Full Terms & Conditions of access and use can be found at
Biotechnology & Biotechnological Equipment
ISSN: 1310-2818 (Print) 1314-3530 (Online) Journal homepage: https://www.tandfonline.com/loi/tbeq20
Transferability of Microsatellite Markers Among
Cool Season Cereals
A. Yıldırım, N. Kandemir, Ö. Ateş Sönmezoğlu & T. Eserkaya Güleç
To cite this article: A. Yıldırım, N. Kandemir, Ö. Ateş Sönmezoğlu & T. Eserkaya Güleç (2009) Transferability of Microsatellite Markers Among Cool Season Cereals, Biotechnology & Biotechnological Equipment, 23:3, 1299-1302, DOI: 10.1080/13102818.2009.10817657 To link to this article: https://doi.org/10.1080/13102818.2009.10817657
© 2009 Taylor and Francis Group, LLC
Published online: 15 Apr 2014.
Submit your article to this journal
Article views: 174
View related articles
1299
Articles
A&eB
Introduction
DnA based markers provide suitable tools for detailed genetic analysis, gene mapping and estimation of genetic diversity. Microsatellites, also known as Simple Sequence Repeats (SSRs), have emerged as an important source of DnA markers (25) and have been successfully applied for detection of genetic diversity (8, 31), genome mapping (12, 22), marker assisted selection of agronomically important traits (11) and genotype differentiation (7, 29). SSR markers have many advantages for genetic studies over other markers. they are highly polymorphic, locus specific and abundant. Besides, they are distributed over the genome and require only small amounts of genomic DnA for analysis. Microsatellite markers have been developed in many crop species such as soybean (1), wheat (12), maize (23), barley (28), rice (32) and potato (19).
in cereals, microsatellites show a much higher level of polymorphism than other marker systems (4, 18, 20, 21). however, development of microsatellite markers is extremely expensive and time-consuming. only about 30% of all primer pairs developed from microsatellite sequences are functional and suitable for genetic analysis (4, 25). therefore, it is important to transfer SSR markers among related species. comparative mapping studies clearly reveal the presence of synteny within the genomes of closely related species, such as wheat (Triticum sp.), barley (Hordeum vulgare), rye (Secale cereale), triticale, rice (Oryza sativa) and maize (Zea
mays L.) (6). Sufficient homology exists among several crop
genomes in the sequences flanking the SSR loci. Thus, primer pairs of a species can be used in related species and this is called “transferability” (14). transferability has been used in many species like wheat and rye (5), wheat, rye and triticale (14), wheat, barley and rye (20). this similarity and genomic
relationship has allowed exchange of SSR primers between crop species.
in this study, our objective was to examine the transferability of some SSR markers among cool season cereals. therefore, 27 wheat and 23 barley SSR markers were selected to test the transferability among barley, oat, rye and wheat.
Materials and Methods
Plant materials
two durum wheat (Selçuklu and Kiziltan) and three bread wheat (Bezostaya, Gönen, Gün), five barley (Steptoe, Morex, triumph, harrington, tokak), two oat (Seydisehir, Faikbey) and two rye (Aslım, Tetra) cultivars were used to determine transferability of barley and wheat SSR markers.
DNA extraction
DnA was extracted from leaf material of each genotype using genomic DNA purification kit (Fermentas Life Sciences, Genomic DNA Purification Kit). Twenty-three barley microsatellite primers (table 2) and twenty-seven wheat microsatellite primers (table 3) have been tested.
