Available online 28 August 2019
0254-0584/© 2019 Elsevier B.V. All rights reserved.
doped Y
2
Ti
2
O
7
and Sm
2
Ti
2
O
7
host crystals
Esra €Oztürk
*, Ebru Sarılmaz
Karamano�glu Mehmetbey University, Faculty of Engineering, Department of Metallurgy and Materials Engineering, Karaman, Turkey
H I G H L I G H T S
�The smart materials, Y1.98Eu0.02Ti2O7 and Sm1.98Eu0.02Ti2O7 were synthesized. �These systems were characterizated with DTA/TG, XRD, SEM/EDX, PL, LCR and d33-m. �The excitation and emission mechanisms and dielectric properties were explained.
A R T I C L E I N F O Keywords: Multi-functional materials Curie temperature Eu3þ Photoluminescence Piezoelectric material A B S T R A C T
Y1.98Eu0.02Ti2O7 and Sm1.98Eu0.02Ti2O7 with a cubic pyrochlore-type structure were synthesized from stoichio-metric mixtures of the constituent oxides using a solid solution reaction method at 1400 �C in an open atmo-sphere. Their crystal structures and lattice constants were determined by X-ray powder diffractometry. To determine the thermal behaviour of the mixtures of the starting materials, differential thermal analysis (DTA) and thermogravimetric (TG) analysis were used. The surface morphology and EDX analysis of the samples were investigated using a scanning electron microscope (SEM). The photoluminescence properties were measured and the red emission was observed from Y1.98Eu0.02Ti2O7 and Sm1.98Eu0.02Ti2O7 phosphors. Both materials show the typical emission bands of Eu3þions. The emission spectra of the Y
1.98Eu0.02Ti2O7 and Sm1.98Eu0.02Ti2O7 phos-phors consist of several intense peaks in the red spectral range, which correspond to the characteristic transitions of Eu3þfrom 5D
0 to 7FJ (J ¼ 2, 3, 4). When Eu3þions are doped to Sm2Ti2O7 host crystal, the life time prolongs 800 times compared with Y2Ti2O7 host crystal and the luminescent intensity was remarkably improved by the incorporation of Eu3þand Y2Ti2O7 host microcrystal. Also, the dielectric constant, loss tangent, piezoelectric charge constant and Curie temperature were investigated by LCR-meter, d33-m and TG/DTA, respectively. Y1.98Eu0.02Ti2O7 has the bigger piezoelectric charge constant and Sm1.98Eu0.02Ti2O7 (1561 �C) has a higher Curie temperature than Y1.98Eu0.02Ti2O7 (1449 �C).
1. Introduction
Piezoelectric materials can be used in a wide range of applications. Pb(Zrx,Ti1-x)O3, (PZT) materials have high piezoelectric coefficients,
dielectric constants and large ferroelectric polarizations [1]. PbTiO3 (PT) material has become the main material for a variety of piezoelectric devices. Among various PT-based ferroelectric materials, Pb (Mg1/3Nb2/3)O3–PbTiO3(PMN–PT) materials have shown high
piezo-electric constant (d33~640 pC/N) [2]. However, PMN-PT material
ex-hibits relatively low Curie temperature (~150 �C) [3]. BiFeO 3 (BF),
other attractive piezoelectric material, has relatively high Curie tem-perature TC~ 1103 K [4]. The BiFeO3–PbTiO3 (BF-PT) exhibits
relatively high Curie temperature Tc~ 600 �C [5].
A2B2O7 type pyrochlores are another attractive compounds family
with excellent piezoelectric properties. Pyrochlore materials are being used in various areas such as photoluminescent materials, sensor ap-plications and catalytic activities [6–8]. Moreover, pyrochlore com-pounds can be used as solid electrolyte and oxygen electrodes in fuel cells [9]. Especially, titanates based pyrochlore materials are being used for the immobilization of actinides, particularly plutonium from dismantled nuclear weapons [10,11]. The pyrochlore structure is a defective fluorite-type structure in which cations having variable charge occupy face-centered cubic positions Pyrochlore Y2Ti2O7 consists of
Y3þ-O2– distorted cubes and Ti4þ-O2– octahedrons [12–14]. Y2Ti2O7 is * Corresponding author.
