RESEARCH
Inorganic wastes in glaze recipes and their
effects on microstructure
Nergis Kılınç Mirdalı1
Received: 12 January 2017 / Revised: 30 May 2017 / Accepted: 9 June 2017 / Published online: 19 June 2017 # Australian Ceramic Society 2017
Abstract This work reports on recycling various amounts of inorganic wastes (scraps of glass packaging waste, key saw-dust, copper slag, and pyrite ash) into artistic ceramic glazes. These waste materials were used in the range of 0.6–20% in artistic glaze compositions. Glazes were composed of a mix-ture of acidic (SiO2, B2O3), basic (Na2O, K2O, CaO, ZnO, PbO), and amphoteric (Al2O3,Cr2O3) oxides and formulated using the Seger method. These glaze compositions were ap-plied over the surface of the porcelain body and fired at 1080 °C and characterized by field emission scanning electron microscope (FESEM) coupled with energy dispersive X-ray spectrometry (EDS). As a consequence, results showed that, different inorganic wastes could be used in artistic glaze com-positions for the obtaining attractive colors and textures. Keywords Utilization . Recycling . Artistic glazes . Waste processing . Microstructure
Introduction
The need for raw materials has grown regularly in par-allel with industrial development all over the world. In common with technological developments and produc-tion of the raw materials, huge amounts of toxic and inorganic hazardous wastes are produced every day. Customarily, these wastes are poured directly into natu-ral areas without enough treatment which cause serious
environmental, economical, political, and administrative problems for the near future.
The ceramics sector can incorporate large amounts of waste materials without relevant process modifications, while taking advantage of the calorific value from waste combustion or incorporating the residue in the internal structure of materials, such that the residue forms part of these materials’ matrix and becomes an inert element [1]. As stated in the literature, pos-sibility of using a wide variety of inorganic wastes has become important aspect in the ceramic sector like as ceramic engobes [2,3], ceramic glazes [4–8], ceramic colorants, or pigments [9–15], glass and glass-ceramics [16–18], bricks and roof tiles [19–26], and ceramic tiles [27–30].
In this study, scraps of glass packaging waste, key sawdust, copper slag, and pyrite ash were used as raw material in order to produce artistic glazes. Since the waste materials contain different coloring oxides such as CuO, Fe2O3, and Cr2O3, they seem to be good candidates for coloring purposes and to promise attractive textures in different glaze compositions. All of the waste materials have changed color in the glazes depending on the glaze compositions.
Materials and methods
Different glaze compositions were formulated using the Seger method and prepared by traditional glaze process-ing. Amount of waste materials were ranged from 0.6 to 20 wt% and they were added into the glaze composi-tions. These glaze compositions labeled as G1, G2, G3, G4, G5, and G6, respectively.
The copper slag and pyrite ash used in the present study were obtained from the Eti Bakır Kastamonu Küre Plant in Turkey. Chemical composition of glass packaging waste and copper slag was determined by X-ray fluorescence (Shimadzu
* Nergis Kılınç Mirdalı nkilinc@cu.edu.tr
1 Faculty of Fine Arts, Department of Ceramic, Çukurova University, Balcalı, 01330 Adana, Turkey
XRF-1800). Chemical composition of pyrite ash was deter-mined by wet chemical analysis. Elemental composition of key sawdust was determined by energy dispersive X-ray spec-trophotometer (EDS). Copper slag is rich in Fe2O3 (57.40 wt%) and SiO2(28.63 wt%) and lower amounts of other metal oxides (less than 10 wt%) as CuO, MgO, ZnO, Na2O, and K2O. Chemical composition (in wt%) of pyrite ash is SiO2 (7.39%), Al2O3 (2.32%), Fe2O3 (64.02%), CaO (6.95%), MgO (0.75%), Na2O (0.09%), K2O (0.25%), MnO (0.02%), TiO2(0.14%), P2O5(0.02%), and Cr2O3(0.004) and calculated LOI (loss on ignition) is 6.93%. Chemical compo-sition (in wt%) of glass packaging waste is SiO2(73.01%), Na2O (11.2%), MgO (0.78%), Al2O3(3%), P2O5(0.21%), B2O3(11.3%), and Fe2O3(0.50%). Elemental composition of key sawdust is Cu (66%), Zn (32.4%), Sn (0.92%), and Ni (0.69%).
Glazes were applied onto commercial biscuit fired and un-glazed porcelain plates with 25.8 cm diameter and fired at 1080 °C for 1 h in oxidizing atmosphere.
