environmental toxicology and pharmacology 39 (2015)787–793
Available
online
at
www.sciencedirect.com
ScienceDirect
j ou rn a l h o m e p a g e : w w w . e l s e v i e r . c o m / l o c a t e / e t a p
Deferasirox-induced
cytogenetic
responses
Mehmet
Arslan
a,∗,
Hasan
Basri
Ila
baArdahanUniversity,SchoolofHealthSciences,DepartmentofNursing,75000Ardahan,Turkey bCukurovaUniversity,FacultyofScienceandLetters,DepartmentofBiology,01330Adana,Turkey
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received4November2014
Receivedinrevisedform
4February2015
Accepted5February2015
Availableonline16February2015
Keywords: Deferasirox Ironchelator Genotoxicity Cytotoxicity Invitro Invivo
a
b
s
t
r
a
c
t
Deferasirox(commerciallyformulatedasExjade®)isoneoftheeffectiveironchelatorsused
intreatmentofironoverloaddiseases.Inthisstudytheeffectofthissubstancefor
chromo-someaberration,sisterchromatidexchangeandmitoticindexwasstudiedbyinvitro(by
usinghumanperipherallymphocytes)andinvivo(byusingrat)analysis.
Deferasiroxincreasedthesisterchromatidexchangefrequencyinalltested
concentra-tionsandperiodsinvitro.Also,inthepresenceofmetabolicactivator,thesubstanceledto
astatisticallysignificantincreaseinthesisterchromatidexchangefrequenciesonlyathigh
concentration.Whileininvitroanalysisthesubstancesignificantlyincreasedabnormal
cellpercentagesinallconcentrations,ininvivostudythesubstanceincreased
chromo-someaberrationsonlyintwoconcentrationsat12htreatment.Intheculturedlymphocytes,
deferasiroxshowedcytotoxicitybysignificantlyreducingproliferationindexandmitotic
indexvalues.Whileinthepresenceofmetabolicactivationitdidnotaffectthe
prolifera-tionindexfrequency,ithadastimulanteffectonthemitoticindexfrequency.Deferasirox
reducedsignificantlythemitoticindexvalueinthebonemarrowcellsespeciallyinhigh
concentrationandshorttreatmentperiod(12h).
©2015ElsevierB.V.Allrightsreserved.
1.
Introduction
Theironoverloadinhumantissuesemergesdueto
hered-itary reasons or chronic blood transfusions (transfusional
hemosiderosis).Asaconsequence,lossofcellviabilityand/or
metabolicdysfunctionsoccur(Pietrangelo,2003).Ifthis
con-ditionisnotdiagnosedandtreatedtimely,certainliver,heart
andendocrineglandsdiseasesmaydevelop(Andrews,1999).
Thereductionofironloadinthetissueprotectsindividual
against having heart diseases, diabetes mellitus, or
pre-ventsprematuredeathinthalassemia patients(Brıttenham
etal.,1994).Thephlebotomymethod(AssiandBaz,2014)or
ironchelating agentsare usedfor removingexcessive iron
∗ Correspondingauthor.Tel.:+904782112687/5136;fax:+904782112973.
E-mailaddresses:mehmetarslan@ardahan.edu.tr,marslan76@gmail.com(M.Arslan).
accumulatinginthetissuesofhemochromatosispatients.The
firstoneofthreetypesofchelatorsfrequentlyusedfor
remov-ingironisdeferoxamine(DFO)thatisappliedintravenously.
Deferiprone,whichisanotheragentusedforsamepurpose,
istakenorally.Deferasirox,whichisaneworalchelator,is
thethirdtypetakenorallyandusedeasilyandeffectivelyfor
controllingtheironload.Deferasiroxhashighspecific
affin-ityforiron.Allpatientsabove2yearssufferingfromsevere
chronicironoverloadcanusedeferasiroxfortherapy(Wang
etal.,2010).
Despiteoftheworthyadvantagesofchelators,their
muta-genic potentials must not be ignored because someother
chelatorssuchasDFOhasbeen showntohaveheavytoxic
andmutagenicpotential(Whittakeretal.,2001).Also,itwas
http://dx.doi.org/10.1016/j.etap.2015.02.001
determinedthatDFOwasnotclastogenicbyitself,butalong
withgamma raysincreasedacentricchromosomefragment
and ring chromosome formation frequency (Juckett et al.,
1998).Inanotherstudy,itwasfoundthatdeferipronecause
dchromosomefragment(Marshall etal.,2003).Tothe
con-trary of the above-mentioned findings, it was shown that
deferiproneconsiderablydecreasedtheDNAdamage
result-ingfromironaccumulation(Andersonetal.,2000).
Althoughdeferasiroxhasmanybenefit–costadvantagesin
treatment,thereisonlyourpreviousinvestigationaboutits
genotoxicnature.Deferasiroxhasshowedgenotoxicand
cyto-toxiceffectsinsomecellsystemsaccordingtoresultsofthat
study(Ilaetal.,2014).Therefore,inthecurrentstudy,human
peripherallymphocyteswere exposedtothe testsubstance
indifferentconcentrations inthe absenceand presenceof
metabolicactivator.Itwasattemptedtoinvestigategenotoxic
andcytotoxiceffectofthesubstancebyinvitrosister
chro-matidexchange(SCE)tests,chromosomeaberration(CA)tests,
alongwithinvivochromosomeaberration(CA)testsonrat
bonemarrowcells.