E-mail address: esracircir@gmail.com (E. €Oztürk).
https://doi.org/10.1016/j.matchemphys.2019.122085
typical A2B2O7 type pyrochlore compounds with cubic structure [15]
and exhibits high melting temperature, high chemical and physical stability. Recently, Y2Ti2O7 has been shown to have potential
applica-tions in many fields, such as candidate materials for actinides immobi-lization [16,17], photocatalysis for splitting water into H2 and O2 [18, 19], dielectric materials [20,21] fluorescent materials [22–25] and solid fuel cells [26,27]. Especially, pyrochlore Y2Ti2O7 is one of the
inter-esting dielectric ceramics and it is dielectric constant (εr) is 54 [28].
Also, Sm2Ti2O7 has a pyrochlore cubic crystal system and is applied as
an electronic material in the past [29,30]. It’s dielectric-permittivity (ε0)
value is 2.52 [31].
For changing the dielectric properties of Y2Ti2O7 and Sm2Ti2O7,
many dopants can be used. Also, for an effective red photoluminescence, there are two ways: (1) the host crystal or the activator ion/ions that shows powerful absorption in the near-UV area; or (2) the luminescent material could transform the near-UV light into red-light. Eu3þ, as an activator ion, creates a very strong red luminescent centre compared to various rare earth (RE) ions in various host crystals [32]. For this reason, Eu3þion doped luminescent materials have attracted much interest for their good red luminescence efficiency and physical and chemical sta-bility [33].
The aim of the present study is to obtain both piezoelectricity and photoluminescence at the same time. Y2Ti2O7 and Sm2Ti2O7 have been
selected as host crystals due to their high chemical and thermal stability. Based on these assumptions, Y1.98Eu0.02Ti2O7 and Sm1.98Eu0.02Ti2O7
were synthesized by solid-state reaction method. In addition to this, characterization, the photoluminescence and piezoelectric properties of Y1.98Eu0.02Ti2O7 and Sm1.98Eu0.02Ti2O7 materials are investigated and
discussed below in detail. In the evaluation of the electrical properties, it is seen that while Y1.98Eu0.02Ti2O7 and Sm1.98Eu0.02Ti2O7 have similar
dielectric constant, Y1.98Eu0.02Ti2O7 has the bigger piezoelectric charge
constant and Sm1.98Eu0.02Ti2O7 has a higher Curie temperature.
2. Experimental
2.1. Synthesis and characterization of phosphors
Nominal compositions of the materials, Y1.98Eu0.02Ti2O7 and
Sm1.98Eu0.02Ti2O7, were prepared from starting materials. An
appro-priate amount of Y2O3 (99.99%), TiO2 (99.99%), Eu2O3 (99.9%) and
Sm2O3 (99.999%) were weighed and ball milled for 2 h with agate balls.
The samples of the milled mixtures of the starting materials were analyzed by the simultaneous differential thermal analysis (DTA) and the thermogravimetric (TG) analysis (Seiko Instruments Inc./Exstar TG/ DTA 630) at a heating rate of 10 �C/min from room temperature to
1300 �C to determine reaction optimizations. The ground mixtures were
pre-fired at 800 �C for 2 h and calcined at 1100 �C, 1200 �C, 1300 �C and
1400 �C 24 h in an open atmosphere. After sintering at 1400 �C, the X-
ray diffraction data of the calcined samples were collected to identify the crystal system. The X-ray diffraction of the samples was performed using a BRUKER AXS D8 ADVANCE model X-ray diffractometer (XRD), which ran at 40 kV and 30 mA (Cu-Kα radiation) in a step-scan mode (0.02�/
2θ). Scanning electron microscope images of samples’ surface and elemental analysis of samples were taken with a LEO 440 model scan-ning electron microscope (SEM) using an accelerating voltage of 20 kV. To investigate the photoluminescence properties of powder samples, the excitation and emission spectra and decay times of the phosphors were measured on a photoluminescence spectrometer (Photon Technology International (PTI), QuantaMaster™ 30). All the measurements were performed at room temperature. After these stages, the fine powders obtained at 1400 �C were pelletized into 10 mm diameter pellets by a
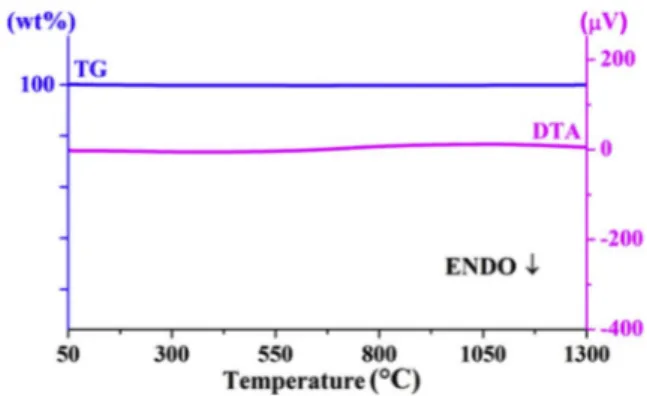
Fig. 1. TheTG/DTA curves of starting materials mixture of Y1.98Eu0.02Ti2O7.
