate certain physiological processes causing postharvest senescence of commodities (Kou et al., 2007).
There is a globally increasing trend to explore innovative strategies that prevent postharvest quality loses, giving priority to methods that reduce decay incidence and avoid side effects on human health resulting from excessive application of synthetic fungicides. Chitosan, as a high-molecular polymer, nontoxic, bioactive agent, has become an appreciated compound due to its fungicidal effects and elicitation of defence mechanisms in plant tissues (Terry and Joyce,2004). Chitosan-based edible coating has been tested for efficacy in prohibiting decay and maintaining shelf-life of perishable produces such as strawberry (Wang and Gao, 2013), peach (Elbarbary and Mostafa, 2014) and plum (Kumar et al., 2017). Studies demonstrated that chitosan reduced decay incidence emerging from Penicillium expansum in apple fruit during storage (De Capdeville et al.,2002). These reports indicate that chitosan offers a great potential as a biodegradable substance that has anti-microbial and eliciting activities without effecting aroma acceptance of horticultural produces (Susenova et al.,2014).
This study was conducted to evaluate the effects of different concentra-tions of chitosan coating on maintenance of quality of berry-detached grapes (cv. ‘Alphonse Lavallée’, an internationally well-known black table grape cultivar with its high quality) during storage at 1°C. Besides, the possibility of chitosan application, as an organic thin layer of protective barrier, was aimed to investigate as an alternative postharvest strategy for chemical-based treatments such as SO2.
Material and methods
Sample preparation and treatments
Grape clusters of organically grown Vitis vinifera L. cv‘Alphonse Lavallée’ were freshly harvested from research area of Selcuk University, Konya, Turkey. The experimental vines received organic cultivation practices with regular winter and summer pruning, drip irrigation and mechanical weed control. About 30 kg grape clusters were collected from research area in early morning. Clusters were immediately transported to laboratory and sorted to obtain homogeneous batches based on colour, size, lack of damage, health and green-ish rachises. Then the clusters were sanitized by dipping into 100 mg L−1 sodium hypochlorite. The detachment operational treatment was performed by cutting the pedicel with a sharp scissor leaving 1–2 mm of cap stem without touching the berries as has been described by Sabir and Sabir (2013).
The chitosan solutions were prepared by dissolving 5.0, 10.0 and 20 g chitosan (Brookfield, Sigma-Aldrich) in 1000 mL distilled water containing 10 mL (v/v) acetic acid (Tezotto-Uliana et al.,2014). Grape berries of three of the groups were dipped into different concentrations of chitosan for 5 min while control fruits immersed into distilled water contain 10 mL acetic acid for same duration. Care was taken to ensure that all berries were completely submerged into the solution. After treatments, berries were dried for 2 h with a mild air movement in order to let the dew on berries evaporate at room temperature. About 200 g of each grape sample was packed in 12 × 15 cm rigid polypropylene cups. The cups were then covered with their original lids. A total of 48 cups were used, 12 of which belonged to each treatment (apart from initial analysis). The packages were stored at 1°C (±0.5°C) 0 with a relative humidity of 90% for 28 days with quality evaluation performed on days 0, 7, 14, 21 and 28. For each treatment, consisting of 12 cups initially, three cups were used in each sampling date for quality analyses.
Weight loss and visual quality assessments
The weight loss (%) during postharvest storage was determined by periodical weighing and calculated by dividing the mass change during storage by the original mass: Weight loss (%) = [(Mi – Ms)/Mi] × 100, where Mi = initial
mass and Ms= mass at examined time (Mattiuz et al.,2009). After weighing,
visual index of the same berry set in each cup was determined using 1–5 scale (excellent = 5, good = 4, slightly dull = 3, <50% brownish or soft berries = 2, >50% brownish or soft berries = 1).
Skin colour
Skin colour of 30 berries per treatment was recorded using a colorimeter (Minolta® CR-400) to obtain the following variables from two equatorial points of berries: L* (lightness), C (chroma) and h° (hue). Lightness values may range from 0 (black) 100 (white). Chroma indicates the purity or intensity of colour, the distance from grey (achromatic) towards a pure chromatic colour and is calculated from the a* and b* values of the CIE Lab scale system, starts from zero for a completely neutral colour, and does not have an arbitrary end, but intensity increases with magnitude. Hue refers to the colour wheel and is measured in angles; green, yellow and red correspond to 180°, 90° and 0°, respectively (McGuire,1992; Peppi et al.,2006).