2.
Materials
and
methods
The test substance of the present study was deferasirox
(Exjade®,obtainedfromapharmacyinAdana/Turkey)(CAS
No.:201530-41-8).Nonmedical ingredientsinExjade®tablet
are:lactose, crospovidone,povidone,sodium laurylsulfate,
microcrystallinecellulose,silica-colloidalanhydrous,
magne-siumstearate(Exjade®–ConsumerMedicineInformation).In
thestudy,short-termgenotoxicitytestswereperformedon
culturedhumanperipherallymphocytesunderinvitroand
invivoconditionswithrat.Sincethetestsubstancewasnot
absolutelywater-soluble,dimethylsulfoxide(DMSO)(CASNo.:
67-68-5)wasusedasasolvent.Thecellsusedfor
quantita-tiveanalyseswereexposedtothetestsubstanceinvarious
concentrationsindifferentperiods,andtheresultswere
com-paredwiththeirowncontrols.OECDguidelinewasconsidered
todoseselectionforinvitrostudies(OECDTG473,paragraph
21,2012).Deferasiroxconcentrationsforinvivo studywere
selectedbasedonitsoralLD50forrats(≥1000mg/kg).
2.1. Material
This study was approved by the Ethics Committee of
the Cukurova University Medical Sciences, Experimental
Research,andApplicationCentre(no.:11,date:2July2010).In
thepresentstudy,Exjade®(itsactivesubstanceisdeferasirox),
whichisan ironchelatingdrug, wasused asthetest
sub-stance,and peripheralblood takenfrom twonon-smoking,
healthy,andvoluntarywomenandtwonon-smoking,healthy,
and voluntary men ofthe same age (23) was used as the
material for in vitro tests. Four healthy 10 to 12 week
rats (two females+two males) were used in in vivo tests.
Colchicine (SigmaC9754), mitomycin-C MMC(CAS No.:
50-07-7),colchicine(3mg/kgb.w.,SigmaC9754),Urethane(ethyl
carbamate;EC;CASNo.:51-79-6)andchromosomemedium
(Pb-Max,Gibco,cat.no.:12557-013)wereusedinthisstudy.
2.2. Method
2.2.1. InvitroCAandSCEassay
HumanperipherallymphocytesweresubjectedtoCAandSCE
teststoinvestigatethegenotoxiceffectsofdeferasirox(aniron
chelator)anddeferasiroxmetabolite.
Wholeblood(0.2mL)fromfourhealthydonors(twofemale
andtwomale,non-smokers,aged23years,bloodsamplesnot
pooled) wasadded to2.5mLchromosomemedium
supple-mentedwith10g/mLsterile5-bromo-2-deoxyuridine(BrdU).
Themediumcontainedphytohaemagglutinin(PHA)for
stim-ulatingofthecellproliferation.Thecultureswereincubated
totaloftime72hat37◦Casfollowing:
The blood culture was treated by 10, 20 and 40mg/kg
concentrationsofdeferasiroxdissolvedindimethylsulfoxide
(DMSO),for24h(deferasiroxwasaddedat48hafterinitiation
timeofincubation)and 48h(deferasiroxwasaddedat24h
afterinitiationtimeofincubation).
Colchicine (0.06g/mL) wasadded to the cultureat the
last 2h (70thh) of incubation time for arrest cell cycle at
metaphase. Thenthe cultures were centrifuged (2000rpm,
5min)andtreatedwithhypotonicsolution(0.4%KCl)for5min
at37◦C,andfixedincoldmethanol:glacialaceticacid(3:1)
for15minatroomtemperature.Treatmentwithfixativewas
repeatedthreetimes,andthenthecellswerespreadonglass
slidesandair-dried.
InordertoCAanalysisthepreparationsweresubjectedto
stainingwith5%Giemsafor24h.Also,forstudyofSCEthe
Flu-orescencePlusGiemsa(FPG)methoddevelopedbySpeit(1984)
andSpeitandHauper(1985)wasused.DMSO(10L/2.7mL)
andMitomycin-C(0.25g/mL)wasconsideredasanegative
controlandapositivecontrol,respectively.DMSOisagood
solventthatdidnotinduceCA.
InordertostudyofdeferasiroxmetaboliteonCAandSCE,
thecellculturetreatedwithdeferasiroxalongwithS9mix
(3-methylcolanthrene-inducedratliversubcellularenzymes).To
mimicinvivoconditions,exogenousmetabolizingsystemwas
usedforemployingcellswhichhaveinadequateendogenous
metaboliccapacity.Inthisway,thepossiblecontributionof
deferasiroxmetabolismonitsgenotoxicitycanbedisplayed
(MaronandAmes,1983).Forthispurpose,thethree
concen-trationsoftestsubstance(10,20and40mg/kg)togetherwith
S9mix(0.5mL)wereaddedthecultureat48thhofthe
incuba-tiontime.After3htreatment,theculturewaswashedtwice
foreliminatingoftheS9mixfromculture.Followingthelast
washing,BrdU-supplementedfreshmediumwasaddedinto
the cells inthetube, andthe cellswere incubated for19h
at37◦C (untilthe70thhofincubationtime).Afterthatthe
cells were treatedbycolchicine,centrifuged and continued
theprocessmentionedabovetostudyofCAandSCE.