PCR amplification
PCR amplifications were performed using the procedure described by Röder (21) with some modifications. PCR reactions were carried out in final volume of 40 µl in a thermal cycler (thermo Px2). PcR mixtures contained 50 ng of genomic DNA, 0.25 µM of each primer, 0.2 µM dNTP mix, 2.5 µM MgCl2, 10x PcR Buffer and 0.5U of taq DnA
polymerase per reaction volume. the PcR cycles were started at 95oc for 5 min. thirty cycles were performed as follows: 1
min at 94oc, 1 min at 50-60oc (depending upon the annealing
temperature of the primers), 1 min at 72oC and a final extension
of five minutes at 72oc. PcR products were separated on 3%
TRANSFERABILITY OF MICROSATELLITE MARKERS
AMONG COOL SEASON CEREALS
A. Yıldırım1, n. Kandemir2, Ö. Ateş Sönmezoğlu2 and t. eserkaya Güleç2
Karamanoglu Mehmetbey University, Faculty of Science, Department of Biology, Karaman, turkey1
University of Gaziosmanpasa, Faculty of Agriculture, Department of Field crops, tokat, turkey2
correspondence to: Ahmet Yildirim e-mail: ahmety55@gmail.com
ABSTRACT
Microsatellite markers are powerful tools for many evolutionary and genetic studies. They provide high level of polymorphism and information in cereal genomic research. However, development of microsatellite markers is extremely expensive and both time and labor consuming because of the requirement of prior sequence knowledge for design of locus specific primers. Therefore, use of microsatellite markers developed for one species could be very valuable for related species. In the present study we investigated the possibility of SSR marker transferability among cool season cereals. Transferability of 27 wheat and 23 barley microsatellites were studied using five wheat, five barley, two oat and two rye cultivars. Transferability of the barley SSR markers to wheat was 69.6% (16/23), to oat 43.5% (10/23) and to rye 52.2% (12/23). Of 27 wheat SSR markers 20 (74%) were amplified in barley, 20 (74%) in oat and 19 (70.4%) in rye. These results show that about two or three out of every four microsatellites can be used in related cereal genera.
1300
metaphore agarose gel and visualized by ethidium bromide. electrophoresis was applied at 90W constant power for two to three hours. 1x tBe was used as a running buffer.
Scoring and amplification analysis
Amplified fragments were visualised by a gel image system (Vilber lourmat). Bands were analyzed using Biocapt software Bio 1D, 11.04 version. The number of amplified fragments in a species was counted (14).
Results and Discussion
twenty (74%) of the 27 wheat SSR markers studied were amplified in barley and oat while 19 (70%) were amplified in rye (Table 1), meaning that more than two-thirds of wheat SSRs can be used for any other cool season genus. Sixteen of the wheat markers (Stm18tgag, Stm264agac, Stm553actc, Stm542acag, Stm560acag, Stm578acag, Stm635acag, Stm643acag, Xgwm374, Xgwm513, Xgwm550, Xwmc49, Xwmc329, Xwmc550, Xwmc608 and Xwmc798) were amplified in all four genera while four markers (Stm91agac, Stm527agac, Xgwm130 and Xgwm389) were amplified in three (Table 3). Four SSR markers were specific to wheat only and were not amplified in other genera. Many studies have indicated that microsatellite primers of a species could be used and amplified in its close relatives (2, 3, 10, 26). Kuleung et al. (14) have shown that about 70% of wheat and rye SSR markers could be amplified in at least one species of wheat, rye and triticale. the percentage of SSR transferability from wheat to rye was 27% (13), while 60% of seven rye markers could be transferable to wheat (20). Another study has shown that about 50% SSR primers were transferable from Triticum to Hordeum (9). transferability of wheat SSRs to other cereal species in the present study is comparable to or higher than those of other studies in the literature. higher transferability rates could be a result of the fact that the SSR markers used in the present study were selected among good quality SSR markers, i.e. single copy and clear amplicon production in the original genus (Fig. 1). Our findings, thus, confirm that half to two-thirds of wheat SSRs are transferable to other cereal species. SSRs can also be transferred from major cereals such as wheat, rice, maize and sorghum to minor grass species such as finger millet (Eleusine
coracana l.) with more than 50% success rate depending upon
the genetic relatedness between genera (30).
Of the 23 barley SSR markers, 16 (70%) were amplified in wheat (Fig. 2a), 10 (43.5%) in oat and 12 (52.2%) in rye (Table 1). in other words, at least half of the SSR markers developed for barley were amplified in all of the studied genera. nine barley SSR markers (Bmag110, Bmag359, Bmag606, ebmac691, Gbms50, Gbms117, Gbms166, hVM40 and SCSSR7759) were amplified in all four genera, while two markers (Bmag369 and Bmag603) – in three genera (Table 2). Five barley SSR markers were specific to barley and were not able to amplify in other genera (Fig. 2b). These findings also confirm the fact that, as with wheat, transfer success rate of
barley SSR markers among different cereal species is around 50%.
