Bugrahan Emsen, Ali Aslan1, Hasan Turkez2,3, Ali Taghizadehghaleh joughi4, Abdullah Kaya Department of Biology, Kamil Özdağ Faculty of Science, Karamanoğlu Mehmetbey University, Karaman, 1Department of Biology Education, Kazim Karabekir Faculty of Education, Atatürk University, 4Department of Medical Pharmacology, Medical Faculty, Atatürk University, 2Department of Molecular Biology and Genetics, Faculty of Science, Erzurum Technical University, Erzurum, Turkey, 3Department of Pharmacy, University “G. d’Annunzio” Chieti‑Pescara, Chieti, Italy For correspondence: Dr. Bugrahan Emsen, Department of Biology, Kamil Özdağ Faculty of Science, Karamanoğlu Mehmetbey University, 70100 Karaman, Turkey. E‑mail: bugrahanemsen@ gmail.com
The anti‑cancer efficacies of diffractaic,
lobaric, and usnic acid: In vitro inhibition
of glioma
ABSTRACT
Aims: Glioblastoma multiforme (GBM) shows the most aggressive invasion among primary brain tumors. In spite of the standard therapy methods such as surgery, radiotherapy, and chemotherapy, the mortalities are high in GBM patients owing to side effects. Some lichen secondary metabolites that have many bioactive functions exhibited anti‑cancer efficacy toward many cancer types. The present study was undertaken to investigate proliferation change, oxidative status and DNA damage potentials of human U87MG‑GBM, and primary rat cerebral cortex (PRCC) cells exposed to three lichen secondary metabolites.
Materials and Methods: Different concentrations of lichen secondary metabolites including diffractaic acid (DA), lobaric acid (LA), and (+)‑usnic acid (UA) were used for the treatments. PRCC cells were obtained from Sprague Dawley®rats. U87MG cell line was preferred as GBM cells.
Results: The results showed that lactate dehydrogenase and 8‑hydroxy‑2’‑deoxyguanosine levels increased in PRCC and U87MG cells in a clear dose‑dependent manner. Inhibitory concentration 50% (IC50) values of LA, DA, and UA were calculated as 9.08, 122.26, 132.69 mg/L for PRCC cells and 5.77, 35.67, 41.55 mg/L for U87MG cells, respectively. Concentration of 10 mg/L of DA and UA demonstrated high anti‑oxidant capacity on healthy PRCC cells.
Conclusions: Overall, obtained data indicated that LA was highly toxic on GBM and PRCC cells. However, DA and then UA had high anti‑oxidant capacity on PRCC cells. These results suggest that further studies that will be held on LA may play a critical role in GBM treatment.
KEY WORDS: Cytotoxicity, genotoxicity, glioblastoma multiforme, lichen, oxidative status, secondary metabolite
INTRODUCTION
Brain cancer, the most common solid tumor of childhood, occurs in the central nervous system and can be seen in every age starting from postpartum period. Brain tumors represent different physiological and morphological characteristics according to each age period. In early ages, tumors mostly settled in the rear cranial fossa are medulloblastomas, ependymomas, astrocytomas, and brainstem gliomas. Type of tumor usually seen in old ages is derived from brain tissue. The most common and most dangerous type of brain cancer in adults is glioblastoma multiforme (GBM) briefly known as glioblastoma.[1,2]
GBM that represented of 15% of all brain tumors is located within the fourth degree of the most dangerous and aggressive tumors in cancer classification established by the World Health
Organization. It was reported that most of GBM patients lost their lives in the 1st year and a half.[1]
There are two types of GBM including primary and secondary. Primary GBM is the most common
and aggressive one and composes 60% of GBM.[3]
Some researchers have identified abnormalities in different chromosomal genes involved in tumorigenesis and suggested that primary brain tumor formation caused by these abnormalities.[4,5]
Unfortunately, a definitive solution to GBM could not be generated for years. Improved treatment methods are inadequate. It was determined that healthy cells damaged as well as cancer
cells showing abnormal proliferation due to Access this article online Website: www.cancerjournal.net DOI: 10.4103/0973-1482.177218
PMID: ***
Quick Response Code:
Cite this article as: Emsen B, Aslan A, Turkez H, Taghizadehghalehjoughi AT, Kaya A. The anti-cancer efficacies of diffractaic,
lobaric, and usnic acid: In vitro inhibition of glioma. J Can Res Ther 2018;14:941-51.
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution‑NonCommercial‑ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non‑commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms. For reprints contact: reprints@medknow.com
chemotherapy, one of the most preferred treatment methods;
therefore, body defense system damages.[6‑8] Radiotherapy,
another method, has also side effect at nonignorable level.[9,10]
In recent years, in addition to chemotherapy and radiotherapy methods, it was aimed to create alternative treatment methods using plant‑based products. A high rate of positive results has been achieved through combined treatment methods. Metabolites in plants ensure that the plants have therapeutic properties.[11‑17]
For many years, it has been benefitted from lichens possessing unique secondary metabolites for many biological activities. The lichens have very high rate of secondary metabolite, and so, they show many biological characters.[18,19] Studies conducted
in previous years showed that lichens had important activities such as anti‑viral,[20] anti‑oxidant,[21‑23] anti‑bacterial,[21,24,25] and
anti‑mutagenic activities.[26‑29] In addition, anti‑cancer activities
of the lichens were reflected in many research results. It was detected that the lichen metabolites had cytotoxic effect against some cancer types such as human melanoma and colon carcinoma,[21,30] breast and lung carcinoma.[22,31,32] However, to
the best of our knowledge, there is no information regarding the anti‑proliferative, genotoxic, and oxidative effects of three lichen secondary metabolites including diffractaic acid (DA), lobaric acid (LA), and (+)‑usnic acid (UA) on primary rat cerebral cortex (PRCC) and U87MG cells in the relevant literature. Hence, this work was set out in order to establish anti‑proliferative (by 3‑[4,5‑dimethylthiazol‑2‑yl]‑2,5‑diphenyltetrazolium bromide [MTT] and lactate dehydrogenase [LDH] assay), oxidative (by measuring total anti‑oxidant capacity [TAC], and total oxidant status [TOS] levels), and genotoxic (by measuring 8‑hydroxy‑2’‑deoxyguanosine [8‑OH‑dG] level) effects of DA,
LA, and UA on PRCC and U87MG‑GBM cells for the 1st time.
MATERIALS AND METHODS
Test compounds
DA (Cas: 436‑32‑8, C20H22O7), LA (Cas: 522‑53‑2, C25H28O8)
and UA (Cas: 125‑46‑2, C18H16O7) were purchased from
Gaia Chemical (New Milford, CT, USA) [Figure 1]. The
metabolites were diluted to different concentrations (2.5, 5, 10, 20, and 40 mg/L) before the experimental setup. Dimethyl sulfoxide (DMSO) + relevant cell culture medium (2% DMSO) was used as negative control (control−).