Rupture force of berry
For skin rupture force, a berry from each equatorial section was cradled in a jig attached to a force gauge (DPS-11; Imada, Northbrook, IL) and the gauge was gently pulled away from the berry until the skin puncture (Fidelibus et al.,2007). The force required to puncture the skin of berry was recorded as the skin rupture force.
Chemical analyses of berry must
Juice from the randomly gathered berries was extracted with a hand press and filtered through cheesecloth and the supernatants were collected for juice analysis. SSC (°Brix) was determined with a hand-held temperature compen-sated refractometer (Atago 9313). TA was quantified by titrating 10 mL of the homogenized berry flesh juice (must) with 0.1 N NaOH to an endpoint of pH 8.1 and expressed as the percentage of tartaric acid (Cefola et al., 2011; Karabulut et al.,2004; Valero et al.,2006). Maturity index (MI) was obtained with soluble solid content/acid content while pH was taken by means of a pH-meter (GLP21+, Crison Instruments, Spain). All assays were performed in triplicate.
Sample extraction for antioxidant and phenol analyses
Grape berry extracts for antioxidant and phenol analyses were prepared using method described by Thaipong et al. (2006) with certain modifications. After removing the stem caps of berries, 5 g grape tissue from a mixture of 15 berries without seeds was homogenized in methanol using Ultra-Turrax homogenizer (IKA, T18 digital, Staufen, Germany) for 1 min and then centrifuged at 4000 × g for 30 min at 5°C. The supernatants were recovered and stored at−20°C in dark colour bottles until analysis.
Total antioxidant capacity
The antioxidant capacity of the sample was found using a ferric reducing antioxidant potential (FRAP) assay according to the method defined by Benzie and Strain (1996). The FRAP reagent was a mixture of 25 mL of acetate buffer pH 3.0, 2.5 mL of 10 mM 2,4,6-trioyridyl-1,3,5-triazine and 2.5 mL of 20 mM ferric chloride hexahydrate. The mixture reaction was started when 0.5 mL of the supernatant was added in 5 mL of FRAP solution. The reaction solution was incubated at ambient temperature for 30 min and then the absorbance was measured at 630 nm. The antioxidant capacity was expressed as micromoles of Trolox equivalents per gram fresh weight (μmole Trolox equivalent/g FW).
Total phenols
Total phenols were determined according to the method of Singleton et al. (1999). A 100 μL aliquot of each extract was mixed with 1.58 mL of water, 100μL of Folin-Ciocalteu’s reagent and 300 μL of sodium carbonate solution (200 g L–1). The absorbance at 760 nm was read after 2 h. The content of total phenols was calculated on the basis of the calibration curve of gallic acid and was expressed as mg gallic acid 100 g–1FW.
Statistical analysis
Data sets from analysed parameters were subjected to analysis of variance. Sources of variation were time of storage, treatments and their interactions. Comparisons of means were performed by Tukey’s multiple range tests at P ≤ 0.05 significance level. All analyses were performed with SPSS software package v. 15.0 for windows.
