https://doi.org/10.1007/s10147-019-01529-4 ORIGINAL ARTICLE
Prognostic significance of solid growth in endometrioid endometrial
adenocarcinoma
Serra Akar1 · Zeliha Esin Çelik2 · Sıddıka Fındık3 · Tolgay Tuyan İlhan1 · Fedi Ercan4 · Çetin Çelik1
Received: 3 May 2019 / Accepted: 14 August 2019 / Published online: 26 August 2019 © Japan Society of Clinical Oncology 2019
Abstract
Background Endometrioid endometrial cancer is the most common histological subtype of endometrial adenocarcinoma. In the FIGO grading scheme, both architectural and nuclear grade are taken into consideration. However, the specific impact of solid growth alone on endometrioid endometrial adenocarcinoma outcome is not well documented. We sought to assess the degree of impact of solid growth on lymphovascular space invasion (LVSI), myometrial invasion, tumor size, FIGO stage, lymph node metastasis (LNM), relapse-free survival (RFS) and disease-specific survival (DSS).
Methods Paraffin blocks of 269 patients treated for endometrioid endometrial cancer were retrospectively analyzed with morphometry for solid growth percentages.
Results A statistically significant cut-off value of 1% solid growth was found for predicting LNM and advanced stage (III or IV), myometrial invasion and LVSI (p < 0.001) and a cut-off value of 8% was found for predicting adverse survival outcome (p < 0.001). The mean DSS was significantly higher in patients with < 6% solid growth compared to patients with 6–50%, 51–75% and > 75% solid growth (p < 0.001). Although, the mean RFS and DSS were lowest in patients with 51–75% solid growth, this did not reach statistical significance in comparison to 6–50% and > 75% (p > 0.05).
Conclusion Although > 75% solid growth was most significantly associated with many of the adverse prognostic factors, this subset did not provide prognostic superiority in predicting adverse survival when compared to subsets within 6–75% solid growth. In conclusion, although no statistically significant difference in survival was found among subdivisions of architectural grades 2 and 3, solid growth, especially ≥ 8%, appeared to be an independent prognostic factor for survival in patients with early-stage endometrioid endometrial cancer.
Keywords Endometrial cancer · Solid growth · Architectural grade · Prognosis · Survival
Introduction
Endometriod tumors comprise the most common histo-logical subtype of endometrial adenocarcinomas and are seen in 75–80% of cases [1]. Early diagnosis and treatment
result in high 5-year overall survival rates [2]. Although grade is not included in the criteria for stage, it is con-sidered to have significant prognostic and therapeutic implications. The grading system currently in use for endometrioid endometrial adenocarcinoma is based on the revised International Federation of Gynecology and Obstetrics (FIGO) system [3]. In this grading scheme, both architectural and nuclear grade are taken into con-sideration, whereby, nuclear atypia unsuitably high for the architectural grade (nuclear grade 3) raises the grade by one. Architectural grade is based on the percentage of non-squamous epithelial solid growth, where grade 1 tumors demonstrate 5% or lower, grade 2 tumors contain 6–50% and grade 3 tumors exhibit higher than 50% solid growth. However, the specific impact of the degree of solid growth alone on endometrioid endometrial adenocarcinoma out-come has not been well described. We sought to assess
* Serra Akar
serraakar@yahoo.com
1 Division of Gynecologic Oncology, Department
of Obstetrics and Gynecology, Selcuk University Hospital, Selcuk University Medical School, Konya 60615, Turkey
2 Department of Pathology, Selcuk University Hospital,
Konya, Turkey
3 Department of Pathology, Meram Faculty of Medicine,
Necmettin Erbakan University, Konya, Turkey
4 Department of Obstetrics and Gynecology, Meram Faculty
the impact of solid growth on lymphovascular space inva-sion (LVSI), myometrial invainva-sion, tumor size, stage, pelvic and para-aortic lymph node metastasis (LNM), malignant peritoneal cytology, adenexal involvement and relapse-free survival (RFS) and disease-specific survival (DSS).