Neuron cell cultures
This study organized at the Medical Experimental Research Center was approved by the Ethical Committee.
Six newborn Sprague Dawley® rats were used obtaining
PRCC cultures. The cerebral cortices were dissociated with Hank’s Balanced Salt Solution (Sigma‑Aldrich, Germany) + trypsin‑ethylenediaminetetraacetic acid (EDTA) (0.25% trypsin, 0.02% EDTA; Sigma‑Aldrich), treated with DNAse type 1 (Sigma‑Aldrich) and centrifuged. After having thrown away the supernatant, fresh medium containing neurobasal (Gibco, Germany) 10% fetal bovine serum (FBS) (Sigma‑Aldrich), 2% B‑27 (Gibco), and 0.1% penicillin‑streptomycin (PAN Biotech, Germany) were added to the residue. Neurons were eventually seeded in 96 well plates, and they were incubated at 37°C in 5% CO2. In this way, each well contained 150 μL medium and 1 × 105 cells.
U87MG‑glioblastoma multiforme cell cultures
We utilized human GBM U87MG cell line used widely as a model for brain cancer. Cells were harvested with 0.25% trypsin‑EDTA and suspended with Roswell Park Memorial Institute 1640 medium (Sigma‑Aldrich) containing 15% FBS, 1% L‑glutamine (Sigma‑Aldrich), and 1% penicillin‑streptomycin. Cells were seeded in 25 mL flasks. After reaching to proper volume, they were seeded in 96 well plates. Thus, each well contained 100 μL medium with 1 × 105 cells.
3‑(4,5‑dimethylthiazol‑2‑yl)‑2,5‑diphenyltetrazolium bromide assay
The cells seeded in 96‑well plates were incubated at 37°C in
a humidified 5% CO2/95% air mixture and exposed to lichen
compounds at different concentrations (2.5, 5, 10, 20, and 40 mg/L) for 48 h. MTT assay was performed by commercially available kit (Cayman Chemical Company, MI, USA). In this assay, MTT moves into the cell owing to owned net positive charge and plasma membrane potential, and it is reduced to a purple formazan intracellular by NAD (P) H oxidoreductases in the cell.[33]
After MTT reagent (10 μL) was added to the cell cultures,
the plate was incubated in CO2 incubator at 37°C for 4 h,
and it was centrifuged at 400 ×g for 10 min. Crystalline solvent solution (100 μL) was added to each well. Crystals of formazan were dissolved with this solution. The intensity of the formazan was measured at 570 nm wavelengths with Multiscan Go microplate reader (Thermo Scientific, USA).
Lactate dehydrogenase release assay
Commercially available kit (Cayman Chemical Company, USA) was used for LDH assay performed in the culture medium. Figure 1: The chemical structures of lichen secondary metabolites
(a) diffractaic acid, (b) lobaric acid, (c) (+)-usnic acid
c
b a
LDH is an enzyme that released to the cell culture medium as a result of rapid cell damage occurred during apoptosis or necrosis events. In kit assay, first, LDH catalyzes the reduction of NAD+ to NADH and H+ by oxidation of lactate to pyruvate.
Second, diaphorase uses the newly formed NADH and H+ to
catalyze the reduction of a tetrazolium salt to highly colored formazan. Formazan amount generated is proportional to the amount of LDH released into the culture medium by the reason of cytotoxicity. LDH activity in the supernatant of culture increases via rising of dead cells.[34,35]
LDH standard (100 μL) was added relevant wells and medium (100 μL) on cells incubated for 48 h was added to other wells. LDH reaction solution (100 μL) was added to each well. The plate was slightly incubated for 30 min via orbital shaker (Labnet, USA) at room temperature. Spectrophotometric reading was performed at 490 nm wavelengths. The positive control was mitomycin‑C chemotherapeutic agent for LDH assays.
Total anti‑oxidant capacity assay
Commercially available kit (Rel Assay Diagnostics, Gaziantep, Turkey) was used for TAC assay on PRCC and U87MG‑GBM cell cultures for 48 h. The aim of kit assay is to reveal anti‑oxidant levels of samples by inhibiting the formation of a free radical, 2,2′‑azino‑bis (3‑ethylbenzothiazoline‑6‑sulfonic acid) compound. Kit assay is calibrated with a stable anti‑oxidant of Vitamin E analog known as Trolox equivalent.
Cells incubated for 48 h were ejected from the incubator. Medium on the precipitated cells was added to relevant wells. Standard solutions in the kit were added to relevant wells. Reagent 1 solution was added to each well. First, spectrophotometric reading was performed at 660 nm wavelengths. After the first reading, reagent 2 was added to each well, and the plate was incubated at room temperature for 10 min. Second, spectrophotometric reading was performed at 660 nm wavelengths. Positive control was ascorbic acid from organic anti‑oxidant compounds for TAC assays.
Total oxidant status assay
Commercially available kit (Rel Assay Diagnostics, Gaziantep, Turkey) was used for TOS assay on PRCC and U87MG‑GBM cell cultures for 48 h. In kit assay, complexes with ferric ion are oxidized to ferrous ion by oxidants presented in the sample. The oxidation reaction is performed by strengthening molecules in the reaction medium. Ferrous ions form a colored structure with chromogen in the acidic environment. The color intensity measured spectrophotometrically is related to the total amount of oxidant molecules in the sample. Kit assay is calibrated with hydrogen peroxide (H2O2).
Cells incubated for 48 h were ejected from the incubator. Medium on the precipitated cells was added to relevant wells. Standard solutions in the kit were added to relevant wells. Reagent 1 solution was added to each well. First,
spectrophotometric reading was performed at 530 nm wavelengths. After the first reading, reagent 2 was added to each well, and the plate was incubated at room temperature for 10 min. Second, spectrophotometric reading was performed at 530 nm wavelengths. The positive control was H2O2, reactive oxygen species for TOS assays.
Oxidative DNA damage assay
Commercially available DNA/RNA Oxidative Damage kit (Cayman Chemical Company, USA) was used for oxidative DNA damage assay in the culture medium. The aim of this assay is to measure the oxidative DNA damage in the cells via calculation of 8‑OH‑dG level. 8‑OH‑dG is the form
of oxidized guanine.[36] Experimental stages were carried
out considering the kit procedure. Positive control was mitomycin‑C chemotherapeutic agent for oxidative DNA damage assays.[37]
Statistical analyses
All the experiments were run in triplicate. Various activities of the metabolites were evaluated with analysis of variance followed by appropriate post hoc test (Duncan test), and the differences were accepted as statistically significant at
P < 0.05. Inhibitory concentration 50% (IC50) values were calculated with probit regression analysis and associated 95% confidence limits for each treatment. Relations among the variables were tested by bivariate correlation analysis. Statistical Package for Social Sciences (SPSS, Version 21.0, IBM Corporation, Armonk, NY, USA) software was used for the calculations.