The seger formulas of the glazes can be seen in Table 1. Surface image of glazes was obtained by
digital camera (Nikon D3200 DSLR Camera with 18– 55-mm lens).
The microstructural studies were performed on fractured and rough glaze surfaces using a field emission scanning elec-tron microscope (FESEM-FEI Quanta 650 model) in back-scattered electron imaging mode (BSE) attached to an energy dispersive X-ray spectrophotometer (EDS). The glazed sam-ples were coated with a thin layer of gold (Au) to a thickness of 1.6 nm and examined at 15 kV.
Results and discussion
After the gloss firing at 1080 °C, crystalline effects were found in all glazes in which the inorganic waste content was 0.6–
Table 1 Molecular formulas of the glazes Molecular formula (Seger)
Basic oxides Neutral oxides Acid oxides Waste material G1
0.25 Na2O 0.30 Al2O3 1.2 SiO2 20% copper slag 0.20 K2O 0.75 B2O3
0.30 CaO 0.25 ZnO G2
1 PbO 0.20 Al2O3 2 SiO2 4% copper slag 0.5 B2O3
2 TiO2
G3 – 2 SiO2 10% scraps of glass packaging waste
1 Na2O 1 B2O3
G4
0.75 Na2O 0.02 Al2O3 2.8 SiO2 10% pyrite ash 0.15 PbO 5%Fe2O35%TiO2 0.75 B2O3
0.10 SnO
G5 0.30 Al2O3 1.2 SiO2 0.6% key sawdust 0.25 Na2O 0.75 B2O3
0.20 K2O 0.30 CaO 0.25 ZnO
G6 – 2 SiO2 20% pyrite ash
0.90 Na2O 1 B2O3 0.10 CaO G1 G2 G3 G4 G5 G6 6cm 6cm 6cm 6cm 6cm 6cm
Fig. 1 Surface appearance and the color of the artistic glazes (glazes were applied onto biscuit fired and unglazed porcelain plates with 25.8 cm diameter and fired at 1080 °C—G1toG6, respectively)
20 wt% (G1to G6, respectively) as shown in Fig.1and Table1 that presents the molecular formulas of the glazes (Seger formula).
All of the glazes are based on boron oxide (B2O3). It reduces the melting temperatures and it influences the esthetic characteristic of the glazes.
Sheet-like crystals were formed in G1, G2, and G6glazes. In B2O3-CaO-ZnO glaze systems that include scraps of glass packaging waste and key sawdust, matt surfaces were formed (G1, G3, G4, G5).
The excessive using of oxides in glaze compositions such as Al2O3, ZnO, and CaO increases glaze viscosity and causes pinhole defects. However, defects like pin holing can be minimized when the viscosity decreased. On the other hand, by using fluxing agents in the glaze composition as lead, feldspar and boron which de-creased viscosity of the zinc based glassy phase and led to the dissolution of some crystals. Scraps from packaging waste glass, pyrite ash, and copper slag give green and brown colors to glaze. The final appearances
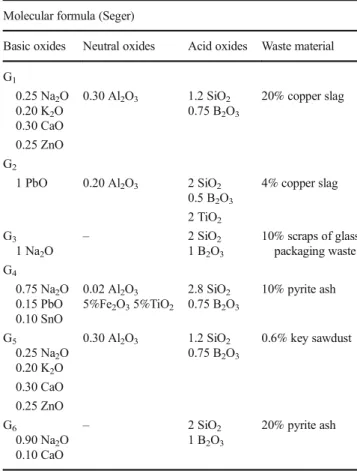
Fig. 2 Representative SEM-BSE image of G1glaze layer with corresponding EDS spectrum from full area (20k×)
Fig. 3 Representative SEM-BSE image of G2 glaze layer with corresponding EDS spectrum from full area (5k×)
Table 2 Approximate elemental compositions (atomic %) from full areas of the fractured and rough glazes obtained with EDS spectrum Elements G1 G2 G3 G4 G5 G6 B 14.35 0.08 9.06 0.11 – – C – 15.76 14.5 – – – O 48.86 48.44 48.45 52.35 56.24 55.6 Na 3.73 2.59 10.3 5.16 4.64 11.05 Mg – – 1.24 1.01 0.52 – Al 6.31 1.36 1.19 0.47 6.3 6.73 Si 19.53 23.72 12.67 28.34 20.3 18.9 Au 0.71 1.42 0.71 1.06 1.12 0.74 S – 0.53 – 0.09 – – Pb – 3.43 – – – – K 3.02 23.72 0.26 0.69 2.62 – Sn – – – 0.03 0.06 – Ca 2.12 1.88 1.34 2.92 4.17 – Ni – – – – 0.07 – Ti – – – 0.06 – – Cr – – 0.18 – – – Fe – 0.33 0.1 6.46 – 6.53 Cu 0.06 0.46 – – 1.5 – Zn 1.3 – – 1.24 2.45 –
of these glazes have patchy, matt, and darker and dif-ferent shades of brown as shown in G3, G4, and G6. The glazes G4and G6contain pyrite ash and have aven-turine effect due to their high content of Fe2O3. Aventurine glazes have randomly scattered laminar crys-tals of high reflectivity. This formation depends on the pyrite ash content, the glaze composition, the viscosity, the thickness of the applied glaze layer, and the firing cycle. Iron from pyrite ash creates aventurine effect in which the glitters are generated by hematite crystals with a dendritic shape or in hexagonal sheets. Glaze which includes key sawdust gives blue color in alkaline glazes. In addition, excessive use of key sawdust is created different shades of blue and metallic effects on G5 glaze.