Materials and methods
A total of 269 patients treated for endometrioid endome-trial adenocarcinoma in Selcuk University Hospital, Konya, Turkey between September 2009 and Janurary 2018 were included in the study. All patients had a histopathological diagnosis of endometrioid endometrial adenocarcinoma fol-lowing surgical treatment. Patients were treated with adju-vant therapy after surgery as deemed appropriate by insti-tutional physicians. Patients with a history of malignancy other than endometrioid endometrial adenocarcinoma or those who received chemotherapy or radiotherapy prior to surgical treatment were excluded from the study. Formalin-fixed paraffin-embedded tissue blocks were retrospectively analyzed for architectural grading. Percentage of solid non-squamous epithelium for each patient was assessed by mor-phometry by two experienced pathologists.
To better analyze the prognostic impact of solid growth percentages, smaller increments of growth percentages were analyzed separately; 0% (absent solid growth), 1–5%, 6–25%, 26–50%, 51–75%, 76–100%. FIGO architectural grade was assigned based on the percentage of non-squa-mous epithelial solid growth, with architectural grade 1 tumors exhibiting 5% or lower, grade 2 showing 6–50% and grade 3 demonstrating greater than 50% solid growth. FIGO architectural grading with the omission of status of nuclear atypia was analyzed in terms of survival.
Patients’ ages and clinicopathological information about LVSI, myometrial invasion, stage according to the FIGO criteria [4], pelvic and para-aortic lymph node metastasis (LNM), adnexal involvement, peritoneal cytology, omental involvement, tumor size, cervical and extrauterine involve-ment were retrieved from definitive pathology reports. Patients with stage I or II disease with positive peritoneal cytology or stages III and IV disease were grouped as hav-ing extrauterine disease. RFS as the interval from the date of completion of primary therapy to clinical or radiological evi-dence of metastatic disease (confirmed by biopsy) and DSS as the interval from the date of diagnosis to time of death due to disease, were calculated from follow-up records and National Death Registry, last checked on 11 June 2019. The study was approved by the Institutional Human Research Ethics Committee of Selcuk University Medical School (Konya, Turkey). Written informed consent was obtained from patients for use of their specimen in the study.
Statistical analysis
The prognostic significance of each category of solid growth in terms of clinicopathological characteristics was compared using the Chi-square test and post-hoc test with Bonferroni adjustments. Percentage of solid growth was converted into dichotic data of low and high for predicting the presence of LVSI, LNM, outer half myometrial inva-sion, advanced stage and adverse survival by cut-off and sensitivity values using the receiver operator characteris-tics curve analyses. Kaplan–Meier analysis was used to investigate the potential association between solid growth and survival outcomes and the log-rank test was used to determine statistical significance. Some of the variables with p value < 0.20 on univariate analyses were included in multivariate analysis (Cox proportional hazards model).
p value less than 0.05 was considered statistically
signifi-cant. All statistical analyses were performed using SPSS 24.0 statistical software package (SPSS Inc., Chicago, IL).
Results
A total of 269 patients with a histopathological diagnosis of endometrioid endometrial adenocarcinoma were included in the study. Mean follow-up was 60 (7–139) months. The mean age of patients was 59.05 ± 10.02 (32–89). There was no statistically significant correlation between patients’ age and the percentage of solid growth (p = 0.11). The clin-icopathological patient characteristics are summarized in Table 1. In total recurrence was observed in 30/269 (11.2%) patients. 24/269 (8.9%) patients died of the disease. Recur-rence was seen in 15/187, 5/37, 8/41 and 2/4 patients with stages I, II, III and IV disease, respectively. Disease-specific death was seen in 11/187, 3/37, 8/41 and 2/4 patients in stages I, II, III and IV, respectively.