RESULTS
Anti‑proliferative activities
Cell viabilities of PRCC and U87MG cells exposed to different concentrations of DA, LA, and UA metabolites were determined by MTT analysis. The results showed that solutions with the highest concentration (40 mg/L) highly inhibited cell proliferation. LA was the most potent cytotoxic agent for PRCC and U87MG cells. While cell viability decreased to 35.09% in PRCC cells exposed to a maximum concentration of LA, this rate was 30.47% in U87MG cells [Figure 2]. According to IC50 values, secondary metabolites were in the ascending order of LA < DA < UA for PRCC and U87MG cells. It was determined that IC50 values calculated for both cell types were statistically (P < 0.05) different from each other [Tables 1 and 2].
Table 1: Inhibitory concentration 50% values for primary rat cerebral cortex cells exposed to different lichen secondary metabolites (mg/L)
Metabolite IC50 (limits) Slope±SE (limits) χ2
Diffractaic acid 122.26b (80.65-225.20) 0.94±0.10 (0.74-1.13) 11.71 Lobaric acid 9.08a (7.03-11.60) 0.61±0.08 (0.46-0.76) 1.54 Usnic acid 132.69c (57.97-902.79) 0.37±0.08 (0.21-0.52) 2.42
Values followed by different superscript letters in the same column differ significantly at P<0.05. IC50=Inhibitory concentration 50%, SE=Standard error
In addition, in order to determine cytotoxic effects of DA, LA, and UA on PRCC and U87MG cells, LDH release test was also used. The present study revealed that metabolite solutions with the highest concentration caused maximum LDH release. The maximum concentration of LDH in PRCC and U87MG cell mediums was detected at treatment
with mitomycin‑C (positive control) (100.32 and 94.95 μU/mL, respectively). In metabolite treatments, for PRCC and U87MG cells, the closest values to LDH activity of mitomycin‑C belonged to a solution with a concentration of 40 mg/L of LA (90.00 and 89.77 μU/mL, respectively). LDH activities of solutions with a concentration of 2.5 and 5 mg/L of DA were not statistically (P > 0.05) different from activities of control− having minimum LDH activity for PRCC
cells [Figure 3].
Anti‑oxidative activities
TAC analysis was carried out to determine TACs of different concentrations of DA, LA, and UA metabolites on PRCC and U87MG cells. It was showed that positive control, ascorbic Table 2: Inhibitory concentration 50% values for U87MG cells
exposed to different lichen secondary metabolites (mg/L)
Metabolite IC50 (limits) Slope±SE (limits) χ2
Diffractaic acid 35.67b (31.01-42.15) 1.66±0.11 (1.46-1.87) 13.25 Lobaric acid 5.77a (4.27-7.34) 0.64±0.08 (0.49-0.80) 3.18 Usnic acid 41.55c (25.42-105.55) 0.41±0.08 (0.26-0.56) 7.32
Values followed by different superscript letters in the same column differ significantly at P<0.05. SE=Standard error, IC50=Inhibitory concentration 50%
Figure 3: Lactate dehydrogenase release level in cells exposed to different lichen secondary metabolites (a) for primary rat cerebral cortex cells, (b) for U87MG cells. Each value is expressed as mean ± standard deviation (n = 3). Values followed by different small letters differ significantly
at P < 0.05
b a
Figure 2: Viability rate in cells exposed to different lichen secondary metabolites (a) for primary rat cerebral cortex cells, (b) for U87MG cells.
Each value is expressed as mean ± standard deviation (n = 3)
b a
acid, had the highest TAC on both cells. When the TAC of metabolites was investigated on PRCC cells, it was determined that solutions with a concentration of 40 mg/L of DA and UA showed greater TAC (37.74 and 37.34 mmol Trolox equivalent/L, respectively) in comparison with other solutions. Furthermore, there was no statistically (P > 0.05) significant difference between TAC levels of concentrations of 10, 20, and 40 mg/L of LA and TAC level possessed by control−. In addition,
it was demonstrated that LA metabolite had the lowest TAC on PRCC cells [Figure 4].
As for TAC activities of metabolites on U87MG cells, it was observed that TAC values for all metabolite concentrations were statistically different (P < 0.05) from control− and ascorbic
acid. Concentrations of 5 mg/L of DA and 2.5 mg/L of UA had the highest TAC (8.25 and 8.49 mmol Trolox equivalent/L, respectively) among all metabolite treatments. The lowest TAC values belonged to concentrations of 2.5 mg/mL of DA (4.97 mmol Trolox equivalent/L), 40 mg/mL of LA (4.96 mmol Trolox equivalent/L), and 5 mg/mL of UA (4.97 mmol Trolox equivalent/L), and these values were not statistically different (P > 0.05) from each other [Figure 4].
Pro‑oxidative activities
TOS analysis was carried out to determine TOS of different concentrations of DA, LA, and UA metabolites on PRCC and U87MG cells. Based on TOS levels on PRCC cells, maximum concentrations of tested metabolites showed the highest TOS activity. Among treatment groups, positive control, H2O2 and then solution at a concentration of 40 mg/L of LA caused a high degree of oxidative stress. Among the tested metabolites, TOS
activity of UA only increased in a concentration‑dependent manner [Figure 5].
TOS data revealed for U87MG cells indicated that the DA and LA caused high TOS activity (68.09 and 70.79 μmol H2O2 equivalent/L, respectively) after (79.76 μmol H2O2
equivalent/L). TOS levels of concentrations of 20 mg/L
of LA were also had high TOS activity (66.98 μmol H2O2
equivalent/L), and there was no statistically (P > 0.05) difference between this value and abovementioned two other high values except for H2O2. On the other hand, it was exhibited that all concentrations of UA were not created highly oxidative stress on U87MG cells. Performed statistical analyses were indicated that TOS activities of DA, LA, and UA on both cell types were significant at the 0.05 level as compared with control treatments [Figure 5].