2.2.2. Invivoassay
TheinvivotestwasperformedaccordingtoTopaktasetal.
(1996). Deferasirox concentrations for in vivo study were
selectedbasedonitsoralLD50forrats(≥1000mg/kg).Forthis
purposetwogroupsofrats(twofemales+twomales;fourrats
intotal)were treatedbythreeconcentrationofdeferasirox
(250,500,and1000mg/kg)viaoralgavage.Bonemarrowcells
were taken out from one group after 12h treatment and
environmental toxicology and pharmacology 39 (2015)787–793
789
colchicine (3mg/kg) was injected intraperitoneally for one
groupat10thtreatmenttimeandforanotherat22nd
treat-menttime(2hbeforetheanimalsweresacrificed).
Afterthat,theanimalsweresacrificedbycervical
disloca-tion,andthefemurswerestrippedproximally,andthebone
marrowcellswereaspiratedin0.9%NaCl(37◦C).Theobtained
suspensionwascentrifuged for5minat2000rpm,and the
bonemarrow pelletwas resuspended in0.4% KCl at 37◦C
for10min,thenthecellswerefixedincoldmethanol:glacial
aceticacid(3:1)for20minatroomtemperature.Thetreatment
withfixativewasrepeatedtwice.Thenthecellswerespread
onglass slidesand air-dried.The slideswere stained with
Giemsa(5%inSorensenbuffer)for15minand100well-spread
metaphasesperanimal(atotalof400metaphasespergroup)
weresubjectedtostudyofstructuralandnumericalCAsby
1000×magnification.MI(mitoticindex)wasalsodetermined
byscoring3000cellsfromeachanimal.
Untreatedanimalgroup(asanegativecontrol),treatedby
DMSO(asasolventgroup),andtreatedbyethylcarbamate(as
positivecontrol)werestudiedinthisresearch.
2.3. Microscopicinvestigationandstatisticalmethod
TheCAwasclassifiedaccordingtotheinternationalsystem
forhumancytogeneticnomenclature(ISCN)(Paz-y-Minoetal.,
2002).Ahundredwell-spreadmetaphasesperdonor(atotalof
400metaphasesperconcentrations)wereexaminedat1000×
magnificationfortheoccurrencesoftheCA.Gapswere not
countedasCA,accordingtoMaceetal.(1978).SCE scoring
wascarried out accordingtoAlbertiniet al.(2000). For the
numberofSCEs,atotalof100cells(25cellsfromeachdonor)
undersecondmetaphaseswereexamined.Theresultswere
usedtodetermine the mean numberofSCEs (SCE/cell).In
addition,atotalof400cells(100cellsfromeachdonor)were
scoredforthedeterminationofthereplicationindex(RI).The
mitoticindex(MI)wasalsodeterminedbyscoring3000cells
fromeachdonor.TheMIexplainstheeffectsofthe
chemi-calsontheG2stageofthecellcycle,andtheRIreflectsthe
effectsofthechemicalsontheSandG2stagesofthecycle.
TheRI was calculated according tothe followingformula:
RI=[(M1×1)+(M2×2)+(M3×3)]/totalscoredcells,whereM1,
M2,and M3are the fractionofcells undergoingtheir first,
second,andthirdmitosisduringthe72-hcellcultureperiod.
Thesignificanceofdifferencesbetweenthe mean SCEs,
RI, MI, structural, numerical, and total CAsin treated
cul-tures and their controls were determined using the t-test.
Dose–responserelationshipsweredeterminedfromthe
cor-relationandregressioncoefficientsforthemeanSCEs,RIand
MI,structural,numerical,andtotalCAs.
3.
Results
3.1. Invitrosisterchromatidexchange(SCE)
AlthoughallconcentrationsincreasedtheSCEfrequencyin
the24htreatmentofthedeferasirox,theSCEfrequencywas
foundtobesignificantlyhigherincomparisontothecontrol
(P<0.05),onlyinthelowestconcentration(10g/mL).
In the48h treatment, allthe usedconcentrations (even
thelowestconcentration)significantlyincreasedtheSCE
fre-quencyincomparisontothecontrol(Table1).