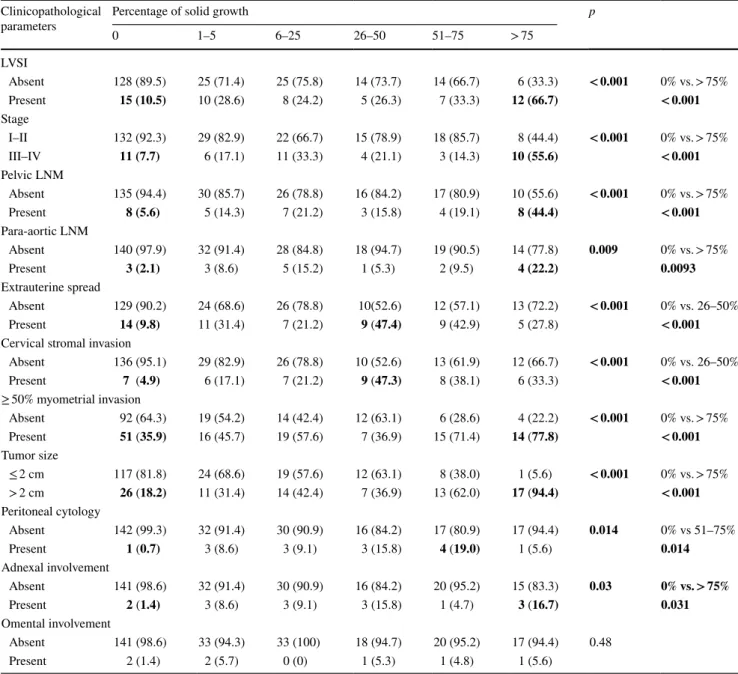
The associations of solid growth percentages with prog-nostic factors are summarized in Table 2. Presence of > 75% tumoral solid growth was significantly associated with pres-ence of LVSI (p < 0.001), advanced stage (p < 0.001), outer half myometrial invasion (< 0.001), pelvic lymph node metastasis (p < 0.001), para-aortic lymph node metastasis (p = 0.009), tumor size ≥ 2 cm (p < 0.001), adnexal involve-ment (p = 0.03). 51–75% tumoral solid growth was sig-nificantly associated with malignant peritoneal cytology (p = 0.014). 26–50% solid growth was significantly asso-ciated with cervical stromal involvement and extrauterine spread (p < 0.001). There was no significant association between solid growth and omental involvement (p = 0.48).
A statistically significant cut-off value of 1% solid growth with 75.8% sensitivity and 65.4% specificity,
71.4% sensitivity and 65.9% specificity, 60.0% sensitiv-ity and 73.0% specificsensitiv-ity, 70.2% sensitivsensitiv-ity and 65.3% specificity was found for predicting pelvic or para-aortic
lymph node metastasis, advanced stage (III or IV), myo-metrial invasion and LVSI, respectively (p < 0.001 for all). A significant cut-off value of 8% solid growth was found for predicting adverse outcome (recurrence and disease-specific death) with a sensitivity of 75.6% and disease-specificity of 73.8% (p < 0.001).
The mean DSS for patients with architectural FIGO grade 1, 2 and 3 tumors were 117.1 (95% CI 112.8–121.4), 87.4 (95% CI 78.7–101.1) and 81.2 (95% CI 66.1–96.3) months, respectively. The mean DSS was significantly higher in patients with architectural FIGO grade 1 tumors com-pared to those with grade 2 (p < 0.001) and grade 3 tumors (p < 0.001). There was no significant difference in survival between patients with architectural grade 2 and 3 tumors (p = 0.29).
The mean RFS for patients with < 6%, 6–25%, 26–50%, 51–75% and > 75% were 111.8 (95% CI 109.6–122.0), 89.4 (95% CI 74.5–104.3), 77.5 (95% CI 51.3–83.7), 67.2 (95% CI 40.3–88.3) and 85.7 (95% CI 69.0–105.2) months, respectively (Fig. 1). There was no statistically significant difference in RFS between patients with 0% and 1–5% solid growth (p = 0.22). There was no difference in RFS between 0% and 1–7% solid growth (p > 0.05). Patients with < 6% solid growth had the highest survival compared to patients with 6–25% (p = 0.027) 26–50% (p = 0.08), 51–75% (p < 0.001) and > 75% (p < 0.044) solid growth. There was no statistically significant difference in RFS among patients with solid growth subsets of 6–25%, 26–50%, 51–75%, > 75% (p > 0.05).