Genotoxicity activities
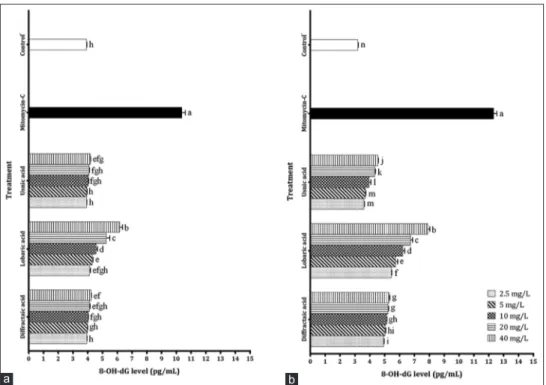
In order to determine levels of oxidative DNA damage of different concentrations of DA, LA, and UA metabolites on PRCC and U87MG cells, 8‑OH‑dG levels occurring in the cells were assessed. 8‑OH‑dG activities showed high positive correlation with concentration for both cells. Based on 8‑OH‑dG levels on PRCC cells, it was revealed that concentrations of 10, 20, and 40 mg/L of LA created highly DNA damage (4.57, 5.25, and 6.16 pg/mL, respectively) compared to other metabolite treatments. It was demonstrated that the values of aforementioned three treatments were statistically (P < 0.05) different from all other treatment values [Figure 6].
Figure 4: Total antioxidant capacity levels of different lichen secondary metabolites on cells (a) for primary rat cerebral cortex cells, (b) for U87MG cells. Each value is expressed as mean ± standard deviation (n = 3). Values followed by different small letters differ significantly at P < 0.05
b a
When levels of oxidative DNA damage occurred by tested metabolites on U87MG cells were examined, it was showed that all concentrations of LA had higher 8‑OH‑dG levels than other metabolite concentrations. Concentration of 40 mg/L of LA showed the closest value to DNA damage level of positive control, mitomycin‑C. UA exhibited the lowest 8‑OH‑dG levels after control−. Difference between the 8‑OH‑dG levels of all
metabolite concentrations and control− was significant at
0.05 the level [Figure 6]. DISCUSSION
Recently, there have been a lot of studies that deal with the
treatment of GBM by using herbal products.[38‑43] However,
Figure 6: 8-hydroxy-2’-deoxyguanosine level in cells exposed to different lichen secondary metabolites (a) for primary rat cerebral cortex cells, (b) for U87MG cells. Each value is expressed as mean ± standard deviation (n = 3). Values followed by different small letters differ significantly
at P < 0.05
b a
Figure 5: Total oxidant status levels of different lichen secondary metabolites on cells (a) for primary rat cerebral cortex cells, (b) for U87MG cells. Each value is expressed as mean ± standard deviation (n = 3). Values followed by different small letters differ significantly at P < 0.05
b a
comparative anti‑proliferative, oxidative, and genotoxic effects of lichen secondary metabolites on cancerous and healthy brain cells have not been studied uniformly. This study demonstrated that DA, UA, and especially LA from lichen secondary metabolites inhibited proliferation of U87MG as cancerous brain cell and PRCC cells as a healthy brain cell. Furthermore, it was detected that inhibition property was oxidative stress and genotoxic‑induced.
Performed binary correlation analyses showed that all metabolite treatments decreased viable cell numbers and
increased LDH and 8‑OH‑dG level in a concentration‑dependent manner in both PRCC and U87MG cells [Tables 3‑8]. Moreover,
calculated IC50 values gave insight about proliferation
inhibitory concentrations on cells [Tables 1 and 2].
While DA metabolite had high IC50 value (122.26 mg/L) on PRCC cells, low IC50 value (35.67 mg/L) on U87MG cells revealed that this metabolite was more toxic on cancer cells [Tables 1 and 2]. These results showed that when DA was used at certain concentrations, it could show toxic effect on cancer cells without damaging the healthy cells. When investigated LDH Table 3: Correlation between different variables for primary rat cerebral cortex cells exposed to diffractaic acid
Cell viability Concentration LDH activity Oxidative DNA damage TAC TOS
Cell viability 1.00a −0.95** −0.91** −0.93** 0.08 −0.88**
Concentration −0.95** 1.00 0.96** 0.97** −0.23 0.96**
LDH activity −0.91** 0.96** 1.00 0.97** −0.15 0.91**
Oxidative DNA damage −0.93** 0.97** 0.97** 1.00 −0.14 0.94**
TAC 0.08 −0.23 −0.15 −0.14 1.00 −0.26
TOS −0.88** 0.96** 0.91** 0.94** −0.26 1.00
aPearson’s correlation coefficient, **Correlation is significant at the 0.01 level. TAC=Total antioxidant capacity, LDH=Lactate dehydrogenase, TOS=Total oxidant status
Table 4: Correlation between different variables for U87MG cells exposed to diffractaic acid
Cell viability Concentration LDH activity Oxidative DNA damage TAC TOS
Cell viability 1.00a −0.99** −0.96** −0.89** 0.27 −0.95**
Concentration −0.99** 1.00 0.96** 0.88** −0.25 0.96**
LDH activity −0.96** 0.96** 1.00 0.94** −0.11 0.93**
Oxidative DNA damage −0.89** 0.88** 0.94** 1.00 −0.15 0.85**
TAC 0.27 −0.25 −0.11 −0.15 1.00 −0.34
TOS −0.95** 0.96** 0.93** 0.85** −0.34 1.00
aPearson’s correlation coefficient, **Correlation is significant at the 0.01 level. TAC=Total antioxidant capacity, LDH=Lactate dehydrogenase, TOS=Total oxidant status
Table 5: Correlation between different variables for primary rat cerebral cortex cells exposed to lobaric acid
Cell viability Concentration LDH activity Oxidative DNA damage TAC TOS
Cell viability 1.00a −0.92** −0.94** −0.94** 0.95** −0.91**
Concentration −0.92** 1.00 0.97** 0.98** −0.90** 0.85**
LDH activity −0.94** 0.97** 1.00 0.98** −0.94** 0.81**
Oxidative DNA damage −0.94** 0.98** 0.98** 1.00 −0.91** 0.86**
TAC 0.95** −0.90** −0.94** −0.91** 1.00 −0.82**
TOS −0.91** 0.85** 0.81** 0.86** −0.82** 1.00
aPearson’s correlation coefficient, **Correlation is significant at the 0.01 level. TAC=Total antioxidant capacity, LDH=Lactate dehydrogenase, TOS=Total oxidant status
Table 6: Correlation between different variables for U87MG cells exposed to lobaric acid
Cell viability Concentration LDH activity Oxidative DNA damage TAC TOS
Cell viability 1.00a −0.90** −0.96** −0.93** 0.79** −0.84**
Concentration −0.90** 1.00 0.98** 0.99** −0.79** 0.79**
LDH activity −0.96** 0.98** 1.00 0.99** −0.85** 0.85**
Oxidative DNA damage −0.93** 0.99** 0.99** 1.00 −0.82** 0.82**
TAC 0.79** −0.79** −0.85** −0.82** 1.00 −0.88**
TOS −0.84** 0.79** 0.85** 0.82** −0.88** 1.00
aPearson’s correlation coefficient, **Correlation is significant at the 0.01 level. TAC=Total antioxidant capacity, LDH=Lactate dehydrogenase, TOS=Total oxidant status
Table 7: Correlation between different variables for primary rat cerebral cortex cells exposed to usnic acid
Cell viability Concentration LDH activity Oxidative DNA damage TAC TOS
Cell viability 1.00a −0.93** −0.93** −0.91** −0.44 −0.89**
Concentration −0.93** 1.00 0.87** 0.94** 0.23 0.95**
LDH activity −0.93** 0.87** 1.00 0.93** 0.62* 0.90**
Oxidative DNA damage −0.91** 0.94** 0.93** 1.00 0.45 0.94**
TAC −0.44 0.23 0.62* 0.45 1.00 0.29
TOS −0.89** 0.95** 0.90** 0.94** 0.29 1.00
aPearson’s correlation coefficient, *Correlation is significant at the 0.05 level; **Correlation is significant at the 0.01 level. TAC=Total antioxidant capacity,