TABLE 1 Amplification of wheat and barley SSR markers in barley, wheat, oat and rye
SSR donor
species Number of markers
Number of amplified markers Wheat Barley Oat Rye
Wheat 27 27 20 20 19
Barley 23 16 23 10 12
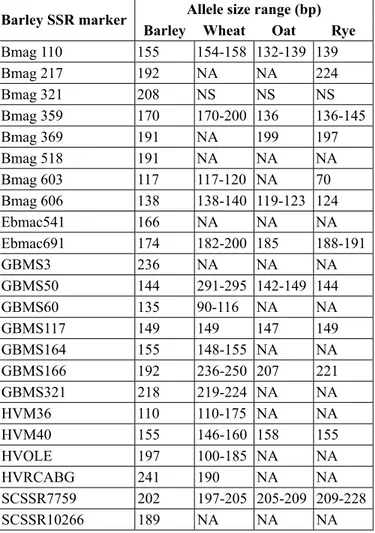
TABLE 2 Some features of barley microsatellites and transferability results
Barley SSR marker Allele size range (bp)
Barley Wheat Oat Rye
Bmag 110 155 154-158 132-139 139 Bmag 217 192 nA nA 224 Bmag 321 208 nS nS nS Bmag 359 170 170-200 136 136-145 Bmag 369 191 nA 199 197 Bmag 518 191 nA nA nA Bmag 603 117 117-120 nA 70 Bmag 606 138 138-140 119-123 124 ebmac541 166 nA nA nA ebmac691 174 182-200 185 188-191 GBMS3 236 nA nA nA GBMS50 144 291-295 142-149 144 GBMS60 135 90-116 nA nA GBMS117 149 149 147 149 GBMS164 155 148-155 nA nA GBMS166 192 236-250 207 221 GBMS321 218 219-224 nA nA hVM36 110 110-175 nA nA hVM40 155 146-160 158 155 hVole 197 100-185 nA nA hVRcABG 241 190 nA nA ScSSR7759 202 197-205 205-209 209-228 ScSSR10266 189 nA nA nA
NA: no amplification; NS: non specific; different band sizes show polymorphism among the genotypes used
Although SSR markers amplified in other genera were not mapped, the sizes of the alleles were generally quite similar to the ones in the original species (Fig. 1 and Fig. 3, Table 3). Therefore, it could be stated that SSRs were amplified from conserved or similar genomic regions in all cereals studied.
1301 TABLE 3
Some features of wheat microsatellites and transferability results
Wheat SSR marker Allele size range (bp)
Wheat Barley Oat Rye
Stm18tgag 244 244 215 206-209 Stm91agac 111 111-113 nS 108 Stm264agac 271 265-271 271 269-271 Stm527acag 164 157 147 nS Stm553actc 50 50-52 34 50-42 Stm542acag 190 193-211 195 193 Stm542tcac 250 nA nA nA Stm560tctg 175 175-178 170 170 Stm578tctg 163 160-166 161 161 Stm635acag 115 113-116 128-134 128 Stm643tgag 180 238 175-185 172-207 Xgwm295 207 nA nS nS WMS595 147 nA 174-180 nA Xgwm46 146 139-146 nS nS Xgwm130 130 nA 132 132 Xgwm374 215 219-221 215 212-215 Xgwm389 133 nA 120 65-77 Xgwm458 117 nA nS nS Xgwm513 165 165-169 165 165 Xgwm550 112 113-119 50 55 Xgwm577 128-131 134 nA nA Xgwm765 195 nA nA nA Xwmc49 200 200-207 219 226 Xwmc329 130 115-128 98 90 Xwmc550 113 113-117 113 116 Xwmc608 86-100 83-97 85-100 85-100 Xwmc798 219 196-200 224-229 224-229
NA: no amplification; NS: non specific; different band sizes show polymorphism among the genotypes used
Fig. 1. PcR products obtained from four wheat SSRs in barley (lane1- wheat
(cv. Selçuklu); lanes 2-6 barley (cvs. Steptoe, Morex, Triumph, Harrington, tokak 2). M: Sigma PcR 50 bp low ladder
Fig. 2. (a) PcR products obtained from two barley SSRs in wheat (lane 1-
barley (cv. Steptoe); lanes 2-6 wheat (cvs. Selçuklu, Kızıltan, Bezostaya, Gönen, Gün). M: Sigma PcR 50 bp low ladder. (b) one of the eSt-derived barley SSRs with no amplification in other genera (lane 1- barley (cv. Steptoe)