The mean DSS for patients with < 6%, 6–25%, 26–50%, 51–75% and > 75% were 117.1 (95% CI 112.8–121.4), 89.6 (95% CI 74.4–104.8), 87.3 (95% CI 69.8–104.8), 71.3 (95% CI 52.4–90.1) and 88.8 (95% CI 72.7–110.8) months, respectively (Fig. 1). There was no statistically significant difference in DSS between patients with 0% and 1–5% solid growth (p = 0.32). There was no difference in DSS between 0% and 1–7% solid growth (p > 0.05). Patients with < 6% solid growth had the highest DSS compared to patients with 6–25%, 26–50%, 51–75% (p < 0.001 for 3 subsets) and > 75% (p = 0.01). Although, the mean RFS and DSS were lowest in patients with 51–75% solid growth, this did not reach statistical significance in comparison to 6–50% and > 75% (p > 0.05). There was no statistically significant difference in DSS of patients among subsets of 6–25%, 26–50%, 51–75%, > 75% solid growth (p > 0.05).
When survival was analyzed based on the cut-off of 8%, patients with < 8% solid growth had a significantly higher mean DSS of 117.3 (95% CI 113.0–121.5) and RFS of 111.9 (95% CI 110.0–122.8) months than patients with ≥ 8% solid growth [DSS 85.5 (95% CI 76.3–94.8), RFS 82.7 (95% CI 72.4–92.0) months]. On multivariate survival analyses, ≥ 8% solid growth was a significant independent adverse prognostic factor along with stage Table 1 Distribution of clinicopathological patient characteristics
a 2 patients had isolated para-aortic LNM, 16 had both pelvic and
para-aortic LNM Clinicopathologic characteristics N (%) Solid growth 0% 143 (53.2) 1–5% 35 (13.0) 6–25% 33 (12.3) 26–50% 19 (7.1) 51–75% 21 (7.8) 76–100% 18 (6.7)
FIGO architectural grade
1 178 (66.2) 2 52 (19.3) 3 39 (14.5) Stage I 187 (69.5) II 37 (13.8) III 41 (15.2) IV 4 (1.5) LVSI Absent 212 (78.8) Present 57 (21.2) LNMa Absent 232 (86.2) Present 37 (13.8) MI < 50% 147 (54.6) ≥ 50% 122 (45.3) Tumor size < 2 cm 181 (67.3) ≥ 2 cm 88 (32.7) Peritoneal cytology Negative 254(94.4) Positive 15(5.6) Adnexa Not involved 254 (94.4) Involved 15(5.6) Extrauterine spread Absent 214 (79.6) Present 55 (20.4)
Cervical stromal invasion
Absent 226 (84.0)
Present 43 (16.0)
Omental involvement
Absent 262 (97.4)
(p = 0.001) (Table 3). This cut-off offered the most sig-nificant predictive value of survival based on the highest Hazard Ratio (HR) compared to a cut-off of 20%, 50% and solid growth grouped into FIGO architectural grades or subsets of < 6%, 6–25%, 26–50%, 51–75%, > 75%. On multivariate DSS analyses by stage, myometrial invasion and solid growth, FIGO architectural grading exhibited prognostic significance with a HR of 2.0 (95% CI 1.2–2.9) (p = 0.004) along with stage [HR 2.1 (95% CI 1.3–3.0)] (p = 0.016) but not myometrial invasion [HR 1.5 (95% CI 0.6–3.5)] (p = 0.38). On multivariate survival analyses by stage, myometrial invasion and solid growth, subgrouping
of solid growth (< 6%, 6–25%, 26–50%, 51–75%, > 75%) exhibited prognostic significance with a HR of 1.3 (95% CI 1.0–1.7) (p = 0.015) along with stage [HR 1.5 (95% CI 1.0–2.4)] (p = 0.010) but not myometrial invasion [HR 1.5 (95% CI 0.6–3.6)] (p = 0.34).