and 8‑OH‑dG activities of DA treatments at low concentrations on PRCC cells, it was determined that the values were not statistically different from control− values. Otherwise, in the
treatments realized on U87MG cells, it was showed that all concentrations were significantly (P < 0.05) different from control− [Figures 3 and 6]. Concentrations of 20 and 40 mg/L
of DA caused highly oxidative stress on both cells [Figure 5]. Furthermore, correlation analyses revealed that there was significantly (P < 0.01) negative correlation between cell viability and TOS activity [Tables 3 and 4]. High anti‑oxidant capacity of the concentration of 10 mg/mL of DA was showed in TAC results [Figure 4]. The results occurred for DA metabolite demonstrated that low concentrations of DA were sufficient to destroy U87MG cells, and these low concentrations did not statistically show the highly toxic effect on PRCC cells. In previous studies carried out by researchers, it was indicated the presence of cytotoxic capacity or high anti‑oxidant effect of DA, depending on the concentration. Bayir et al.[44] reported
that DA had a protective role against indomethacin‑induced gastric lesions in the study performed on rats and according to them; this effect was realized by reducing of oxidative stress. Another study about the gastric protective property of DA belonged to Sepulveda et al.[45] Analgesic and anti‑pyretic effects
of DA were also reported.[46] Odabasoglu et al.[47] exhibited
that DA‑induced apoptosis and anti‑oxidant system as a pro‑apoptotic active ingredient. Similar ones to the present study results about anti‑oxidant capacity of DA treatments were reported by Karagoz et al.[48] They revealed that although
low concentrations of DA showed protective feature against stomach, liver, kidney, small and large intestine cells of healthy mice, they had high anti‑tumor activity on Ehrlich ascitic tumor cells. Cytotoxic and anti‑proliferative effects of DA on melanoma,[49] keratinocytes,[50] breast cancer, colon and cervical
cells[51] were other studies reported.
LA was metabolite showed the highest cytotoxic effect on PRCC and U87MG cells owing to the lowest and close IC50 values (9.08 and 5.77 mg/L, respectively) [Tables 1 and 2]. The cytotoxic activity of LA was caused by oxidative stress [Figure 5, Tables 5 and 6]. High LDH enzyme and 8‑OH‑dG base level released from the cells suggested about how damaged to the cells [Figures 3 and 6]. When examined the anti‑oxidant capacity of LA, highly negative correlation was determined between concentration and TAC level for the both cells [Tables 5 and 6]. Moreover, concentrations of 10, 20, and 40 mg/L of LA were not statistically (P > 0.05) showed a different TAC value from
control− [Figure 4]. The results obtained for LA revealed that
this metabolite was a highly toxic agent on PRCC and U87MG cells. Further studies on LA metabolite suggested that positive results could be obtained in GBM treatment through methods that will be performed targeting only the cancerous cells. In the past years, anti‑bacterial and cell anti‑proliferative activities performed using the toxic effect of LA are remarkable. At the same time, the presence of antioxidant capacity of LA was determined in some researches. Bhattarai et al.[52] were
reported that LA metabolite isolated from Stereocaulon alpinum had anti‑oxidant and anti‑bacterial capacities. Another study about anti‑oxidant property of LA was detected by Thadhani
et al.[53] They revealed that LA had superoxide, nitric oxide
2,2‑diphenyl‑1‑picrylhydrazyl radical scavenging activities. Studies inhibited proliferations of the cells exposed to LA were also available. In the experiment carried out on human platelets, it was exhibited that LA showed the high level of inhibitory activity.[54] In another cytotoxicity assay, it was
examined the effect of LA on erythroleukemia and breast cancer cells and was found highly cell death.[55] Anti‑fungal[56]
and anti‑mycotic[57] activity studies of LA featured toxic
properties of this metabolite once again.
Difference among the IC50 values of UA calculated for
PRCC (132.69 mg/L) and U87MG (41.55) mg/L cells was high. These results suggested that using of UA at low concentrations caused low levels of side effects in GBM treatment. The data of LDH activity experiment revealed that LDH release amount of UA on the cancer cells was greater than healthy cells [Figure 3] and extracellular LDH activity also increased in a concentration‑dependent manner on the both cells [Tables 7 and 8]. There was significantly (P > 0.05) no difference between 8‑OH‑dG level caused by concentrations of 2.5, 5, 10, and 20 mg/L of UA and control− value [Figure 6].
Positive correlations were very high between oxidative stress and concentration; oxidative stress, and 8‑OH‑dG level on PRCC cells (correlation coefficients >0.90). In the assays on PRCC cells, correlation coefficients between several binary variables (cell viability‑concentration; cell viability‑LDH activity; cell viability‑oxidative DNA damage; and cell viability‑TOS) were calculated as ≤−0.89 and thus, it was detected that there were highly negative correlations between relevant variables [Table 7]. Based on TOS activity of UA on U87MG cells, no significant correlation was found between cell viability and TOS [Table 8]. Because values of TOS level by UA metabolite on U87MG cells were floating [Figure 5]. When Table 8: Correlation between different variables for U87MG cells exposed to usnic acid
Cell viability Concentration LDH activity Oxidative DNA damage TAC TOS
Cell viability 1.00a −0.89** −0.89** −0.91** 0.38 0.17
Concentration −0.89** 1.00 0.90** 0.94** −0.28 −0.37
LDH activity −0.89** 0.90** 1.00 0.98** −0.25 0.04
Oxidative DNA damage −0.91** 0.94** 0.98** 1.00 −0.25 −0.10
TAC 0.38 −0.28 −0.25 −0.25 1.00 0.08
TOS 0.17 −0.37 0.04 −0.10 0.08 1.00
investigated TAC values of UA on PRCC cells, it was determined that TAC level increased until solution with 10 mg/L and then it was observed a decrease in TAC level with increase in concentration [Figure 4]. Since TAC level increased up to a certain concentration level for PRCC cells, moderate positive correlation at P < 0.05 level occurred between TAC and LDH activity [Table 7]. Concentration with the highest TAC level of UA on U87MG cells was 2.5 mg/L, and this value created the highest TAC level for all treatments performed on cancer cells [Figure 4]. When overall data about UA examined, it was indicated that in GBM treatment process, healthy cells will be damaged in minimum level and number of cancerous cells will decrease with using of the concentrations owned high TAC level on healthy cells.