Fig. 2. TheTG/DTA curves of starting materials mixture of Sm1.98Eu0.02Ti2O7.
Fig. 3. The XRD pattern of Y1.98Eu0.02Ti2O7.
uniaxial press with 250 MPa pressure using 2 wt % of an aqueous solu-tion of 5 wt % of polyvinyl alcohol (PVA) as a binder and then heat treatment at 1400 �C for 4 h was applied to eliminate PVA. The relative
density of the sintered specimens was measured by the Archimedes method using the thickness and diameter of pelletized samples. All samples had a relatively high density (>95%). Thus, for the electrical measurements, silver paste (99,9%) was coated on both sides of the sintered samples and fired at 650 �C for 1 h to form electrodes. Silver-
coated pellets were used to carry out the capacitance (c) and loss tangent (tan δ) using an LCR-meter (Hioki IM3536) to calculate the dielectric properties. An electric field of 9–18 kV/mm was applied to the coated and pelletized samples in a silicon oil bath at 120 �C for 30 min.
After 24 h, the piezoelectric charge co-efficient (d33) of samples were
measured with a Berlincourt d33-m. The Curie temperature (Tc) of
samples were determined with the simultaneous differential thermal analysis (DTA) and thermogravimetric (TG) analysis (Seiko Instruments Inc./Exstar TG/DTA 630).
3. Results and discussion
3.1. Thermal, structural and morphological characterization
The thermal behaviour of the mixtures of the starting materials composed of oxides was carried out between 50 �C and 1300 �C for the
nominal compositions of Y1.98Eu0.02Ti2O7 and Sm1.98Eu0.02Ti2O7
(Figs. 1–2). The TG curves are very similar to each other and no mass loss was determined due to the fact that all starting materials are oxide compounds. In Fig. 1., there is no endothermic or exothermic peak while an endothermic peak which is attributed to phase formation Sm1.98Eu0.02Ti2O7 was observed at 1270 �C in Fig. 2.
Fig. 3 shows the XRD pattern of Y1.98Eu0.02Ti2O7 powder calcined at
1400 �C for 24 h in an open atmosphere. The observed XRD pattern was
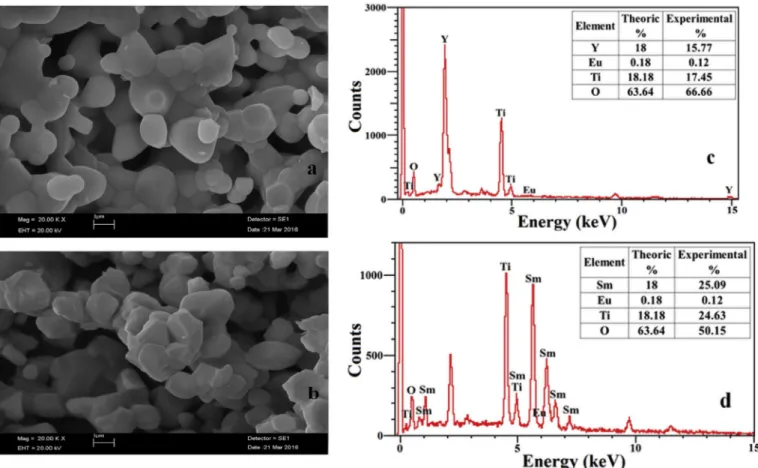
Fig. 5. The SEM image of (a) Y1.98Eu0.02Ti2O7, (b) Sm1.98Eu0.02Ti2O7, the EDX spectra of (c) Y1.98Eu0.02Ti2O7 (d) Sm1.98Eu0.02Ti2O7.