Itwasdeterminedthatwhentheculturedlymphocytecells
wereexposedtothedeferasiroxinthepresenceofmetabolic
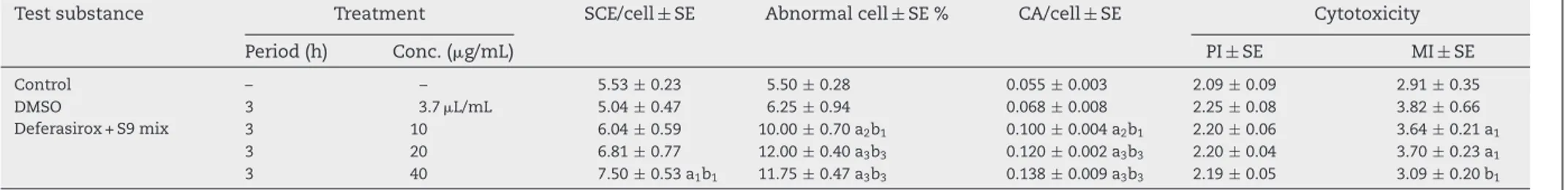
activator,onlythehighestconcentrationledtoSCEincreases,
andtheincreasesweresignificantincomparisontothe
nega-tivecontrolandthesolventcontrol(P<0.05)(Table2).
3.2. Invitrochromosomeaberration(CA)
Inthe24happlication,deferasiroxincreasedCAsinallthree
concentrations,buttheabnormalityincreasedeterminedin
highconcentrations(20and40g/mL)wasstatistically
signif-icant(Table1).Inthe48happlication,theCAsformationsinall
dosesinvestigatedwerefoundsignificantlyhigherin
compar-isontothenegativecontrol.Inthisapplication,twoincreases
inhighdosewerefoundsignificantlyhigherincomparisonto
thesolventcontrol(P<0.05)(Table1).Thetestsubstanceinthe
presenceofexogenousmetabolicactivator(S9mix)
demon-stratedimportantgenotoxiceffectsinallconcentrations.The
observedCAfindingsweresignificantlyhigherincomparison
tobothcontrols(P<0.01orP<0.001).Theratesof
abnormal-itypercellshowedsimilaritieswiththeabnormalcellrates
(Table2).
3.3. Invivochromosomeaberration(CA)
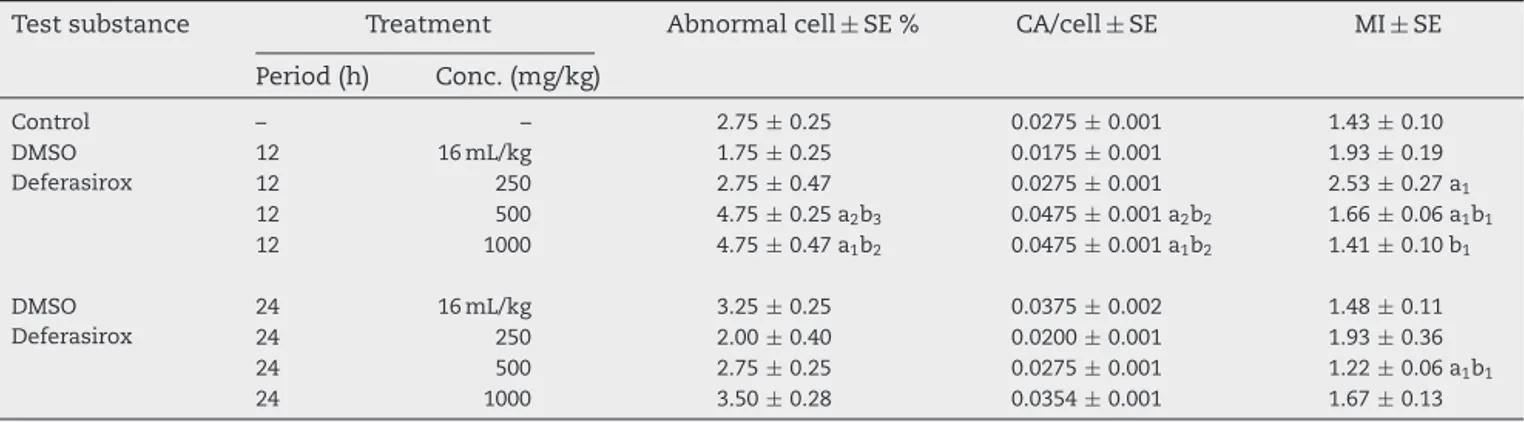
In in vivo study, two high deferasirox concentrations (500
and 1000mg/kg) used in treatment of rats caused
signifi-cantchromosomeaberrationsat12hoftreatment(Table3),
Whilethelowestconcentrationofthesubstancedidnotshow
anysignificanteffectonchromosomeaberration.The
high-estconcentrationofdeferasiroxcausedtopartialincreaseof
chromosomeaberrationat24htreatment,buttheeffectwas
notstatisticallysignificant.
3.4. EffectofdeferasiroxonDNAreplicationand mitosis
IninvitroteststhedeferasiroxshowedasignificantPI
(pro-liferationindex) reductioninallconcentrations inthe 48h
treatment, though in the 24htreatment, all concentration
exceptthelowestonedecreasedthereplicationrate
signifi-cantlyincomparisontothenegativecontrolandthesolvent
control(P<0.05)(Table1).However,deferasiroxdidnotshow
anysignificantincreaseinthePIvalueswhenitwasusedalong
withmetabolicactivator(S9mix)(Table2).