Among patients with stage I and II disease, on multi-variate survival analyses, ≥ 8% solid growth was a signifi-cant independent adverse prognostic factor (p < 0.001) of survival along with LVSI (p = 0.014) (Table 4). On multi-variate survival analyses of patients with stage III and IV disease, ≥ 8% solid growth was not a significant independ-ent adverse prognostic factor (p > 0.05) (Table 5). Table 2 Association of solid growth with clinicopathological patient characteristics
Values in bold indicate statistically significant differences Clinicopathological
parameters Percentage of solid growth0 1–5 6–25 26–50 51–75 > 75 p
LVSI Absent 128 (89.5) 25 (71.4) 25 (75.8) 14 (73.7) 14 (66.7) 6 (33.3) < 0.001 0% vs. > 75% Present 15 (10.5) 10 (28.6) 8 (24.2) 5 (26.3) 7 (33.3) 12 (66.7) < 0.001 Stage I–II 132 (92.3) 29 (82.9) 22 (66.7) 15 (78.9) 18 (85.7) 8 (44.4) < 0.001 0% vs. > 75% III–IV 11 (7.7) 6 (17.1) 11 (33.3) 4 (21.1) 3 (14.3) 10 (55.6) < 0.001 Pelvic LNM Absent 135 (94.4) 30 (85.7) 26 (78.8) 16 (84.2) 17 (80.9) 10 (55.6) < 0.001 0% vs. > 75% Present 8 (5.6) 5 (14.3) 7 (21.2) 3 (15.8) 4 (19.1) 8 (44.4) < 0.001 Para-aortic LNM Absent 140 (97.9) 32 (91.4) 28 (84.8) 18 (94.7) 19 (90.5) 14 (77.8) 0.009 0% vs. > 75% Present 3 (2.1) 3 (8.6) 5 (15.2) 1 (5.3) 2 (9.5) 4 (22.2) 0.0093 Extrauterine spread Absent 129 (90.2) 24 (68.6) 26 (78.8) 10(52.6) 12 (57.1) 13 (72.2) < 0.001 0% vs. 26–50% Present 14 (9.8) 11 (31.4) 7 (21.2) 9 (47.4) 9 (42.9) 5 (27.8) < 0.001
Cervical stromal invasion
Absent 136 (95.1) 29 (82.9) 26 (78.8) 10 (52.6) 13 (61.9) 12 (66.7) < 0.001 0% vs. 26–50% Present 7 (4.9) 6 (17.1) 7 (21.2) 9 (47.3) 8 (38.1) 6 (33.3) < 0.001 ≥ 50% myometrial invasion Absent 92 (64.3) 19 (54.2) 14 (42.4) 12 (63.1) 6 (28.6) 4 (22.2) < 0.001 0% vs. > 75% Present 51 (35.9) 16 (45.7) 19 (57.6) 7 (36.9) 15 (71.4) 14 (77.8) < 0.001 Tumor size ≤ 2 cm 117 (81.8) 24 (68.6) 19 (57.6) 12 (63.1) 8 (38.0) 1 (5.6) < 0.001 0% vs. > 75% > 2 cm 26 (18.2) 11 (31.4) 14 (42.4) 7 (36.9) 13 (62.0) 17 (94.4) < 0.001 Peritoneal cytology Absent 142 (99.3) 32 (91.4) 30 (90.9) 16 (84.2) 17 (80.9) 17 (94.4) 0.014 0% vs 51–75% Present 1 (0.7) 3 (8.6) 3 (9.1) 3 (15.8) 4 (19.0) 1 (5.6) 0.014 Adnexal involvement Absent 141 (98.6) 32 (91.4) 30 (90.9) 16 (84.2) 20 (95.2) 15 (83.3) 0.03 0% vs. > 75% Present 2 (1.4) 3 (8.6) 3 (9.1) 3 (15.8) 1 (4.7) 3 (16.7) 0.031 Omental involvement Absent 141 (98.6) 33 (94.3) 33 (100) 18 (94.7) 20 (95.2) 17 (94.4) 0.48 Present 2 (1.4) 2 (5.7) 0 (0) 1 (5.3) 1 (4.8) 1 (5.6)