Fig. 6. The excitation and emission spectras of (a) Y1.98Eu0.02Ti2O7, (b) Sm1.98Eu0.02Ti2O7.
compared with the PDF card number 01-074-9633 and there was no impurity phase. The result shows that the obtained XRD pattern is in good agreement with the PDF card number 01-074-9633, indicating the formation of the cubic pyrochlore phase of the crystal structure with unit cell parameters a ¼ b ¼ c ¼ 10.161 Å, α ¼β ¼ γ:90�. Fig. 4 presents X-ray
diffraction patterns of Sm1.98Eu0.02Ti2O7 sample. Pyrochlore phase
Sm2Ti2O7 is the single component of the pattern. All the diffraction
peaks are in accordance with PDF card number 01-072-9772. The pyrochlore phase Sm1.98Eu0.02Ti2O7 lattice belongs to the cubic crystal
system and its unit cell parameters are a ¼ b ¼ c ¼ 10.231 Å,
α ¼β ¼ γ:90�. The cubic pyrochlore unit cell parameter (a) of
Sm1.98Eu0.02Ti2O7 is bigger than the unit cell parameter (a) of
Y1.98Eu0.02Ti2O7 due to Sm3þion having larger ionic radii than Y3þion.
The SEM images of Y1.98Eu0.02Ti2O7 and Sm1.98Eu0.02Ti2O7 are
shown in (Fig. 5 a and b) and the surface morphologies of samples can be seen. The samples present a ball-like structure with pretty crystallinity prepared at 1400 �C. The samples have regular particles with a particle
size of less than 3.0 μm. In Fig. 5a, the hexagonal-shaped micro crystals
clearly can be seen. A2B2O7 type pyrochlore crystals adopt a cubic
crystal system with the space group Fd-3m. There are two kinds of ox-ygen ions, O and O0in the pyrochlore structure. The trivalent A3þcations
in the A2B2O7 type pyrochlore are surrounded by eight oxygen anions
(6Oþ2O’). The tetravalent B4þcations are surrounded by six oxygen
anions (O) [34,35].
In Fig. 5-c and d, EDX analysis of samples are shown. The results show that there are no impurity atoms and samples consist of yttrium/ samarium, titanium, oxygen and europium elements. Also the theoret-ical and experimental percentage of elements are quite close, clearly indicating the presence of appropriate Eu3þand Sm3þions incorporated
into the phosphors.
3.2. Photoluminescence properties
Fig. 6a shows the excitation and emission spectra of Y1.98Eu0.02Ti2O7.
The excitation spectrum contains three maximum. A broad band near 266 nm is the charge-transfer state (CTS) band, due to an electron transferred from the O2 2p orbital to the empty 4f orbital of Eu3þ,
which may be ascribed as ligand-to-Eu3þ charge-transfer transitions
(LMCT). The typical Eu3þ-activated phosphors always show strong CTS
transition band absorption around 200–300 nm. The other peaks at 384 nm and 461 nm are associated with typical intra-4f 7F0→5L6 and
7F0→5D1 transitions of the Eu3þion. The emission spectrum showed the
typical emissions of Eu3þions at 601 nm and 706 nm, and the emission
peaks can be assigned to the 5D0→7F2 and 5D0→7F4 transitions, respectively. It is known that the 4f energy levels of Eu3þion are strongly
affected by the crystal field of host crystal due to the 5s2 5p6 electrons
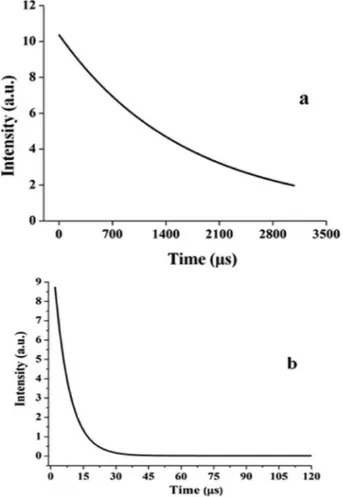
Fig. 7. The decay curves of (a) Y1.98Eu0.02Ti2O7, (b) Sm1.98Eu0.02Ti2O7.