Thedeferasiroxhadaninhibiting effectonmitotic
divi-sion(MI).In24htreatmentofcellculture,themitoticindexes
decrease obviously and significantly. Thebiggestreduction
occurredinthehighestconcentration.AlthoughalloftheMI
reductionsoccurringinthatperiodweresignificantin
com-parison tothe negativecontrol, the MI falling inthe high
concentrationwassignificantincomparisontoboththe
con-trol and the solventcontrol.In the48happlicationin this
test,significantdecreasesoccurredinMIvaluesinallofthe
appliedconcentrationsincomparisontothecontrolandthe
solventcontrol(Table1).Asdistinctfromtheprevioustests,
e n v i r o n m e n t a l t o x i c o l o g y a n d p h a r m a c o l o g y 3 9 ( 2 0 1 5 ) 787–793
Table1–Theinvitrosisterchromatidexchange,abnormalcellpercentage,CA/cellratio,proliferationindex(PI),andmitoticindex(MI)valuescausedbythedeferasirox inthehumanperipherallymphocytesintheabsenceofmetabolicactivator(S9mix).
Testsubstance Treatment SCE/Cell±SE Chromosomeaberration Cytotoxicity
Period(h) Conc.(g/mL) Abnormalcell±SE% CA/cell±SE PI±SE MI±SE
Control – – 5.06±0.23 3.75±0.47 0.037±0.002 2.28±0.10 3.69±0.26 DMSO 24 3.7L/mL 5.46±0.36 9.50±0.64 0.095±0.003 2.22±0.10 3.25±0.27 Deferasirox 24 10 6.45±0.34a1 5.00±0.70b2 0.050±0.001b2 2.26±0.04 2.84±0.16a1 24 20 5.62±0.46 16.25±1.18a2b1 0.172±0.004a2b1 2.05±0.03a2b1 2.78±0.19a1 24 40 6.43±0.46 18.75±0.85a3b2 0.190±0.004a2b2 1.90±0.09a1b1 1.83±0.17a2b2 DMSO 48 3.7L/mL 4.99±0.23 7.25±0.75 0.070±0.003 2.51±0.08 3.84±0.38 Deferasirox 48 10 6.33±0.21a2b1 10.50±1.32a1 0.105±0.002a1 2.31±0.03b1 2.83±0.22a1b1 48 20 6.56±0.45a1 13.25±1.31a2b1 0.132±0.002a2b1 1.63±0.06a2b2 1.51±0.17a3b3 48 40 8.18±0.66a1 22.00±2.86a2b1 0.270±0.008a2b1 1.37±0.11a2b2 0.99±0.10a3b3
a:significantfromcontrol;b:significantfromsolventcontrol(DMSO).a1b1:P≤0.05;a2b2:P≤0.01;a3b3:P≤0.001.
Table2–Theinvitrosisterchromatidexchange,abnormalcellpercentage,CA/cellratio,proliferationindex(PI),andmitoticindex(MI)valuescausedbythedeferasirox inthehumanperipherallymphocytesinthepresenceofmetabolicactivator(S9mix).
Testsubstance Treatment SCE/cell±SE Abnormalcell±SE% CA/cell±SE Cytotoxicity
Period(h) Conc.(g/mL) PI±SE MI±SE Control – – 5.53±0.23 5.50±0.28 0.055±0.003 2.09±0.09 2.91±0.35 DMSO 3 3.7L/mL 5.04±0.47 6.25±0.94 0.068±0.008 2.25±0.08 3.82±0.66 Deferasirox+S9mix 3 10 6.04±0.59 10.00±0.70a2b1 0.100±0.004a2b1 2.20±0.06 3.64±0.21a1 3 20 6.81±0.77 12.00±0.40a3b3 0.120±0.002a3b3 2.20±0.04 3.70±0.23a1 3 40 7.50±0.53a1b1 11.75±0.47a3b3 0.138±0.009a3b3 2.19±0.05 3.09±0.20b1
environmental toxicology and pharmacology 39 (2015)787–793
791
Table3–Theinvivoabnormalcellpercentage,CA/cellratio,andmitoticindex(MI)valuescausedbythedeferasiroxin thebonemarrowcellsofrats.
Testsubstance Treatment Abnormalcell±SE% CA/cell±SE MI±SE
Period(h) Conc.(mg/kg) Control – – 2.75±0.25 0.0275±0.001 1.43±0.10 DMSO 12 16mL/kg 1.75±0.25 0.0175±0.001 1.93±0.19 Deferasirox 12 250 2.75±0.47 0.0275±0.001 2.53±0.27a1 12 500 4.75±0.25a2b3 0.0475±0.001a2b2 1.66±0.06a1b1 12 1000 4.75±0.47a1b2 0.0475±0.001a1b2 1.41±0.10b1 DMSO 24 16mL/kg 3.25±0.25 0.0375±0.002 1.48±0.11 Deferasirox 24 250 2.00±0.40 0.0200±0.001 1.93±0.36 24 500 2.75±0.25 0.0275±0.001 1.22±0.06a1b1 24 1000 3.50±0.28 0.0354±0.001 1.67±0.13
a:significantfromcontrol;b:significantfromsolventcontrol(DMSO).a1b1:P≤0.05;a2b2:P≤0.01;a3b3:P≤0.001.
influenceoftheexogenousmetabolicactivator(P<0.05). How-ever,theMI valuedetermined inthe highestconcentration herewasstatisticallylowerincomparisontothesolvent con-trol(P<0.05)(Table2).