Results and discussion
Berry weight loss and visual quality
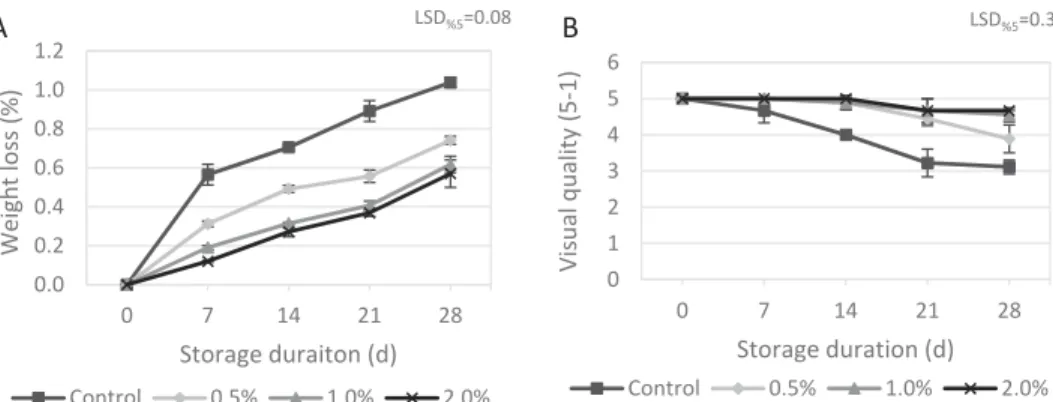
Changes in weight loss for samples of ‘Alphonse Lavallée’ throughout the cold storage period are shown in Figure 1(a). The rate of weight loss gradually increased along with the prolonged storage time at 1°C. The weight loss value of control berries was always significantly higher than those of chitosan coating treatments. As for the application concentrations, the high-est rate of (2%) chitosan was more effective than others with a significant differences detected during the storage. At the end of the storage, the highest loss in weight was found in control berries (1.10%), while the lowest value was determined in 2% (0.56%) which was closely followed by 1% rate (0.57%). The values were similar to those reported by Sabir at al. (2011)
who studied quality maintenance of detached grapes cv. ‘Razaki’ during storage at 0°C for 28 days. The greatest change that takes place in horticul-tural produces during the storage is loss of water which ultimately causes loss in weight (Kader,2002). As the moisture content of fresh commodities, such as grapes, is generally higher than 80%, loss in water content during storage is inevitable (Sagar and Suresh Kumar, 2010). Therefore, preventing the water loss of the produces is an essential issue that solely affects the success of the storage. In the present study, chitosan coating had markedly positive effects on delaying weight loss during the storage.
Visual quality is a prime consideration in extending postharvest life of mini-mally processed horticultural produces. Decay incidence and shrivelling are major factors determining the visual quality of fresh produces. In the present study, grape berries did not display decay problems regardless of treatments. Therefore, pathogen identification was not needed. During the first week of the storage period, no significant change occurred in berry visual quality, except for control where little reduction was detected (Figure 1(b)). Later, the berries belonging to control group underwent a noticeable decrease in visual quality around the second weeks. Chitosan coating, regardless of application concentrations, was capable of maintaining the visual quality up to the 21st day. At the end of the storage, the control berries with a least berry appearance value of 3.1 were not marketable due to slight shrivelling development on berry surface, whereas the visual quality of the berries subjected to chitosan coating at 1% (4.7) or 2% (4.8) were almost similar to initial quality. Previous studies demonstrated that even a minimum loss in water content may sharply affect berry visual quality of table grapes, leading to brown-ing, wilting and shrivelling (Cappellini et al.,1986; Sabir et al.,2011). Actually, the highest decrease in visual quality occurred in control berries, in a similar pattern to that of weight losses in this study. These simultaneous changes in appearance and weight loss values corroborate the mentioned reports.
0.0 0.2 0.4 0.6 0.8 1.0 1.2 0 7 14 21 28 Weight loss (%) Storage duraiton (d) LSD%5=0.08 Control 0.5% 1.0% 2.0% A 0 1 2 3 4 5 6 0 7 14 21 28 Visual quality (5-1) Storage duration (d) LSD%5=0.33 Control 0.5% 1.0% 2.0% B
Figure 1.Weight loss (A) and visual quality (B) changes of berries during the prolonged storage (1–5 score) as influenced by treatments. Error bar stands for the standard deviation of the mean of triplicate determinations.
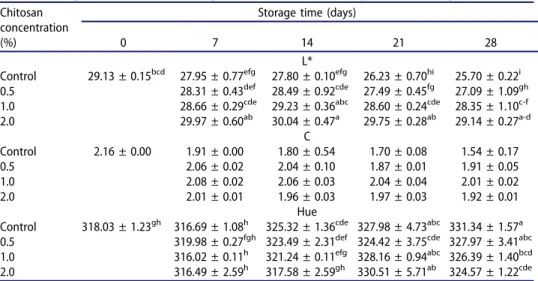
Colour and rupture force of berry
Development of different colour coordinates on berries of the different treatments during the cold storage is presented in Table 1. L* and C values gradually decreased, while Hue angle increased along with the storage dura-tion. All the applied concentrations of chitosan coating delayed the changes in L*and C and Hue values, though the differences in C values were insig-nificant. The highest decreases in L*, C and Hue values occurred in control while the lowest changes were determined in higher doses of chitosan coat-ing. From overall results, it is obvious that chitosan coating was markedly effective on extending the harvest colour of berry skin by slowing down the browning process, a common problem seen in grape storage (Sabir et al.,
2011) caused by non-enzymatic and enzymatic reactions as reported by different authors (González-Barrio et al.,2005; Pastor et al., 2011), studying on postharvest colour response of table grapes to various treatments.