Table 1
The thickness (t) and diameter (∅) and relative densities of pelletized samples.
Thickness, t, (mm) Diameter, ∅, (cm) Experimental density, ρex, (g/cm3) Theoretical density, ρteo (g/cm3) Relative density, ρre
Y1.98Eu0.02Ti2O7 1.1 1.0 5.04 4.883 0.97
Sm1.98Eu0.02Ti2O7 1.1 1.0 6.31 6.309 0.99
Fig. 8. The TG/DTA curves and Curie temperature of Y1.98Eu0.02Ti2O7.
spectra of Y1.98Eu0.02Ti2O7 shows a sharp and intensitive peak with a
maximum at 601 nm corresponding to electric dipole transition (5D0→7F2). This is attributed to Eu3þions occupying the non-inversion
center of symmetry [37]. Fig. 6b shows the excitation and emission spectra of Sm1.98Eu0.02Ti2O7. The band around 293 nm is assigned to the
O2 → Eu3þCTB, resulting from an electron transfer from the ligand O2
(2p6) orbital to the empty state of 4f of Eu3þion. Another excitation peak
at 421 nm belongs to 7F0→5D3 transition of Eu3þ. In the emission spectra of Sm1.98Eu0.02Ti2O7, two emission peaks were observed at 655 nm and
704 nm. These emission peaks are atributed to 5D0→7F3 and 5D0→7F4
transitions of Eu3þions, respectively [38,39].
The attribution of Eu3þtransitions also can be identified via the
measurement of a luminescent lifetime. Fig. 7-a and b show the lumi-nescence decay curves of Y1.98Eu0.02Ti2O7 and Sm1.98Eu0.02Ti2O7 by
monitoring the most intensitive emission under the excitation of CTB. The decay curves can be well fitted by the exponential function: I ¼ A1exp(-t/τ1)þ C
where I is phosphorescence intensity A1, C are constants t is time, and τ1
is the lifetime for the exponential components. The decay times of Y1.98Eu0.02Ti2O7 and Sm1.98Eu0.02Ti2O7 phosphors were calculated with
the above equation as 1656.0 μs and 1.999 μm. It is clearly shown that
Y1.98Eu0.02Ti2O7 phosphors have has 800 times longer lifetime than
Sm1.98Eu0.02Ti2O7.
3.3. Dielectric and piezoelectric properties
The thickness (t) and diameter (∅) of pelletized phosphors were measured and the densities were calculated using the Archimedes’ method. The densities and dimensions of the pellets of Y1.90Eu0.10Zr2O7
and Y1.90Sm0.10Zr2O7 are shown in Table 1. Due to the pelletized
sam-ples having >95% relative density, the samsam-ples’ densities are acceptable for dielectric and piezoelectric measurements. The loss tangent (tan δ) and capacitance (c) of the pelletized samples coated with silver paste were measured and dielectric constant (εr) was calculated using the
equation.
εr¼c.t/A.ε0
Then, pelletized samples were applied to an electric field of 9–18 kV/ mm in a silicon oil bath at 120 �C for 30 min. After 24 h, the piezoelectric
charge constant (d33) of all samples was measured by a d33-m. The Curie
temperature (Tc) of sintered powder samples at 1400 �C for 4 h was
determined with a TG/DTA system (Figs. 8 and 9). The capacitance (c), dielectric constant (εr), loss tangent (tan δ), piezoelectric charge
con-stant (d33) and Curie temperature are listed in Table 2. The first
endo-termic peak’s temperature in the termogram belongs to any phase transitions attributed to Curie temperature because of the material loses its piezoelectric property at temperatures above Curie temperature [40,
41]. When evaluating electrical properties, while Y1.98Eu0.02Ti2O7 and
Sm1.98Eu0.02Ti2O7 have similar dielectric constant, Y1.98Eu0.02Ti2O7 has
the bigger piezoelectric charge constant and Sm1.98Eu0.02Ti2O7 has a
higher Curie temperature.