In the in vivo tests, the deferasirox had heterogeneous
effectsonmitoticdivisionofbonemarrowcells.Inthe12h
application,thedeferasiroxledtosignificantMIdecreasesin
highdosesincomparisontothesolventcontrol.Inthe24h
application,the testsubstancedecreasedthemitoticindex
significantly incomparison to the control and the solvent
controlonlyinoneconcentration (500mg/kgb.w.)(P<0.05)
(Table3).
Inaddition,itwasdeterminedthatthedifferences
occur-ringincytotoxicityvaluesasaresultofincreaseindosewere
notstatisticallysignificant(P>0.05).
Tosumupthepropertiesofthedeferasiroxregarding
cyto-toxicity,itsloweddownreplicationanddivisionratesinthe
in vitro tests when no metabolic activator was used, but
increasedthem,thoughslightly, incomparisontothe
con-trolintheteststhatcontainedmetabolicactivator.Although
fluctuationswereobservedinMIvaluesintheinvivotests,
thevalueswereparallelwiththeresultsoftheinvitrotests
includingS9mix.
4.
Discussion
4.1. Clastogeniceffectofdeferasirox
There are limited studies about genotoxic effects of iron
chelators. Manystudies based ondifferent testingsystems
reportedthatthedeferoxamin(DFO),amemberofiron
chela-tors including deferasirox, removed the metal in the cell,
and thus had an antigenotoxic effect on the DNA
frag-mentsderiving from hydroxylradical occurringasa result
ofFenton/Haber–Weissreaction(Cooganetal.,1986;Stinson
etal.,1992;Bealletal.,1996;Zhangetal.,1996;Andersonetal., 2000;Witteetal.,2000;Chakrabartietal.,2001).Tothe
con-traryofthesefindings,astudyindicatedthatDFOwerehighly
toxicandmutagenicirrespectiveofthepresenceorabsence
ofS9inL5178Yratlymphomacells (Whittakeretal.,2001).
Itwasfoundthatdeferipronedidnothaveasmuch
clasto-geniceffectasDFOhad,andledtochromosomefractionless
frequentlythandeferoxamine(Marshalletal.,2003).In
addi-tion,itwasdeterminedthatDFOwasnotclastogenicbyitself,
butincreasedacentricfragmentandringchromosome
forma-tionfrequencyalongwithgammarays(Juckettetal.,1998).
In general, iron chelators remove the excessive iron in
the cell, and thus minimize iron-mediated hydroxyl
radi-cal formation reactions, which reduce the DNA fragments
and proliferative effects caused by the said radical.
How-ever, infew studiesit wasreported thatthe ironchelators
were genotoxic (Juckett et al., 1998;Whittaker et al., 2001;
Marshalletal.,2003).Accordingtotheresultsofthecurrent
study,thedeferasiroxgenerallyincreasedtheSCEfrequency.
Theseincreasesoccurredinthe48htreatmentperiodwithout
metabolicactivatorandathighconcentrationswithmetabolic
activator.Theseresultssuggestthatthetestsubstanceleads
toDNA fragments.ThehighCAfrequenciesemerging
irre-spectiveofthepresenceofmetabolicactivatorsupportthis
theory,too.However,CAvaluesbecameevidentinratbone
marrowcellsinacellcycle(12h),butinthe24hprocess,the
CAsdisappeared.Itcanbeexplainedbyeitherthatthetest
substanceeliminatedfromthebodythroughmetabolization
(thehalf-lifeofthedrugis8–16hfollowingtheoral
applica-tion)orthattheabnormalcellsunderwentselection.
4.2. Cytotoxiceffectofdeferasirox
Accordingtotheproliferationindex(PI)data,thedeferasirox
decreased the replication rate in the absence ofmetabolic
activation most probably as a result of genotoxicity, but
the decrease in the PI value disappeared in the presence
ofthe metabolic activation.Metabolic activation decreased
theantiproliferativeeffectofdeferasirox.Wethinkthatthe
decrease in the replication rate was a slowing down
aris-ing from DNA damage. In connection with this situation,
mitotic index wasdecreased. A similar situation was seen
in thefollowing study (Sedigh-Ardekani et al., 2013). In all
concentrations and application periods of deferasirox, the
mitoticindexesdecreasedconsiderably.Suchslowingdownis
stronglyconsistentwiththeCAresults.However,these
find-ingschangedsomewhatinthetestcarriedoutwithS9mix.In
invivotest,MIvaluesexhibitinverseproportiontotheCA
for-mations.Theseresultsareparallelwiththeresultsofthe
of chelators. For example, deferiprone functioned as an
antioxidantinastudyonhumanumbilicalveinendothelium
cellsunderinvitroconditions,andthusitwassuggestedthat
itmightreduceatherogenesis(Matthewsetal.,1997).