Water loss during the storage results in decrease in skin rupture force which directly influences the market quality of the berries. As can be seen in
Figure 2, skin rupture force of the berries gradually decreased with storage duration. However, chitosan coatings, with a concentration-depended effect, significantly extended the skin rupture force of detached grapes. Higher concentration of the chitosan solutions were more influential while skin rupture force of control berries underwent a dramatic decrease around the end of storage duration. Periodical analyses indicated that chitosan coatings always saved the berries from bruising and rupturing of berry skin.
Table 1.Changes in L*, C and Hue angle values of berries throughout the storage period.
Chitosan concentration (%)
Storage time (days)
0 7 14 21 28
L*
Control 29.13 ± 0.15bcd 27.95 ± 0.77efg 27.80 ± 0.10efg 26.23 ± 0.70hi 25.70 ± 0.22i
0.5 28.31 ± 0.43def 28.49 ± 0.92cde 27.49 ± 0.45fg 27.09 ± 1.09gh
1.0 28.66 ± 0.29cde 29.23 ± 0.36abc 28.60 ± 0.24cde 28.35 ± 1.10c-f
2.0 29.97 ± 0.60ab 30.04 ± 0.47a 29.75 ± 0.28ab 29.14 ± 0.27a-d C Control 2.16 ± 0.00 1.91 ± 0.00 1.80 ± 0.54 1.70 ± 0.08 1.54 ± 0.17 0.5 2.06 ± 0.02 2.04 ± 0.10 1.87 ± 0.01 1.91 ± 0.05 1.0 2.08 ± 0.02 2.06 ± 0.03 2.04 ± 0.04 2.01 ± 0.02 2.0 2.01 ± 0.01 1.96 ± 0.03 1.97 ± 0.03 1.92 ± 0.01 Hue
Control 318.03 ± 1.23gh 316.69 ± 1.08h 325.32 ± 1.36cde 327.98 ± 4.73abc 331.34 ± 1.57a
0.5 319.98 ± 0.27fgh 323.49 ± 2.31def 324.42 ± 3.75cde 327.97 ± 3.41abc
1.0 316.02 ± 0.11h 321.24 ± 0.11efg 328.16 ± 0.94abc 326.39 ± 1.40bcd
2.0 316.49 ± 2.59h 317.58 ± 2.59gh 330.51 ± 5.71ab 324.57 ± 1.22cde
LSD for L*: 0.90; C: N.S.; hue: 4.25. Means of triplicate measurements are presented with standard deviations.