action method. Both materials show the typical emission bands of Eu3þ ions. The emission spectra of the Y1.98Eu0.02Ti2O7 and Sm1.98Eu0.02Ti2O7
phosphors consist of several intense peaks in the red spectral range, which correspond to the characteristic transitions of Eu3þfrom 5D0 to 7F
J (J ¼ 2, 3, 4). When Eu3þions are doped to Sm2Ti2O7 host crystal, the
life time prolongs 800 times compared with Y2Ti2O7 host crystal. In
addition, the luminescent intensity was remarkably improved by the incorporation of Eu3þ and Y2Ti2O7 host micro-crystal. Additionally,
Y1.98Eu0.02Ti2O7 and Sm1.98Eu0.02Ti2O7 phosphors show piezoelectric
properties and have a high Curie temperature. Because Y1.98Eu0.02Ti2O7
and Sm1.98Eu0.02Ti2O7 have both photoluminescence and piezoelectric
properties, they are a good candidate for opto-electronic applications.
Acknowledgements
The authors would like to thank TUBITAK (The Scientific and Technological Research Council of Turkey) for the support of the project numbered 114Z438.
References
[1] I. Kanno, H. Kotera, K. Wasa, Measurement of transverse piezoelectric properties of PZT thin films, Sens. Actuators A. 107 (2003) 68–74.
[2] J. Kelly, M. Leonard, C. Tantigate, A. Safari, Effect of composition on the electro- mechanical properties of (1-x)Pb(Mg1/3Nb2/3)O3–xPbTiO3 ceramics, J. Am. Ceram.
Soc. 80 (1997) 957–964.
[3] D. La-Orauttapong, B. Noheda, Z.-G. Ye, P.M. Gehring, J. Toulouse, D.E. Cox, G. Shirane, Phase diagram of the relaxor ferroelectric (1-x)Pb(Mg1/3Nb2/3)
O3–xPbTiO3, Phys. Rev. B. 65 (2002) 144101.
[4] H. Zhang, W.F. Liu, P. Wu, X. Hai, S.Y. Wang, G.Y. Liu, G.H. Rao, Unusual magneticbehaviors and electrical properties of Nd-doped BiFeO3 nanoparticles
calcined atdifferent temperatures, J. Nanoparticle Res. 16 (2014) 2205. [5] H. Amorín, C. Correas, C.M. Fern�andez-Posada, O. Pe~na, A. Castro, M. Alguer�o,
Multiferroism and enhancement of material properties across the morphotropicphase boundary of BiFeO3-PbTiO3, J. Appl. Phys. 115 (2014)
104104-1–104104-9.
[6] M.A. Subramanian, G. Aravamudan, G.V. Subba Rao, Oxide pyrochlores-a review, Prog. Solid State Chem. 15 (1983) 55e143.
[7] O. Porat, C. Heremans, H.L. Tuller, Stability and mixed ionic electronic conduction in Gd2 (Ti1- xMox)2 O7 under anodic conditions, Solid State Ion. 94 (1997) 75e83. [8] M. Hanawa, Y. Maraoka, T. Tayama, T. Sakakibara, J. Yamanra, Z. Horoi,
Superconductivity at 1 K in Cd2Re2O7, Phys. Rev. Lett. 87 (2001) 187001. [9] T.A. Vanderah, Talking ceramics, Science 298 (2002) 1182e4.
[10] J.B. Goodenough, R.N. Castellano, Defect pyrochlores as catalyst supports, J. Solid State Chem. 44 (1982) 44.
[11] A.E. Ringwood, S. Kesson, N.B. Ware, W.A. Hibberson, Immobilisation of high level nuclear reactor wastes in SYNROC, Nature 278 (1979) 219e23.