Discussion
Endometrioid endometrial adenocarcinomas are graded according to the FIGO grading system, which currently incorporates nuclear grading into the architectural grading
scheme [1]. Architectural grading is based on the percent-age of non-squamous solid growth patterns. However, the impact of solid growth itself on disease outcomes includ-ing relapse-free survival (RFS) and disease-specific sur-vival (DSS) has not been well documented. Since grade is an important prognosticator and guides therapeutic Fig. 1 Relapse-free and disease-specific survival in endometrioid endometrial adenocarcinoma in relation to solid growth percentages
Table 3 Univariate and multivariate survival analyses by solid growth, stage, myometrial invasion in endometrioid endometrial cancer
p values in bold indicate statistical significance
LVSI lymphovascular space invasion, MI myometrial invasion, HR hazard ratio
No. of cases Univariate analysis Multivariate analysis
DSS RFS DSS RFS
Events (N) p Events (N) p HR (95% CI) p HR (95% CI) p
Stage Early 224 19 < 0.001 19 < 0.001 3.7 (1.8–7.9) 0.001 2.8 (1.5–5.2) 0.016 Late 45 5 11 Solid growth < 8% 192 9 < 0.001 12 < 0.001 3.5 (1.5–7.4) 0.001 2.4 (1.2–4.9) 0.012 8–100% 77 15 18 MI < 50% 147 8 0.002 10 0.003 1.4 (0.6–3.4) 0.31 1.2 (0.3–2.8) 0.34 ≥ 50% 122 16 20
management, there is a need to clarify the exact relation-ship between solid growth and prognosis. This study, to our knowledge, is unique in its investigation of the rela-tionship between solid growth percentages and prognosis. The primary strength of this study lies in the analysis of prognostic impact of solid growth percentages in relation to a wide range of clinicopathological patient characteris-tics and survival. The review of solid growth in the form of percentages enabled the identification of a cut-off value for predicting prognosis and re-grouping solid growth into smaller subdivisions of architectural FIGO grades.
Previous studies have investigated solid growth in rela-tion to a limited number of prognostic factors in the form of two-tiered (≤ 50 vs. > 50% solid growth) systems or in increments of 10%, of which the latter did not include sur-vival analyses [5–7]. Taylor et al. investigated solid growth
in increments of 10% in 85 patients in relation to myometrial invasion and LVSI. 20% was chosen as the delineation value that was found to have similar or better prognostic value than the 3-tiered grading system [7].