Some data about SSR polymorphisms were obtained, although limited number of genotypes was used (four genotypes for wheat and barley, two for oat and rye). Metaphore agarose gels used to detect amplicon length polymorphisms can distinguish DnA fragments differing in length by 2%. considering the average amplicon size of about 200 bp, metaphore agarose gel can differentiate polymorphisms of four or more base differences. thus, amplicon differing by four or more bases were accepted polymorphic. Fifteen of the 20 wheat SSRs amplified in barley showed polymorphisms among four barley genotypes. Of 20 and 18 amplified wheat SSRs in oat and rye, five and three SSR markers were polymorphic between the two genotypes, respectively (Fig. 3a, b).
Fig. 3. PcR products obtained with wheat and barley SSRs in oat and rye
(lanes 1a-1b wheat (cv. Selçuklu) and lane 1c barley (cv. Steptoe); lanes 2-3 oat (cvs. Seydisehir, Faikbey); lanes 4-5 rye (cvs. Aslım, Tetra)
Of 16 barley SSR markers amplified in wheat, 14 showed polymorphism among four wheat genotypes. there were four polymorphisms in 10 amplified barley SSRs in two oat genotypes, and three polymorphisms in 12 amplified barley SSRs in two rye genotypes (Fig. 3c). thus, it can be said that at least in wheat and barley, polymorphism rates of transferred SSR markers are quite high. other SSR transfer studies among cereals indicate low levels of SSR polymorphisms in another cereal species (9, 16, 30). lower polymorphism rates in at least some of the studies in literature (9) were due to the fact that they used EST-origin SSRs and amplified highly conserved regions, which have high level of transferability but low level of polymorphism. in this study, only two eSt-derived barley SSRs were used (ebmac541, ebmac691).
1302
Microsatellites have many advantages for molecular genetic studies and studies of relationships. however, the major bottleneck is the development of new markers. this process is difficult, costly and laborious. Comparative maps suggest that a marker of one genus/species is likely to be present in another related genus/species (26, 27), because DNA sequences flanking the SSR loci are highly conserved among closely related species (14).
Conclusions
this study suggests that microsatellite markers are highly transferable among cereals. our results showed that some of SSR primers produced amplification products with good quality in wheat, barley, oat and rye. Besides, we have found quite high polymorphism rates despite the limited number of genotypes tested. These findings indicate that SSR markers could be transferred among cereal species and can be used in gene mapping and marker assisted selection studies. transferable SSR markers are especially important when there are polymorphic marker shortages in specific regions of the genome. Markers from comparable regions of related genomes can be used. obviously, this approach would be a lot easier and cheaper than developing new SSR primers.
REFERENCES
1. Akkaya M., Bhagwat A., Cregan P. (1992) Genetics, 132, 1131-1139.
2. Bal E.B., Akkaya M.S. (2001) turkish Journal of Biology, 26, 9-12.
3. Brown M., Hopkins M., Mitchell S., Smior M., Wang T., Duncan R., Gonfzle-Candelas F. (1996) theoretical and Applied Genetics, 93, 190-198.
4. Bryan G.J., Collins A.J., Stephenson P., Orry A., Smith J.B., Gale M.D. (1997) theoretical and Applied Genetics, 94, 557-563.