ItwasfoundthattheapplicationofironsalttotheKaposi
sarcomacell culturesstrongly stimulated the development
inthesaidcells.Thestimulated growthwasinhibitedwith
deferiprone and DFO inthe same culture(Simonart et al.,
1998).
Itwas foundthatsuchchelatorsas1,10-phenanthroline
(OP),desferrioxaminemesylate,and deferipronehada
pro-tectiveeffectagainstthetoxicitycausedbythecompoundof
9,10-phenanthraquinone(PQ)(maincomponentinthediesel
exhaustparticles)inhumanlungepithelialcells.Here,PQled
toiron-mediated oxidative damage(Sugimoto et al., 2005).
Whenthe antiproliferativeeffectsofsuchironchelatorsas
DFXandO-trensoxonhumanhepatocarcinomacelllineand
humanhepatocyteculture were compared, the deferasirox
wasfoundtoplayamoreactiverolethanO-trensoxinthat
itinducedtheDNAfragmentation,inhibitedDNAreplication,
and reduced cell vitality. Itwas reported that these
chela-torsblockaded thecell cycleatthe phasesofG0–G1andS
respectively. Based on theseresults, it was suggestedthat
thedeferasiroxmighthaveaverystrongantitumoraleffect
incancertreatment(Chantrell-Groussardetal.,2006).
Simi-larly,inanotherstudy,itwasstatedthattheprogressofthe
cellcycledependedonintracellularironlevel,andthe
chela-torsreducedcellproliferationthroughremovingiron.Itwas
mentionedthatsuchantiproliferativeeffectcouldbeinhibited
inthe presence ofexogenous iron(Pires et al., 2006). DFO
activatedp53-mediated check points inthe cultured blood
lymphocytes, and stimulated apoptosis in human
periph-eralbloodlymphocytesviamitochondriadamage(Kimetal.,
2007).
Apartfromthat,theHIV-1replicationinducedbyexcessive
ironisinhibitedbyDFObecausethechelatorpreventsthe
pro-liferationofthevirusthroughremovingtheironintheinfected
cells.Itisstronglyrecommendedtofocusonthedevelopment
ofironchelatorsforanti-retroviraldrugsinthefuture(Debebe
etal.,2007).Anotherstudyreportedthatsuchironchelatorsas
DFO,deferasirox,andciclopiroxolamineblockedWntsignal
andcelldevelopmentinthecolorectalcancercellline(Song
etal.,2011).
Accordingtoliterature,DFXismostprominentiron
chela-torascomparedwithotherones.However,thereisnotenough
informationonthegenotoxicprofileofthedeferasirox.Tofill
thisgap,thesetestswereperformedandimportantfindings
wereobtained.Amongsuchfindings,themostimportantone
isthatthedeferasiroxhasaclastogeniccharacter.Apartfrom
that,theagentledtoevidentreductionsinPIandMIvaluesin
parallelwiththegenotoxicityvalues.Basedontheseresults,it
canbesaidthatthetestsubstanceexhibitedan
antiprolifera-tivecharacterassociatedwithgenotoxicity.Thischaracterwas
similarwithsomechemicalagents(mitomycinC,bleomycin,
etc.)usedinthecancertreatment.Itmustberememberedthat
adrugmaytreatthetargetdiseaseononehand,butstimulate
genotoxiceffectsontheotherhand.Althoughsomeimportant
findingswereobtainedinthepresentstudy,thedeferasirox
shouldalsobetestedthroughothertestingsystemsandalong
withtumorcellsinparticular.
Whiledeferasiroxisusedasachelator,owingto
antipro-liferativeeffect, it canbeagoodcandidatetotreatmentof
tumors.
Conflict
of
interest
Theauthorsdeclarethattherearenoconflictsofinterest.
Transparency
document
TheTransparencydocumentassociatedwiththisarticlecan
befoundintheonlineversion.
Acknowledgements
WewishtothanktheCukurovaUniversityScientificResearch
Commissionforsupportingourstudythroughprojectgrants
no.FEF2010D11.WewouldliketothankEbrahimValipourfor
helpingtoeditourmanuscriptlanguagecarefully.
r
e
f
e
r
e
n
c
e
s
Albertini,R.J.,Anderson,D.,Douglas,G.R.,Hagmar,L.,Hemminki,
K.,Merlo,F.,Natarajan,A.T.,Norppa,H.,Shuker,D.E.G.,Tice,
R.,Waters,M.D.,Aitio,A.,2000.IPCSguidelinesforthe
monitoringofgenotoxiceffectsofcarcinogensinhumans. Mutat.Res.463,111–172.
Anderson,D.,Yardley-Jones,A.,Vives-Bauza,C.,Chua-Nusorn,
W.,Cole,C.,Webb,J.,2000.Effectofironsalts,haemosiderins,
andchelatingagentsonthelymphocytesofathalassaemia patientwithoutchelationtherapyasmeasuredinthecomet assay.Teratog.Carcinog.Mutagen.20,251–264.
Andrews,N.,1999.Disordersofironmetabolism.N.Engl.J.Med.
342,364.