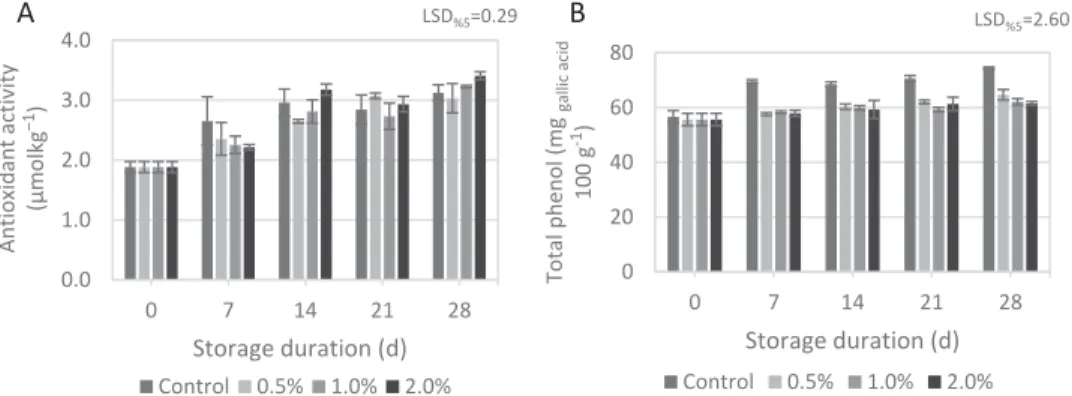
Bioactive components
Postharvest treatments together with cold storage are required to maintain bioactive components in fruits (Antunes et al., 2003). In the present study, changes in antioxidant activity (Figure 3(a)) and total phenol (Figure 3(b)) were determined during the storages. There was a significant and apparent increase in antioxidant activity of grape berries, regardless of treatments up to the 14th day. Afterwards, minor increases were detected until the end of storage. On the 28th day, antioxidant activity of berries coated with 2% chitosan was significantly higher than the others. Total phenol content was significantly affected by the treatments. From the beginning of the storage till the end, total phenol was significantly higher in control berries with an analogue pattern recorded by Champa et al. (2015) and Sabir and Sabir (2017), while chitosan coating markedly delayed the changes in berry phenol content. Grape berries possess high antioxidant capacity due to high phenol content. Thus, retaining the phenol content is an essential issue in extending
0.0 0.5 1.0 1.5 2.0 2.5 0 7 14 21 28 Skin rupture force Storage duration (d) LSD%5=0.06 Control 0.5% 1.0% 2.0%
Figure 2.Skin rupture force changes of berries during the prolonged storage as influenced by
treatments. Error bar stands for the standard deviation of the mean of triplicate determinations.
0.0 1.0 2.0 3.0 4.0 0 7 14 21 28 Antioxidant activity (µmolkg −1 ) Storage duration (d) LSD%5=0.29 Control 0.5% 1.0% 2.0% A 0 20 40 60 80 0 7 14 21 28 Total phenol (mg gallic acid 100 g -1) Storage duration (d) LSD%5=2.60 Control 0.5% 1.0% 2.0% B
Figure 3.Antioxidant capacity (A) and total phenol (B) changes of berries during the prolonged storage as influenced by treatments. Error bar stands for the standard deviation of the mean of triplicate determinations.
the postharvest quality of detached grapes. It should be noted that antiox-idant potential of a produce is not always determined by phenolic com-pounds alone. Exceptionally, some other non-phenolic comcom-pounds could have more potent antioxidant activities. Furthermore, the antioxidant poten-tial of plants is influenced by several factors including the presence or absence of co-antioxidants and transition metal ions as indicated by Kasote et al. (2015). Phenol content of the control berries drastically increased at early stages of storage probably due to accelerated respiration physiology. However, phenol analyses imply that chitosan treatments efficiently mini-mized the undesired syneresis of phenols.
Chemical analyses of berry must
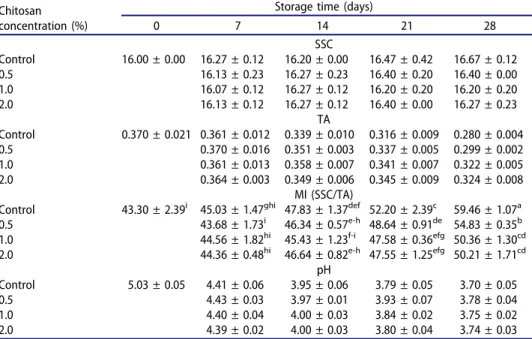
Changes in SSC, TA, pH and MI values of grape juice in response to the different treatments during the prolonged cold storage are shown inTable 2. Generally, SSC underwent a slight but insignificant increase through the storage. Using various controlled atmospheres of CO2 combined with O2,
Crisosto et al. (2002) concluded that none of the treatments affected the SSC, TA and MI values of berries of‘Red globe’ grape, although a general increase in SSC is seen during storage of horticultural produces (Qin et al.,2015; Sabir and Sabir, 2013). TA content of berries progressively decreased during storage with the highest decrease in control, though the differences were
Table 2.Changes in SSC (°Brix), TA (%), MI and pH of berries throughout the storage period.