The approximate elemental compositions from full areas of the fractured and rough glazes are shown in Table2.
The microstructure and elemental analysis of the fractured surfaces of the glazed samples were characterized by SEM-EDS and shown in Figs.2,3,4,5,6, and7.
It was observed from the micrographs that, crystalline materials occurred in glaze matrix and they were not homogenously distributed. Figure 2 shows the SEM mi-crograph and EDS analysis of the G1 glaze. Its com-posed of Na2O, Al2O3, B2O3, CaO, ZnO, and copper slag as shown in Table 1. EDS analysis confirmed the presence of Na, Al, B, Ca, and Zn elements indicating of full area of the G1 glaze. Sheet-like laminar crystals were formed in G2 glaze. EDS analysis from full area of the G2 glaze showed that this glaze consisted of high percentage of Si, B, Pb, and O.
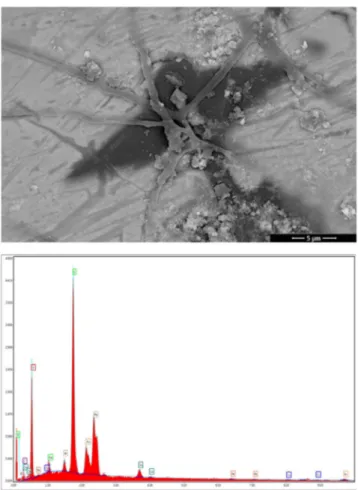
Crack formation in G3 and G4 glazes is due to the difference in coefficient of the thermal expansion between the glaze and body and it can be seen in Figs.4and5. In the G4glaze composition, TiO2 is a well-known nucleat-ing agent for zinc containnucleat-ing crystalline glazes. Figure 5
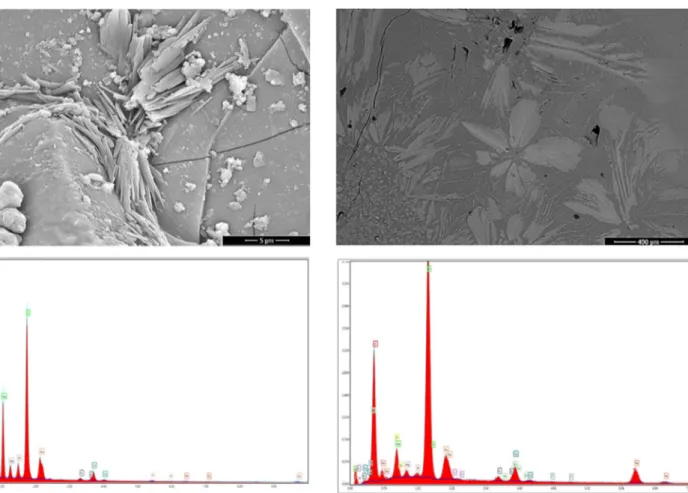
shows SEM-EDS analysis of G4glaze and this glaze con-tains key sawdust. In G5 glaze (Fig. 6), a well-separated and variegated surface was achieved due to B2O3, CaO, ZnO, and key sawdust.