5. Caudrado A., Schwarzacher T. (1998) chromosoma,
107, 587-594.
6. Devos K.M., Millan T., Gale M.D. (1993) theoretical and
Applied Genetic, 85, 784-792.
7. Donini P., Stephenson P., Bryan G.J., Koebner R.M.D. (1998) Genetic Resources and crop evolution, 45, 415-421.
8. Dreisigacker S., Zhang P., Warburton M.L., Skovmand B., Hoisington D., Melchinger A.E. (2005) crop Science, 45, 653-661.
9. Gupta P.K., Rustgi S., Sharma S., Singh R., Kumar N., Balyan H.S. (2003) Molecular Genetics and Genomics,
270(4), 315-323.
10. Hernandes P., Oorado G., Laurie D., Martin A., Snape J. (2001) theoretical and Applied Genetics, 102, 616-622.
11. Jena K.K., Mackill D.J. (2008) crop Science, 48(4), 1266-1276.
12. Korzun V., Röder M., Worland A.J., Börner A. (1997) Plant Breeding, 116, 227-232.
13. Korzun V., Malyshev S., Voylokov A.V., Boerner A. (2001) theoretical and Applied Genetics, 102, 709-717. 14. Kuleung C., Baenziger P.S., Dweikat I. (2003) theoretical
and Applied Genetics, 108(6), 1147-1150.
15. Litt M., Luty J.A. (1989) American Journal of human Genetics, 44, 397-401.
16. Palop M., Palacios C., Gonzales-Candelas F. (2000) conservation Genetics, 1, 177-179.
17. Peakall R., Gilmore S., Keys W., Morgante M., Rafalski A. (1998) Molecular Biology evolution, 15, 1275-1287. 18. Plaschke P., Ganal M.W., Röder M.S. (1995) theoretical
and Applied Genetics, 91, 1001-1007.
19. Provan J., Powell W., Waugh R. (1996) theoretical and Applied Genetics, 92, 1078-1084.
20. Röder M.S., Plaschke P., König S.U., Börner A., Sorrells M.E., Tanksleya S.D., Ganal M.W. (1995) Molecular Gen Genetics, 246, 327-333.
21. Röder M.S., Korzun V., Wendehake K., Plaschke J., Tixier M., Leroy P. and Ganal M.W. (1998) Genetics, 149(4), 2007-2023.
22. Russell J., Fuller J., Young G., Thomas B., Taramino G. (1997) Genome, 40, 442-450.
23. Senior M.L., Heun M. (1993) Genome, 36, 884-889. 24. Sim C.H., Mahani M.C., Choong C.Y., Salma I. (2005)
Fruits, 60, 379-385.
25. Tautz D., M.Trick, Dover G.A. (1986) nature, 322, 652-656.
26. Tikhanov A.P., San Miguel P.J., Nakajima Y., Gorenstein N.M., Bennetzen J.F., Avramova Z. (1999) Proc. nat. Acad. Sci. USA, 96, 7409-7414.
27. Van Deynze A.E., Sorrells M.E., Park W.D., Ayres N.M., Fu H., Cartinhour S.W., Paul E., McCouch S.R. (1998)
theoretical and Applied Genetics, 97, 356-369.
28. Varshney R.K., Marcel T.C., Ramsay L., Russell J., Röder M.S., Stein N., Waugh R., Langridge P., Niks R.E., Graner A. (2007) theoretical and Applied Genetics, 114(6), 1091-1103.
29. Virk P.S, Pooni H.S., Syed N.H., Kearsey M.J. (1999) theoretical and Applied Genetics, 98, 462-464.
30. Wang M.L., Barkley N.L., Yu J., Dean R., Newman M.L., Dunn M.L., Sorrells M., Pederson G.A. (2005) Plant Genetic Resources, 3(1), 45-57.
31. Wei Y.M., Hou Y.C., Yan Z.H., Wu W., Zhang Z.Q., Liu D.C., Zheng Y.L. (2005) Journal Applied Genetics, 46(1), 3-9.
32. Wu K., Tanksley S.D. (1993) Molecular and General Genetics, 241, 225-235.