Several clinical issues of interest such as whether adju-vant therapy can be avoided in a subset of grade 2 or 3 patients with lower solid growth remain critical as further treatment with radiotherapy or combined-modality treatment have serious side effects. In this study, while solid growth > 75% was most significantly associated with majority of the adverse prognosticators such as LVSI, advanced stage, pelvic and para-aortic LNM, outer half myometrial invasion, adnexal involvement, ≥ 2 cm tumor size, this increment of solid growth did not portend poorer survival compared to subsets of lower solid growth (6–25%, 26–50%, 51–75%). Solid growth of 25–50% and 51–75% were significantly Table 4 Univariate and multivariate survival analyses by solid growth, LVSI, myometrial invasion in Stage I and II endometrioid endometrial cancer
p values in bold indicate statistical significance
LVSI lymphovascular space invasion, MI myometrial invasion, HR hazard ratio
No. of cases Univariate analysis Multivariate analysis
DSS RFS DSS RFS
Events (N) p Events (N) p HR (95% CI) p HR (95% CI) p
LVSI Absent 205 9 < 0.001 13 < 0.001 3.6 (1.2–10.0) 0.014 2.4 (1.1–4.8) 0.044 Present 19 5 7 Solid growth < 8% 170 4 < 0.001 7 < 0.001 7.2 (2.5–15.0) < 0.001 4.6 (1.7–11.6) 0.012 8–100% 54 10 13 MI < 50% 142 4 0.002 7 0.003 1.5 (0.7–3.6) 0.32 1.4 (0.6–2.7) 0.42 ≥ 50% 82 10 13
Table 5 Univariate and multivariate survival analyses by solid growth, LNM, tumor size in Stage III and IV endometrioid endometrial cancer
p values in bold indicate statistical significance HR hazard ratio
No. of cases Univariate Analysis Multivariate Analysis
DSS RFS DSS RFS
Events (N) p Events (N) p HR (95% CI) p HR (95% CI) p
LNM Absent 8 2 0.029 2 0.19 1.7 (1.1–2.9) 0.012 2.2 (0.4–10.1) 0.24 Present 37 8 8 Tumor size 1–11 cm 45 10 0.007 10 0.035 1.3 (1.0–1.7) 0.031 1.2 (0.9–1.6) 0.04 Solid growth < 8% 22 5 0.25 5 0.34 8–100% 23 5 5
associated with cervical involvement, extrauterine spread and positive peritoneal cytology, respectively and this may explain the lack of a significant difference in survival out-comes among patients with > 75%, 51–75% and 25–50% solid growth. These results are consistent with previous find-ings that showed no significant relationship between tumor grade and extrauterine spread [8]. Localization rather than grade could be associated with cervical extension, which is a predictor of extrauterine spread and is associated with poor survival.
In this study, the prognostic significance of absent (0%) solid growth, was analyzed separately, which to our knowl-edge was not attempted in previous studies. Absent solid growth was seen in the majority of patients and was most significantly associated with favorable outcomes on all clin-icopathological parameters assessed. A cut-off of 1% was found for predicting presence of LNM, advanced stage, LVSI and outer half myometrial invasion, suggesting that the existence of any solid growth may be indicative of the presence of adverse prognosticators. This is in line with a previous study showing that even a small degree (< 5%) of solid growth in moderately differentiated tumors had poorer outcome than moderately differentiated tumors without solid growth [9].
Furthermore, based on our results, a novel cut-off of 8% solid growth was found for most significantly predicting sur-vival and < 8% solid growth was associated with the most favorable RFS and DSS as there was no difference in sur-vival outcomes between 0 and 1–7% solid growth. As such, < 8% solid growth in endometrioid endometrial adenocar-cinoma may be regarded as a favorable prognosticator of survival. When grouped according to this cut-off value, the solid growth had higher prognostic significance than solid growth categorized into FIGO architectural grades or as sub-divisions of FIGO architectural grades, as reflected by the highest hazard ratio in multivariate survival analyses.
The long follow-up time and the relatively large number of patient samples that included high-grade tumors of endo-metrioid histology can be regarded as additional strengths of the study.
A limitation of the study was our inability to definitively explain for the inconsistency regarding the significant asso-ciation of > 75% solid growth with adverse prognostic fac-tors but not with adverse survival compared to patients with lower subsets of solid growth. In addition, while 1% solid growth was the significant cut-off value for predicting the presence of LNM, advanced stage, LVSI and outer half myo-metrial invasion, the cut-off for predicting adverse survival was 8% and we failed to explain for the differences in these cut-off values.