Fig. 5 Representative SEM-BSE image of G4 glaze layer with corresponding EDS spectrum from full area (100×)
Fig. 4 Representative SEM-BSE image of G3glaze layer with corresponding EDS spectrum from full area (5k×)
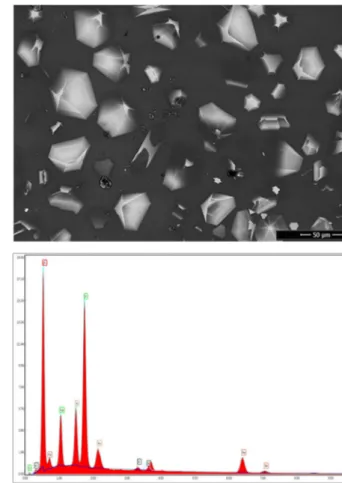
Laminar and hexagonal-shaped crystals were ob-served in G6glaze (Fig. 7). EDS analysis from full area of the G6 glaze showed that this glaze consisted of high percentage of Fe and O (Table 2). Thus, it is most probably the crystalline phase of magnetite (Fe3O4) or hematite (Fe2O3), respectively. Pyrite ash known as the iron-rich waste and can be used as coloring agent in the ceramic glazes. So, the use of pyrite ash in the glaze composition enables obtaining glazes with an aventurine effect. Aventurine glazes can be also obtained with Cr, Cu, Fe, and U, by crystallizing the metal or the oxide.
Conclusions
This work showed that it is possible to utilize inorganic waste materials for coloring purposes and obtaining dec-orative effects. It is proved that, a number of inorganic waste materials can be used for coloring purposes and obtaining attractive textures for decorative purposes in
different glaze compositions. The change in all glazes is related with the amount of inorganic waste material, glaze compositions, and firing temperatures. The recycling of industrial waste into artistic glazes can be environmental solution from technological, ecological, and economic point of view. In this way, possible pol-lution and public health problems can be prevented.
Acknowledgements This work was supported by the Çukurova University Scientific Research Projects Coordination Unit (Project No: GSF2014BAP1). The author gratefully acknowledges the Çukurova University Scientific Research Projects Coordination Unit and the contri-bution of authorities and staffs of Eti Bakır Kastamonu Küre Plant for providing copper slag and pyrite ash.
References
1. Eliche-Quesada, D., Corpas-Iglesias, F.A., Pérez-Villarejo, L., Iglesias-Godino, F.J.: Recycling of sawdust, spent earth from oil Fig. 7 Representative SEM-BSE image of G6 glaze layer with corresponding EDS spectrum from full area (500×)
Fig. 6 Representative SEM-BSE image of G5 glaze layer with corresponding EDS spectrum from full area (1000×)
filtration, compost and marble residues for brick manufacturing. Const and Build Mat. 34, 275–284 (2012)
2. Dal Bó, M., Bernardin, A.M., Hotza, D.: Formulation of ceramic engobes with recycled glass using mixture design. J Clean Prod. 69, 243–249 (2014)
3. Nandi, V.S., Raupp-Pereira, F., Montedo, O.R.K., Oliveira, A.P.N.: The use of ceramic sludge and recycled glass to obtain engobes for manufacturing ceramic tiles. J Clean Prod. 86, 461–470 (2015) 4. Da Silva, R.C., Pianaro, S.A., Tebcherani, S.M.: Preparation and
characterization of glazes from combinations of different industrial wastes. Ceram Int. 38-4, 2725–2731 (2012)
5. Schabbach, L.M., Bolelli, G., Andreola, F., Lancellotti, I., Barbieri, L.: Valorization of MSWI bottom ash through ceramic glazing pro-cess: a new technology. J Clean Prod. 23(1), 147–157 (2012) 6. Karasu, B., Çakı, M., Yeşilbaş, Y.G.: The effect of albite wastes on
glaze properties and microstructure of soft porcelain zinc crystal glazes. J Eur Ceram Soc. 21(8), 1131–1138 (2001)
7. Pekkan, K., Karasu, B.: Evaluation of Borax solid wastes in pro-duction of frits suitable for fast single-fired wall tile opaque glass-ceramic glazes. Bull Mater Sci. 33(2), 135–144 (2012)
8. Yalçın, N., Sevinç, V.: Utilization of bauxite waste in ceramic glazes. Ceram Int. 26(I5), 485–493 (2000)
9. Pereira, O.C., Bernardin, A.M.: Ceramic colorant from untreated iron ore residue. J Hazard Mater. 233–234, 103–111 (2012) 10. Hajjaji, W., Costa, G., Zanelli, C., Ribeiro, M.J., Seabra, M.P.,