[12] D. Hollmann, O. Merka, L. Schwertmann, R. Marschall, M. Wark, A. Brückner, Active sites for light driven proton reduction in Y2Ti2O7 and CsTaWO6 pyrochlore
catalysts detected by in situ EPR, Top. Catal. 58 (2015), 58 769.
[13] V. Badjeck, M.G. Walls, L. Chaffron, J. Malaplate, K. March, New insights into the chemical structure of Y2Ti2O7–δ nanoparticles in oxide dispersion-strengthened
steels designed for sodium fast reactors by electron energy-loss spectroscopy, J. Nucl. Mater. 456 (2015), 456 292.
[14] L.T. Yang, Y. Jiang, G.R. Odette, W.C. Zhou, Z.M. Liu, Y. Liu, Nonstoichiometry and relative stabilities of Y2Ti2O7 polar surfaces: a density functional theory prediction,
Acta Mater. 617 (2013) 260.
[15] P.J. Wild, C.R.A. Catlow, Defects and Diffusion in Pyrochlore Structured Oxides Solid State Ion, vol. 112, 1998, pp. 173–183.
[16] L.J. Chen, X. Su, Y.H. Li, First-principles study on cation-antisite defects of stannate and titanate pyrochlores, OALib 1 (2014) 1–8.
[17] M. Jafar, S.N. Achary, N.P. Salke, A.K. Sahu, R. Rao, A.K. Tyagi, X-ray diffraction and Raman spectroscopic investigations on CaZrTi2O7-Y2Ti2O7 system: delineation
of phase fields consisting of potential ceramic host materials, J. Nucl. Mater. 475 (2016) 192–199.
[18] G. Ravi, S. Mansouri, S. Palla, M. Vithal, Synthesis of Y2Ti2O7-xNy with visible light
responsive photocatalytic activity, Indian J. Chem. 54 (2015) 20–26. [19] R. Abe, M. Higashi, K. Sayama, Y. Abe, H. Sugihara, Photocatalytic activity of
R3MO7 and R2Ti2O7 (R¼ Y, Gd, La; M ¼Nb, Ta) for water splitting into H2 and O2,
J. Phys. Chem. 110 (2016) 2219–2226.
[20] J.Y. Ding, Y. Xiao, Z.F. Wang, L.X. Wang, Q.T. Zhang, Effects of additives on dielectric properties of Y2Ti2O7 ceramics, J. Inorg. Mater. 26 (2011) 327–331. [21] J.Y. Ding, Y. Xiao, Y. Lu, T.T. Tao, Q.T. Zhang, Effects of BaO sintering additive on
dielectric properties of Y2Ti2O7 microwave dielectric ceramics, Rare Met. 30
(2011) 624–627.
[22] Y.M. Yin, H. Wang, M.M. Xing, Y. F, Y. Tian, X.L. Shen, W.G. Yu, X.X. Luo, Upconversion luminescence of Y2Ti2O7:Er3þunder 1550 and 980 nm excitation,
J. Rare Earths 35 (2017) 230–233.
[23] Z.S. Chen, M. Wang, H.Q. Wang, Z.G. Le, G.L. Huang, L.X. Zou, Z.R. Liu, D. Y. Wang, Q.K. Wang, W.P. Gong, Fabrication of Y2Ti2O7:Yb3þ,Ho3þnanoparticles
by a gel-combustion approach and up converting luminescent properties, J. Alloy. Compounds 608 (2014) 165–169.
[24] M. Saif, M. Shebl, A. Mbarek, A.I. Nabeel, R. Maalej, R. Shokry, Synthesis of non- toxic phosphor material based on pyrochlore-type dititanate (Eu3þ/Y
2Ti2O7),
J. Photochem. Photobiol., A 301 (2015) 1–5.
[25] A. Garbout, S. Bouattour, A.W. Kolsi, Synthesis, X-ray diffraction investigations and spectroscopic analysis of yttrium-substituted pyrochlore oxides Y2-2xBi2xTi2O7 via a
sol-gel process, J. Cryst. Growth 307 (2007) 219–228.