Chitosan concentration (%)
Storage time (days)
0 7 14 21 28 SSC Control 16.00 ± 0.00 16.27 ± 0.12 16.20 ± 0.00 16.47 ± 0.42 16.67 ± 0.12 0.5 16.13 ± 0.23 16.27 ± 0.23 16.40 ± 0.20 16.40 ± 0.00 1.0 16.07 ± 0.12 16.27 ± 0.12 16.20 ± 0.20 16.20 ± 0.20 2.0 16.13 ± 0.12 16.27 ± 0.12 16.40 ± 0.00 16.27 ± 0.23 TA Control 0.370 ± 0.021 0.361 ± 0.012 0.339 ± 0.010 0.316 ± 0.009 0.280 ± 0.004 0.5 0.370 ± 0.016 0.351 ± 0.003 0.337 ± 0.005 0.299 ± 0.002 1.0 0.361 ± 0.013 0.358 ± 0.007 0.341 ± 0.007 0.322 ± 0.005 2.0 0.364 ± 0.003 0.349 ± 0.006 0.345 ± 0.009 0.324 ± 0.008 MI (SSC/TA)
Control 43.30 ± 2.39i 45.03 ± 1.47ghi 47.83 ± 1.37def 52.20 ± 2.39c 59.46 ± 1.07a
0.5 43.68 ± 1.73i 46.34 ± 0.57e-h 48.64 ± 0.91de 54.83 ± 0.35b 1.0 44.56 ± 1.82hi 45.43 ± 1.23f-i 47.58 ± 0.36efg 50.36 ± 1.30cd 2.0 44.36 ± 0.48hi 46.64 ± 0.82e-h 47.55 ± 1.25efg 50.21 ± 1.71cd pH Control 5.03 ± 0.05 4.41 ± 0.06 3.95 ± 0.06 3.79 ± 0.05 3.70 ± 0.05 0.5 4.43 ± 0.03 3.97 ± 0.01 3.93 ± 0.07 3.78 ± 0.04 1.0 4.40 ± 0.04 4.00 ± 0.03 3.84 ± 0.02 3.75 ± 0.02 2.0 4.39 ± 0.02 4.00 ± 0.03 3.80 ± 0.04 3.74 ± 0.03
LSD for SSC: ns; TA: ns; MI: 2.61; pH: ns (ns: not significant). Means of triplicate measurements are presented
insignificant. Chitosan at all doses suppressed the decrease in TA for ‘Alphonse Lavallée’ grape berries, indicating the possible restricting effect of coating operation on berry respiration and eventually catabolism of organic acids. Slight increases in SSC and decreases in TA resulted in significant increases in MI which indicates postharvest senescence of com-modities. After a 28-day storage, the highest MI was determined in control, while chitosan at all concentration was effective on delaying MI. The pH also showed a general remarkable decrease along with the storage time similar to the results of Sabir and Sabir (2013). Relatively lower TA decrease courses, with a subsequent lower MI among the overall chitosan-coated grapes, indicate that treatments apparently retarded postharvest physiological senes-cence of grape berries during the prolonged storage.
Conclusion
To generalize, chitosan coating at all doses significantly maintained the overall quality of detached grapes (cv. ‘Alphonse Lavallée’) by delaying loss in berry weight and berry skin rupture force. Chitosan was also effective on maintaining the initial berry surface colour, phenol content and many other chemical components. Overall investigations imply that about one week storage life increase might be achieved with chitosan coating as compared to control (non-coated berries). Higher doses were more effective with similar results on overall quality features of berries. Therefore, 1% concen-tration of chitosan solution can be recommended to apply considering the increasing cost of higher doses which may sometimes be a limiting factor when grape price is very low. The present study pays specific attention to detached grapes, by adjusting a potentially environment-friendly protection means, the utilization of chitosan solution as an edible coating barrier to moisture and resist to water vapour diffusion during the cold storage, offer-ing a good adherence to berry surface.
References
Antunes, L.E.C., F.J. Duarte, and C.M. Souza.2003. Postharvest conservation of blackberry fruits. Pesq. Agropec. Bras. 38:413–419. doi:10.1590/S0100-204X2003000300011.
Benzie, I.F.F., and J.J. Strain.1996. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”, The FRAP assay. Anal. Biochem. 239:70–76. doi:10.1006/abio.1996.0286. Cappellini, R.A., M.J. Ceponis, and G.W. Lightner.1986. Disorders in table grape shipments
to the New York market. Plant Dis. 70:1075–1079. doi:10.1094/PD-70-1075.