Dondi, M., Labrincha, J.A.: An overview of using solid wastes for pigment industry. J Eur Ceram Soc. 32- 4, 753–764 (2012) 11. Riella, G., Bernardin, A.M.: Inorganic pigment made from the
recycling of coal mine drainage treatment sludge. J Environ Manag. 88-4, 1280–1284 (2008)
12. Costa, G., Ribeiro, M.J., Labrincha, J.A., Dondi, M., Matteucci, F., Cruciani, G.: (a) Malayaite ceramic pigments prepared with galvan-ic sludge as coloring agent. Dyes Pigments. 78, 157–164 (2008) 13. Costa, G., Della, V.P., Ribeiro, M.J., Oliveira, A.P.N., Monrŏs, G.,
Labrincha, J.A.: (b) Synthesis of black ceramic pigments from sec-ondary raw materials. Dyes Pigments. 77, 137–144 (2008) 14. Legodi, M.A., de Waal, D.: The preparation of magnetite, goethite,
hematite and maghemite of pigment quality from mill scale iron waste. Dyes Pigments 74 (2006)
15. Prim, S.R., Folgueras, M.V., de Lima, M.A., Hotza, D.: Synthesis and characterization of hematite pigment obtained from a steel waste industry. J Hazard Mater. 192, 1307–1313 (2011)
16. Erol, M., Genç, A., Öveçoğlu, M.L., Yücelen, E., Küçükbayrak, S., Taptık, Y.: Characterization of a glass-ceramic produced from ther-mal power plant fly ashes. J Eur Ceram Soc. 20, 2209–2214 (2000)
17. Appendino, P., Ferraris, M., Matekovits, I., Salvo, M.: Production of glass-ceramic bodies from the bottom ashes of municipal solid waste incinerators. J Eur Ceram Soc. 24, 803–810 (2004) 18. Boccaccini, A.R., Bucker, M., Bossert, J.: Glass and glass-ceramics
from coal fly-ash and waste glass. Tile & Brick Int. 12, 515–518 (1996)
19. Raut, S.P., Ralegaonkar, R.V., Mandavgane, S.A.: Development of sustainable construction material using industrial and agricultural solid waste: a review of waste-create bricks. Const. and Build. Mat. 25, 4037–4042 (2011)
20. Pérez-Villarejo, L., Martínez-Martínez, S., Carrasco-Hurtado, B., Eliche-Quesada, D., Ureña-Nieto, P.J., Sánchez-Soto, C.: Valorization and inertization of galvanic sludge waste in clay bricks. Appl Clay Sci. 105–106, 89–99 (2015)
21. Monteiro, S.N, Vieira, C.M.F: On the production of fired clay bricks from waste materials: a critical update. Const. and Build. Mat. 68, 599–610 (2014)
22. Muñoz Velasco, P., Morales Ortíz, M.P., Mendívil Giró, M.A., Muñoz Velasco, L.: Fired clay bricks manufactured by adding wastes as sustainable construction material—a review. Const Build Mat. 63, 97–107 (2014)
23. Hu, H., Deng, Q., Li, C., Xie, Y., Dong, Z., Zhang, W.: The recov-ery of Zn and Pb and the manufacture of lightweight bricks from zinc smelting slag and clay. J Hazard Mater. 271, 220–227 (2014) 24. Dondi, M., Guarini, G., Raimondo, M., Zanelli, C.: Recycling PC
and TV waste glass in clay bricks and roof tiles. Waste Manag. 29, 1945–1951 (2009)
25. Torres, P., Fernandes, H.R., Olhero, S., Ferreira, J.M.F.: Incorporation of wastes from granite rock cutting and polishing industries to produce roof tile. J Eur Ceram Soc. 29, 23–30 (2009) 26. Monteiro, S.N., Peçanha, L.A., Vieira, C.M.F.: Reformulation of roofing tiles body with addition of granite waste from sawing op-erations. J Eur Ceram Soc. 24, 2349–2356 (2004)
27. Bayer Öztürk, Z., Eren Gültekin, E.: Preparation of ceramic wall tiling derived from blast furnace slag. Ceram Int. 41, 12020–12026 (2015)
28. Olgun, A., Erdoğan, Y., Ayhan, Y., Zeybek, B.: Development of ceramic tiles from coal fly ash and tincal ore waste. Ceram Int. 31, 153–158 (2005)
29. Souza, A.J., Pinheiro, B.C.A., Holanda, J.N.F.: Recycling of gneiss rock waste in the manufacture of vitrified floor tiles. J Environ Manag. 91(I3), 685–689 (2010)
30. Baruzzo, D., Minichelli, D., Bruckner, S., Fedrizzi, L., Bachiorrini, A., Maschio, S.: Possible production of ceramic tiles from marine dredging spoils alone and mixed with other waste materials. J Hazard Mat. 134(1–3), 202–210 (2006)