[26] J.K. Gill, O.P. Pandey, K. Singh, Ionic conductivity, structural and thermal properties of pure and Sr2þdoped Y
2Ti2O7 pyrochlores for SOFC, Solid State Sci. 13
(2011) 1960–1966.
[27] H.E. Hung, C.K. Hao, C.S. Lee, The stability and ionic conductivity in Cr doped Y2Ti2O7 in the search for potential SOFC electrolyte materials, ECS Trans. 58
(2013) 175–184.
[28] T.T. Tao, L.X. Wang, Q.T. Zhang, Study on the composite and properties of Y2O3-
TiO2 microwave dielectric ceramics, J. Alloy. Comp. 486 (2009) 606. [29] M. Jafar, P. Sengupta, S.N. Achary, A.K. Tyagi, Phase evolution and
microstructural studies in CaZrTi2O7 (zirconolite)–Sm2Ti2O7 (pyrochlore) system, J. Eur. Ceram. Soc. 34 (2014) 4373–4381.
[30] M.E. Rabanal, A. V�arez, U. Amador, E.A. Dompablo, F. Garcia-Alvarado, Structure and reaction with lithium of tetragonal pyrochlore-like compound Sm2Ti2O7,
J. Mater. Process. Technol. 92–93 (1999) 529–533.
[31] A.B. Rinkevich, Y.A. Pakhomov, D.V. Perov, Millimeter waveband dielectric properties of nanocomposite 3d and rare-earth titanates, Mater. Today: Proc. 14 (2019) 144–147.
[32] E. €Oztürk, N. Ozpozan Kalaycioglu, E. Uzun, Investigation of luminescence properties of Eu3þ, Dy3þand Gd3þdoped MgAl2Si2O8 red-emitting phosphors,
J. Chin. Chem. Soc. 62 (2015) 47–51.
[33] S. Som, S. Das, S. Dutta, H.G. Visser, M.K. Pandey, P. Kumar, R.K. Dubey, S. K. Sharma, Synthesis of strong red emitting Y2O3:Eu3þphosphor by potential
chemical routes: comparative investigations on the structural evolutions, photometric properties and Judd–Ofelt analysis, RSC Adv. 87 (2015) 70887–70898.
[34] N. Pailh�e, M. Gaudon, A. Demourgues, (Ca2þ, V5þ) co-doped Y
2Ti2O7 yellow
pigment, Mater. Res. Bull. 44 (2009) 1771–1777.
[35] P.J. Wilde, C.R.A. Catlow, Defects and diffusion in pyrochlore structured oxides, Solid State Ion. 112 (1998) 173–183.
[36] M. Saif, Luminescence based on energy transfer in silica doped with lanthanide titania (Gd2Ti2O7:Ln3þ) [Ln3þ ¼ Eu3þ or Dy3þ], J. Photochem. Photobiol. A Chem. 205 (2009) 145–150.
[37] M. Saif, Synthesis of down conversion, high luminescent nano-phosphor materials based on new developed Ln3þ:Y2Zr2O7/SiO2 for latent fingerprint application, J. Lumin. 135 (2013) 187–195.
[38] Y.C. Li, Y.H. Chang, Y.F. Lin, Y.S. Chang, Y.J. Lin, Synthesis and luminescent properties of Ln3þ(Eu3þ, Sm3þ, Dy3þ)-doped lanthanum aluminum germanate
LaAlGe2O7 phosphors, J. Alloy. Comp. 439 (2007) 367–375.
[39] F. Zhang, Y. Wang, Y. Tao, VUV spectroscopic properties of Ba2Gd2Si4O13:Re3þ
(Re3þ¼Ce3þ, Tb3þ, Dy3þ, Eu3þ, Sm3þ), Mater. Res. Bull. 48 (2013) 1952–1956. [40] M.K. Mani, G. Viola, J. Hall, S. Grasso, M.J. Reece, Observation of Curie transition
during spark plasma sintering of ferromagnetic material, J. Magn. Magn. Mater. 382 (2015) 202–205.
[41] G. Ferk, M. Drofenik, D. Lisjak, A. Hamler, Z. Jagli�ci�c, D. Makovec, Synthesis and characterization of Mg1þxFe2-2xTixO4 nanoparticles with anadjustable Curiepoint,