Cefola, M., B. Pace, D. Buttaro, P. Santamaria, and F. Serio.2011. Postharvest evaluation of soilless-grown table grape during storage in modified atmosphere. J. Sci. Food Agr. 91:2153–2159. Champa, W.A.H., M.I.S. Gill, B.V.C. Mahajan, and N.K. Arora.2015. Preharvest salicylic acid
treatments to improve quality and postharvest life of table grapes (Vitis vinifera L.) cv. Flame Seedless. J. Food Sci. Technol. 52:3607–3616.
Crisosto, C.H., D. Garner, and G. Crisosto.2002. Carbon dioxide-enriched atmospheres during cold storage limit losses from Botrytis but accelerate rachis browning of ‘Redglobe’ table grapes. Postharvest Biol. Technol. 26:181–189. doi:10.1016/S0925-5214(02)00013-3. De Capdeville, G., C.L. Wilson, S.V. Beer, and J.R. Aist.2002. Alternative disease control agents
induce resistance to blue mold in harvested ‘Red Delicious’ apple fruit. Phytopathology. 92:900–908. doi:10.1094/PHYTO.2002.92.8.900.
Elbarbary, A.M., and T.B. Mostafa.2014. Effect of g-rays on carboxymethyl chitosan for use as antioxidant and preservative coating for peach fruit. Carbohyd. Polym. 104:109–117. doi:10.1016/j.carbpol.2014.01.021.
Fidelibus, M.W., K.A. Cathline, and J. Burns. 2007. Potential abscission agents for raisin, table, and wine grapes. Hort Sci. 42:1626–1630.
González-Barrio, R., M. Salmenkallio-Marttila, F.A. Tomás-Barberán, E. Cantos, and J.C. Espín.2005. Etiology of UV-C-induced browning in var. Superior white table grapes. J. Agric. Food Chem. 53:5990–5996. doi:10.1021/jf0504115.
Jang, M., L. Cai, G.O. Udeani, K.V. Slowing, C.F. Thomas, C.W. Beecher, H.H. Fong, N. R. Farnsworth, A.D. Kinghorn, R.G. Mehta, et al. 1997. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 275:218–220. doi: 10.1126/science.275.5297.218.
Kader, A.A.2002. Postharvest biology and technology: An overview, p. 145–148. In: Kader A.
A. (ed.). Postharvest Technology of Horticultural Crops. Publication 3311. University, CA. Karabulut, O.A., F.M. Gabler, M. Mansour, and J.L. Smilanick.2004. Postharvest ethanol and hot water treatments of table grapes to control gray mold. Postharvest Biol. Tech. 34:169–177. doi:10.1016/j.postharvbio.2004.05.003.
Kasote, D.M., S.S. Katyare, M.V. Hegde, and H. Bae. 2015. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Biol. Sci. 11:982–991. doi: 10.7150/ijbs.12096.
Kou, L., Y. Lou, and X. Liu.2007. Effects of mild heat treatment on microbial growth and product quality of packaged fresh-cut table grapes. J. Food Sci. 72:567–573. doi:10.1111/ j.1750-3841.2007.00503.x.
Kumar, P., S. Sethi, R.R. Sharma, M. Srivastav, and E. Varghese. 2017. Effect of chitosan coating on postharvest life and quality of plum during storage at low temperature. Sci. Hortic. 226:104–109. doi:10.1016/j.scienta.2017.08.037.
Mattiuz, B., A.C.A. Miguel, V.C. Galati, and J.C. Nachtigal.2009. Efeito da temperatura no armazenamento de uvas apirênicas minimamente processadas. Rev. Bras. Frutic. 31:44–52. doi:10.1590/S0100-29452009000100008.
McGuire, R.1992. Reporting of objective color measurements. Hort Sci. 27:1254–1255.
Min, Z., L. Chunli, H. Yanjun, T. Qian, and W. Haiou.2001. Preservation of fresh grapes at ice-temperature-high-humidity. Inter. Agrophys. 15:139–143.