Another limitation of the study includes our inability to correlate these findings on a molecular level and test for certain molecular mutations that may have a role in the
progression of endometrial tumors. For example, among patients with grade 3 endometrioid endometrial cancer, those carrying POLE exonuclease domain mutations did not have disease progression while about 33% of patients with wild type POLE exonuclease domain experienced dis-ease progression [10]. In addition, the presence of POLE exonuclease domain mutations was a significant prognostic parameter for progression-free survival [10, 11].
Although > 75% solid growth was most significantly associated with many of the adverse prognostic factors, this subset did not provide prognostic superiority in predicting adverse survival when compared to subsets within 6–75% solid growth. In fact, solid growth of ≥ 8% was found to carry the highest significance for prognostic impact on adverse survival. As such, ≥ 8% solid growth should war-rant vigilance in the follow-up of patients with endometrioid endometrial cancer. In conclusion, although no statistically significant difference in survival was found among subdivi-sions of architectural grades 2 and 3, solid growth, especially ≥ 8%, appeared to be an independent prognostic factor for survival in patients with early-stage endometrioid endome-trial cancer.
Author contributions SA: data gathering, data analysis, writing of manuscript. EÇ: data gathering, data analysis. SF: data gathering, data analysis. TTİ: data gathering. FE: data gathering. ÇÇ: supervision, contributed to the writing of manuscript.
Compliance with ethical standards
Conflict of interest S. Akar declares no conflict of interest. E. Çelik declares no conflict of interest. S. Fındık declares no conflict of inter-est. T. T. İlhan declares no conflict of interinter-est. F. Ercan declares no conflict of interest. Ç. Çelik declares no conflict of interest.
Ethical approval This article does not contain any studies with human participants or animals performed by any of the authors. This was a retrospective study of paraffin-blocks and was approved by the Institu-tional Research Ethics Committee.
References
1. WHO Classification of tumours of the female reproductive organs, 4, Kurman RJ, Carcangiu ML, Herrington CS, Young RH (eds) (2014) World Health Organization. pp 126, 150
2. Siegel RL, Miller KD, Jemal A (2017) Cancer statistics, 2017. CA Cancer J Clin 67:7
3. Shepherd JH (1989) Revised FIGO staging for gynaecological cancer. Br J Obstet Gynaecol 96:889–892
4. Pecorelli S (2009) Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet 105:103 5. Scholten AN, Smit VT, Beerman H et al (2004) Prognostic
signifi-cance and interobserver variability of histologic grading systems for endometrial carcinoma. Cancer 100(4):764–772
6. Lax SF, Kurman RJ, Pizer ES et al (2000) A binary architectural grading system for uterine endometrial endometrioid carcinoma
has superior reproducibility compared with FIGO grading and identifies subsets of advance-stage tumors with favorable and unfavorable prognosis. Am J Surg Pathol 24(9):1201–1208 7. Taylor RR, Zeller J, Lieberman RW et al (1999) An analysis of
two versus three grades for endometrial carcinoma. Gynecol Oncol 74(1):3–6
8. DiSaia PJ, Creasman WT, Boronow RC et al (1985) Risk factors and recurrent patterns in Stage I endometrial cancer. Am J Obstet Gynecol 151(8):1009–1015
9. Alm P, Gudmundsson T, Mårtensson R et al (1995) Identifica-tion of small areas of solid growth has a strong prognostic impact in differentiated endometrial carcinomas. A histopathologic and morphometric study. Int J Gynecol Cancer 5(2):87–93
10. Meng B, Hoang LN, McIntyre JB et al (2014) POLE exonuclease domain mutation predicts long progression-free survival in grade
3 endometrioid carcinoma of the endometrium. Gynecol Oncol 134(1):15–19. https ://doi.org/10.1016/j.ygyno .2014.05.006
11. Billingsley CC, Cohn DE, Mutch DG et al (2016) Prognostic sig-nificance of POLE exonuclease domain mutations in high-grade endometrioid endometrial cancer on survival and recurrence: a subanalysis. Int J Gynecol Cancer 26(5):933–938. https ://doi. org/10.1097/IGC.00000 00000 00068 1
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.