As with other tested compounds, certain concentrations determined toxic effects of UA, are available. Researchers have determined toxic concentrations in cytotoxicity studies and achieved desired results on many cell types. Anti‑tumor activities of UA on melanoma, colon cancer,[58] leukemia, cervical cancer,[49,59]
gastric, prostate, lung cancer,[60] brain metastatic prostatic
cancer, breast cancer[61] cells were investigated by researchers.
Backorová et al.[62] proved apoptosis‑inducing activity of UA
against colon and ovarian cancer cells and thus, they displayed another activity of UA on the cancerous cells. It was observed in a study carried out on mouse hepatocytes that UA caused oxidative stress in some cells, and hence necrosis took place.[63]
Anti‑bacterial[58,64‑66] and insecticidal[67‑69] activities of UA also
evidenced the presence of toxic effect of this metabolite in other scientific fields. Many researchers practiced anti‑oxidative studies on UA detected presence of anti‑oxidant capacity in the present study.[65,70,71] Furthermore, UA possessed anti‑mutagenic,[72]
analgesic and anti‑pyretic,[46] and protective effects against
pulmonary inflammation[73] besides the anti‑oxidant capacity. All
of these data showed that UA is a very important compound for human health when used at the particular doses.
CONCLUSIONS
In summary, three lichen secondary metabolites including DA, LA, and UA were analyzed in this study and our present findings indicated that LA highly inhibited proliferation of PRCC and U87MG cells. High impact level on cancer cells as well as the side effects of LA will lead to further studies. Similarly, other tested metabolites, DA and UA also showed cytotoxic activity on the both cells. At the same time, high anti‑oxidant mechanisms of low concentrations of these two metabolites suggest that they will be able to utilize in GBM treatment process.
Financial support and sponsorship
Karamanoğlu Mehmetbey University, Scientific Research Fund Project (01‑D‑13).
Conflicts of interest
There are no conflicts of interest.
REFERENCES
1. Bruce JN, Kennedy B. Glioblastoma multiforme. eMedicine Oncology 2010;1:1‑41.
2. Ahman HA, Sedky M. Pediatric cancer. In: Tuncer M, editor. Cancer Report. Ankara: MN Medical & Nobel Publishing Company; 2010. p. 274‑8.
3. Luwor RB, Stylli SS, Kaye AH. The role of Stat3 in glioblastoma multiforme. J Clin Neurosci 2013;20:907‑11.
4. Urbanska K, Sokolowska J, Szmidt M, Sysa P. Glioblastoma multiforme – An overview. Contemp Oncol (Pozn) 2014;18:307‑12. 5. Farrell CJ, Plotkin SR. Genetic causes of brain tumors:
Neurofibromatosis, tuberous sclerosis, von Hippel‑Lindau, and other syndromes. Neurol Clin 2007;25:925‑46.
6. Perrino C, Schiattarella GG, Magliulo F, Ilardi F, Carotenuto G, Gargiulo G, et al. Cardiac side effects of chemotherapy: State of art and strategies for a correct management. Curr Vasc Pharmacol 2014;12:106‑16.
7. Chan HK, Ismail S. Side effects of chemotherapy among cancer patients in a Malaysian General Hospital: Experiences, perceptions and informational needs from clinical pharmacists. Asian Pac J Cancer Prev 2014;15:5305‑9.
8. Mavrogenis AF, Papagelopoulos PJ, Romantini M, Angelini A, Ruggieri P. Side effects of chemotherapy in musculoskeletal oncology. J Long Term Eff Med Implants 2010;20:1‑12.
9. Pettersson A, Turesson I, Persson C, Johansson B. Assessing patients’ perceived bother from the gastrointestinal side effects of radiotherapy for localized prostate cancer: Initial questionnaire development and validation. Acta Oncol 2014;53:368‑77.
10. Verrone JR, Alves Fde A, Prado JD, Boccaletti KW, Sereno MP, Silva ML,
et al. Impact of intraoral stent on the side effects of radiotherapy for
oral cancer. Head Neck 2013;35:E213‑7.
11. Shen T, Wan W, Yuan H, Kong F, Guo H, Fan P, et al. Secondary metabolites from Commiphora opobalsamum and their antiproliferative effect on human prostate cancer cells. Phytochemistry 2007;68:1331‑7. 12. Russo A, Cardile V, Piovano M, Caggia S, Espinoza CL,
Garbarino JA. Pro‑apoptotic activity of ergosterol peroxide and (22E)‑ergosta‑7,22‑dien‑5alpha‑hydroxy‑3,6‑dione in human prostate cancer cells. Chem Biol Interact 2010;184:352‑8.
13. Efferth T, Herrmann F, Tahrani A, Wink M. Cytotoxic activity of secondary metabolites derived from Artemisia annua L. towards cancer cells in comparison to its designated active constituent artemisinin. Phytomedicine 2011;18:959‑69.
14. Turkez H, Togar B, Di Stefano A, Taspinar N, Sozio P. Protective effects of cyclosativene on H2O 2‑induced injury in cultured rat primary cerebral cortex cells. Cytotechnology 2015;67:299‑309.
15. Türkez H, Celik K, Togar B. Effects of copaene, a tricyclic sesquiterpene, on human lymphocytes cells in vitro. Cytotechnology 2014;66:597‑603. 16. Turkez H, Aydin E, Aslan A. Xanthoria elegans (Link) (lichen) extract
counteracts DNA damage and oxidative stress of mitomycin C in human lymphocytes. Cytotechnology 2012;64:679‑86.
17. Sadi G, Emsen B, Kaya A, Kocabas A, Çinar S, Kartal DI. Cytotoxicity of some edible mushrooms extracts over liver hepatocellular carcinoma cells in conjunction with their antioxidant and antibacterial properties. Pharmacogn Mag 2015;11 Suppl 1:S6‑18.
18. Brodo IM, Sharnoff SD, Sharnoff S. About the lichens. In: Lichens of North America. New Haven & London: Yale University Press; 2001. p. 3‑113. 19. Boustie J, Grube M. Lichens – A promising source of bioactive
secondary metabolites. Plant Genet Resour 2005;3:273‑87. 20. Karagöz A, Aslan A. Antiviral and cytotoxic activity of some lichen
extracts. Biologia 2005;60:281‑6.