Pastor, C., L. Sánchez-González, A. Marcilla, A. Chiralt, M. Cháfer, and C. González-Martínez.2011. Quality and safety of table grapes coated with hydroxypropyl methylcellu-lose edible coatings containing propolis extract. Postharvest Biol. Technol. 60:64–70. doi:10.1016/j.postharvbio.2010.11.003.
Peppi, M.C., M.W. Fidelibus, and N. Dokoozlian.2006. Abscisic acid application timing and concentration affect firmness, pigmentation and color of‘Flame Seedless’ grapes. Hort Sci. 41:1440–1445.
Qin, X., H. Xiao, C. Xue, Z. Yu, R. Yang, Z. Cai, and L. Si.2015. Biocontrol of gray mold in grapes with the yeast Hanseniaspora uvarum alone and in combination with salicylic acid or sodium bicarbonate. Postharvest Biol. Technol. 100:160–167. doi:10.1016/j.postharvbio.2014.09.010.
Sabir, A., F.K. Sabir, and Z. Kara.2011. Effects of modified atmosphere packing and honey dip treatments on quality maintenance of minimally processed grape cv. Razaki (V. vinifera L.) during cold storage. J. Food Sci. Tech. 48:312–318. doi:10.1007/s13197-011-0237-z. Sabir, F.K., and A. Sabir.2013. Quality response of table grapes (Vitis vinifera L.) during cold
storage to postharvest cap stem excision and hot water treatments. Int. J. Food Sci. Technol. 48:999–1006. doi:10.1111/ijfs.2013.48.issue-5.
Sabir, F.K., and A. Sabir.2017. Postharvest quality maintenance of table grapes cv.‘Alphonse Lavallée’ by exogenous applications of salicylic acid, oxalic acid and MAP. Erwerbs-Obstbau. 59:211–219. doi:10.1007/s10341-016-0314-6.
Sagar, V.R., and P. Suresh Kumar.2010. Recent advances in drying and dehidration of fruits and vegetables: A review. J. Food Sci .Technol. 47:15–26. doi:10.1007/s13197-010-0114-1. Singleton, V.L., R. Orthofer, and R.M. Lamuela-Raventos.1999. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth. Enzymol. 299:152–178.
Solari-Godiño, A., I. Lindo-Rojas, and S. Pandia-Estrada. 2017. Determination of phenolic compounds and evaluation of antioxidant capacity of two grapes residues (Vitis vinifera) of varieties dried: Quebranta (red) and Torontel (white). Cogent Food & Agric. 3:1361599. doi:10.1080/23311932.2017.1361599.
Susenoa, N., E. Savitria, L. Sapeia, and K.S. Padmawijaya. 2014. Improving shelf-life of Cavendish banana using chitosan edible coating. Procedia Chem. 9:113–120. doi:10.1016/j. proche.2014.05.014.
Terry, L.A., and D.C. Joyce. 2004. Elicitors of induced disease resistance in postharvest horticultural crops: A brief review. Postharvest Biol. Technol. 32:1–13. doi: 10.1016/j. postharvbio.2003.09.016.
Tezotto-Uliana, J.V., P.F. Gabriela, G.M. Geerdink, and R.A. Kluge.2014. Chitosan applica-tions pre- or postharvest prolong raspberry shelf-life quality. Postharvest Biol. Technol. 91:72–77. doi:10.1016/j.postharvbio.2013.12.023.
Thaipong, K., U. Boonprakob, K. Crosby, and D.H. Byrne. 2006. Comparison of ABTS, DPPH, RAP, and ORAC assays for estimating antioxidant activity from guava extracts. J. Food Compos. Anal. 19:669–675. doi:10.1016/j.jfca.2006.01.003.
Valero, D., J.M. Valverde, D. Martinez-Romero, F. Guillen, S. Castillo, and M. Serrano.2006. The combination of modified atmosphere packaging with eugenol or thymol to maintain quality, safety and functional properties of table grapes. Postharvest Biol. Technol. 41:317–327. doi:10.1016/j.postharvbio.2006.04.011.
Wang, S.Y., and H. Gao. 2013. Effect of chitosan-based edible coating on antioxidants, antioxidant enzyme system, and postharvest fruit quality of strawberries (Fragaria x ananassa Duch.). LWT-Food Sci .Technol. 52:71–79. doi:10.1016/j.lwt.2012.05.003.