21. Grujicic D, Stošic I, Kosanic M, Stanojkovic T, Rankovic B, Miloševic‑Djordjevic O. Evaluation of in vitro antioxidant, antimicrobial, genotoxic and anticancer activities of lichen Cetraria
22. Ghate NB, Chaudhuri D, Sarkar R, Sajem AL, Panja S, Rout J, et al. An antioxidant extract of tropical lichen, Parmotrema reticulatum, induces cell cycle arrest and apoptosis in breast carcinoma cell line MCF‑7. PLoS One 2013;8:e82293.
23. Paudel B, Datta Bhattarai H, Prasad Pandey D, Seoun Hur J, Gyu Hong S, Kim IC, et al. Antioxidant, antibacterial activity and brine shrimp toxicity test of some mountainous lichens from Nepal. Biol Res 2012;45:387‑91.
24. Kosanic M, Rankovic B. Antioxidant and antimicrobial properties of some lichens and their constituents. J Med Food 2011;14:1624‑30. 25. Kosanic M, Ranković B, Stanojković T, Rančić A, Manojlović N.
Cladonia lichens and their major metabolites as possible natural
antioxidant, antimicrobial and anticancer agents. LWT Food Sci Technol 2014;59:518‑25.
26. Agar G, Gulluce M, Aslan A, Bozari S, Karadayi M, Orhan F. Mutation preventive and antigenotoxic potential of methanol extracts of two natural lichen. J Med Plants Res 2010;4:2132‑7.
27. Kotan E, Alpsoy L, Anar M, Aslan A, Agar G. Protective role of methanol extract of Cetraria islandica (L.) against oxidative stress and genotoxic effects of AFB1 in human lymphocytes in vitro. Toxicol Ind Health 2011;27:599‑605.
28. Alpsoy L, Aslan A, Kotan E, Agar G, Anar M. Protective role of two lichens in human lymphocytes in vitro. Fresenius Environ Bull 2011;20:1661‑6.
29. Turkez H, Dirican E. A modulator against mercury chloride‑induced genotoxic damage: Dermatocarpon intestiniforme (L.). Toxicol Ind Health 2012;28:58‑63.
30. Ari F, Ulukaya E, Oran S, Celikler S, Ozturk S, Ozel MZ. Promising anticancer activity of a lichen, Parmelia sulcata Taylor, against breast cancer cell lines and genotoxic effect on human lymphocytes. Cytotechnology 2015;67:531‑43.
31. Singh N, Nambiar D, Kale RK, Singh RP. Usnic acid inhibits growth and induces cell cycle arrest and apoptosis in human lung carcinoma A549 cells. Nutr Cancer 2013;65 Suppl 1:36‑43.
32. O’Neill MA, Mayer M, Murray KE, Rolim‑Santos HM, Santos‑Magalhães NS, Thompson AM, et al. Does usnic acid affect microtubules in human cancer cells? Braz J Biol 2010;70:659‑64.
33. Berridge MV, Herst PM, Tan AS. Tetrazolium dyes as tools in cell biology: New insights into their cellular reduction. Biotechnol Annu Rev 2005;11:127‑52.
34. Haslam G, Wyatt D, Kitos PA. Estimating the number of viable animal cells in multi‑well cultures based on their lactate dehydrogenase activities. Cytotechnology 2000;32:63‑75.
35. Wolterbeek HT, van der Meer AJ. Optimization, application, and interpretation of lactate dehydrogenase measurements in microwell determination of cell number and toxicity. Assay Drug Dev Technol 2005:675‑82.
36. Gan W, Nie B, Shi F, Xu XM, Qian JC, Takagi Y, et al. Age‑dependent increases in the oxidative damage of DNA, RNA, and their metabolites in normal and senescence‑accelerated mice analyzed by LC‑MS/MS: Urinary 8‑oxoguanosine as a novel biomarker of aging. Free Radic Biol Med 2012;52:1700‑7.
37. Türkez H, Geyikoglu F, Yousef MI. Ameliorative effect of docosahexaenoic acid on 2,3,7,8‑tetrachlorodibenzo‑p‑dioxin‑induced histological changes, oxidative stress, and DNA damage in rat liver. Toxicol Ind Health 2012;28:687‑96.
38. Deng JY, Chen SJ, Jow GM, Hsueh CW, Jeng CJ. Dehydroeburicoic acid induces calcium‑ and calpain‑dependent necrosis in human U87MG glioblastomas. Chem Res Toxicol 2009;22:1817‑26.
39. Hahm SW, Park J, Son YS. Opuntia humifusa partitioned extracts inhibit the growth of U87MG human glioblastoma cells. Plant Foods Hum Nutr 2010;65:247‑52.
40. Jung HW, Ghil SH. A Torilis japonica extract exerts anti‑proliferative activities on the U87MG human glioblastoma cell line. Mol Med Rep 2010;3:1041‑5.
41. Jeong JC, Kim JW, Kwon CH, Kim TH, Kim YK. Fructus ligustri lucidi
extracts induce human glioma cell death through regulation of Akt/mTOR pathway in vitro and reduce glioma tumor growth in U87MG xenograft mouse model. Phytother Res 2011;25:429‑34. 42. Wang SG, Huang MH, Li JH, Lai FI, Lee HM, Hsu YN. Punicalagin
induces apoptotic and autophagic cell death in human U87MG glioma cells. Acta Pharmacol Sin 2013;34:1411‑9.
43. Markiewicz‑Zukowska R, Borawska MH, Fiedorowicz A, Naliwajko SK, Sawicka D, Car H. Propolis changes the anticancer activity of temozolomide in U87MG human glioblastoma cell line. BMC Complement Altern Med 2013;13:50.
44. Bayir Y, Odabasoglu F, Cakir A, Aslan A, Suleyman H, Halici M,
et al. The inhibition of gastric mucosal lesion, oxidative stress and
neutrophil‑infiltration in rats by the lichen constituent diffractaic acid. Phytomedicine 2006;13:584‑90.
45. Sepulveda B, Chamy MC, Piovano M, Areche C. Lichens: Might be considered as a source of gastroprotective molecules? J Chil Chem Soc 2013;58:1750‑2.
46. Okuyama E, Umeyama K, Yamazaki M, Kinoshita Y, Yamamoto Y. Usnic acid and diffractaic acid as analgesic and antipyretic components of
Usnea diffracta. Planta Med 1995;61:113‑5.
47. Odabasoglu F, Yildirim OS, Aygun H, Halici Z, Halici M, Erdogan F,
et al. Diffractaic acid, a novel proapoptotic agent, induces with olive
oil both apoptosis and antioxidative systems in Ti‑implanted rabbits. Eur J Pharmacol 2012;674:171‑8.
48. Karagoz I, Ozaslan M, Guler I, Uyar C, Yalim T, Kazanci U, et al. In
vivo antitumoral effect of diffractaic acid from lichen metabolites on
swiss albino mice with Ehrlich ascites carcinoma: An experimental study. Int J Pharmacol 2014;10:307‑14.
49. Brandão LF, Alcantara GB, Matos Mde F, Bogo D, Freitas Ddos S, Oyama NM, et al. Cytotoxic evaluation of phenolic compounds from lichens against melanoma cells. Chem Pharm Bull (Tokyo) 2013;61:176‑83.
50. Kumar KC, Müller K. Lichen metabolites 2. Antiproliferative and cytotoxic activity of gyrophoric, usnic, and diffractaic acid on human keratinocyte growth. J Nat Prod 1999;62:821‑3.
51. Brisdelli F, Perilli M, Sellitri D, Piovano M, Garbarino JA, Nicoletti M,
et al. Cytotoxic activity and antioxidant capacity of purified lichen
metabolites: An in vitro study. Phytother Res 2013;27:431‑7. 52. Bhattarai HD, Kim T, Oh H, Yim JH. A new pseudodepsidone from
the Antarctic lichen Stereocaulon alpinum and its antioxidant, antibacterial activity. J Antibiot (Tokyo) 2013;66:559‑61.
53. Thadhani VM, Choudhary MI, Ali S, Omar I, Siddique H, Karunaratne V. Antioxidant activity of some lichen metabolites. Nat Prod Res 2011;25:1827‑37.
54. Bucar F, Schneider I, Ogmundsdóttir H, Ingólfsdóttir K. Anti‑proliferative lichen compounds with inhibitory activity on 12(S)‑HETE production in human platelets. Phytomedicine 2004;11:602‑6.
55. Ogmundsdóttir HM, Zoëga GM, Gissurarson SR, Ingólfsdóttir K. Anti‑proliferative effects of lichen‑derived inhibitors of 5‑lipoxygenase on malignant cell‑lines and mitogen‑stimulated lymphocytes. J Pharm Pharmacol 1998;50:107‑15.
56. Thadhani VM, Choudhary MI, Khan S, Karunaratne V. Antimicrobial and toxicological activities of some depsides and depsidones. J Natl Sci Found Sri Lanka 2012;40:43‑8.
57. Morita H, Tsuchiya T, Kishibe K, Noya S, Shiro M, Hirasawa Y. Antimitotic activity of lobaric acid and a new benzofuran, sakisacaulon A from Stereocaulon sasakii. Bioorg Med Chem Lett 2009;19:3679‑81. 58. Manojlovic N, Rankovic B, Kosanic M, Vasiljevic P, Stanojkovic T.
Chemical composition of three Parmelia lichens and antioxidant, antimicrobial and cytotoxic activities of some their major metabolites. Phytomedicine 2012;19:1166‑72.
59. Schinkovitz A, Kaur A, Urban E, Zehl M, Páchniková G, Wang Y, et al. Cytotoxic constituents from Lobaria scrobiculata and a comparison of two bioassays for their evaluation. J Nat Prod 2014;77:1069‑73. 60. Nguyen TT, Yoon S, Yang Y, Lee HB, Oh S, Jeong MH, et al. Lichen
951
Journal of Cancer Research and Therapeutics - Volume 14 - Issue 5 - July-September 2018
effects on human cancer cells through the induction of apoptosis and suppression of tumorigenic potentials. PLoS One 2014;9:e111575. 61. Bazin MA, Le Lamer AC, Delcros JG, Rouaud I, Uriac P, Boustie J, et al.
Synthesis and cytotoxic activities of usnic acid derivatives. Bioorg Med Chem 2008;16:6860‑6.
62. Backorová M, Jendželovský R, Kello M, Backor M, Mikeš J, Fedorocko P. Lichen secondary metabolites are responsible for induction of apoptosis in HT‑29 and A2780 human cancer cell lines. Toxicol In
Vitro 2012;26:462‑8.
63. Han D, Matsumaru K, Rettori D, Kaplowitz N. Usnic acid‑induced necrosis of cultured mouse hepatocytes: Inhibition of mitochondrial function and oxidative stress. Biochem Pharmacol 2004;67:439‑51. 64. Mitrovic T, Stamenkovic S, Cvetkovic V, Tošic S, Stankovic M,
Radojevic I, et al. Antioxidant, antimicrobial and antiproliferative activities of five lichen species. Int J Mol Sci 2011;12:5428‑48. 65. Pavithra GM, Vinayaka KS, Rakesh KN, Junaid S, Dileep N,
Kekuda PT, et al. Antimicrobial and antioxidant activities of a macrolichen Usnea pictoides G. Awasthi (Parmeliaceae). J Appl Pharm Sci 2013;3:154‑60.
66. Sisodia R, Geol M, Verma S, Rani A, Dureja P. Antibacterial and antioxidant activity of lichen species Ramalina roesleri. Nat Prod Res 2013;27:2235‑9.
67. Emsen B, Bulak Y, Yildirim E, Aslan A, Ercisli S. Activities of two major lichen compounds, diffractaic acid and usnic acid against Leptinotarsa
decemlineata Say, 1824 (Coleoptera: Chrysomelidae). Egypt J Biol Pest
Control 2012;22:5‑10.
68. Yildirim E, Aslan A, Emsen B, Cakir A, Ercisli S. Insecticidal effect of
Usnea longissima (Parmeliaceae) extract against Sitophilus granarius
(Coleoptera: Curculionidae). Int J Agric Biol 2012;14:303‑6.
69. Yildirim E, Emsen B, Aslan A, Bulak Y, Ercisli S. Insecticidal activity of lichens against the maize weevil, Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). Egypt J Biol Pest Control 2012;22:151‑6.
70. Toledo Marante FJ, García Castellano A, Estévez Rosas F, Quintana Aguiar J, Bermejo Barrera J. Identification and quantitation of allelochemicals from the lichen Lethariella canariensis: Phytotoxicity and antioxidative activity. J Chem Ecol 2003;29:2049‑71.
71. Atalay F, Halici MB, Mavi A, Cakir A, Odabaşoğlu F, Kazaz C, et al. Antioxidant phenolics from Lobaria pulmonaria (L.) Hoffm. and Usnea
longissima Ach. lichen species. Turk J Chem 2011;35:647‑61.
72. He X, Hu Y, Winter J, Young GP. Anti‑mutagenic lichen extract has double‑edged effect on azoxymethane‑induced colorectal oncogenesis in C57BL/6J mice. Toxicol Mech Methods 2010;20:31‑5.
73. Su ZQ, Mo ZZ, Liao JB, Feng XX, Liang YZ, Zhang X, et al. Usnic acid protects LPS‑induced acute lung injury in mice through attenuating inflammatory responses and oxidative stress. Int Immunopharmacol 2014;22:371‑8.