Assi,T.B.,Baz,E.,2014.Currentapplicationsoftherapeutic
phlebotomy.BloodTransfus.12,75–83.
Beall,H.D.,Liu,Y.,Siegel,D.,Bolton,E.M.,Gibson,N.W.,Ross,D.,
1996.RoleofNAD(P)H:quinoneoxidoreductase
(DT-diaphorase)incytotoxicityandinductionofDNAdamage bystreptonigrin.Biochem.Pharmacol.51,645–652.
Brıttenham,G.M.,Griffith,P.M.,Nienhuis,A.W.,Mclaren,C.E.,
Young,N.S.,Tucker,E.E.,Allen,C.J.,Farrell,D.E.,Harris,J.W.,
1994.Efficacyofdeferoxamineinpreventingcomplicationsof
ironoverloadinpatientswiththalassemiamajor.N.Engl.J. Med.331,567–573.
Chakrabarti,S.K.,Bai,C.,Subramanian,K.S.,2001.DNA–protein
crosslinksinducedbynickelcompoundsinisolatedrat lymphocytes:roleofreactiveoxygenspeciesandspecific aminoacids.Toxicol.Appl.Pharmacol.170,153–165.
Chantrell-Groussard,K.,Gaboriau,F.,Pasdeloup,N.,Havouıs,R.,
Nick,H.,Pierre,J.L.,Brissot,P.,Lescoat,G.,2006.Thenew
orallyactiveironchelatorICL670Aexhibitsahigher antiproliferativeeffectinhumanhepatocyteculturesthan
O-trensox.Eur.J.Pharmacol.541,129–137.
Coogan,T.P.,Osenblum,I.Y.,Barsotti,D.A.,1986.
Bleomycin-inducedDNA-stranddamageinisolatedmale germcells.Mutat.Res.162,215–218.
Debebe,Z.,Amosova,T.,Jerebtsova,M.,Kurantsin-mılls,J.,Nıu,
X.,Charles,S.,Richardso,D.R.,Ray,P.E.,Gordeuk,V.R.,Nekhai,
S.,2007.IronchelatorsICL670and311inhibitHIV-1
environmental toxicology and pharmacology 39 (2015)787–793
793
Exjade®–ConsumerMedicineInformation
(www.novartis.com.au/CMIPDF/exj.pdf).
Ila,H.B.,Topaktas,M.,Arslan,M.,Buyukleyla,M.,2014.Signsof
deferasiroxgenotoxicity.Cytotechnology66(4),647–654.
Juckett,M.B.,Shadley,J.D.,Zheng,Y.,Klein,J.P.,1998.
Desferrioxamineenhancestheeffectsofgammaradiationon clonogenicsurvivalandtheformationofchromosomal aberrationsinendothelialcells.Radiat.Res.149,330–337.
Kim,B.M.,Choi,J.Y.,Kim,Y.J.,Woo,H.D.,Chung,H.W.,2007.
Desferrioxamine(DFX)hasgenotoxiceffectsoncultured humanlymphocytesandinducesthep53-mediateddamage response.Toxicology229,226–235.
Mace,J.M.L.,Daskal,Y.,Wray,W.,1978.Scanning-electron
microscopyofchromosomeaberrations.Mutat.Res.52, 199–206.
Maron,D.M.,Ames,B.N.,1983.Revisedmethodsforthe
Salmonellamutagenicitytest.Mutat.Res.113,173–215.
Marshall,R.,Tricta,F.,Galanello,R.,Leoni,G.,Kirkland,D.,Minto,
S.,Spino,M.,2003.Chromosomalaberrationfrequenciesin
patientswiththalassaemiamajorundergoingtherapywith deferiproneanddeferoxamineinacomparativecrossover study.Mutagenesis18,457–463.
Matthews,A.J.,Vercellotti,G.M.,Menchaca,H.J.,Bloch,P.H.,
Michalek,V.N.,Marker,P.H.,Murar,J.,Buchwald,H.,1997.Iron
andatherosclerosis:inhibitionbytheironchelatordeferiprone (L1).J.Surg.Res.73,35–40.
Paz-y-Mino,C.,Bustamante,G.,Sanchez,M.E.,Leone,P.E.,2002.
Cytogeneticmonitoringinapopulationoccupationally exposedtopesticidesinEcuador.Environ.HealthPerspect. 110,1077–1080.
OECDTG473,2012.InVitroMammalianChromosomal
AberrationTest.
Pietrangelo,A.,2003.Haemochromatosis.Gut52,ii23–ii30.
Pires,V.S.,Gaboriau,F.,Nascimento,S.,Dassonville,A.,Lescoat,
G.,Desplat,V.,Rochette,J.,Jarry,C.,Sonnet,P.,2006.
Modulationofcellproliferationinratlivercellculturesby newcalixarenes.J.EnzymeInhib.Med.Chem.21,261–270.
Sedigh-Ardekani,M.,Saadat,I.,Saadat,M.,2013.Propranolol
inducedchromosomalaberrationsinChinesehamsterovary cellline.Mol.Biol.Res.Commun.2(1–2),11–18.
Simonart,T.,Noel,J.C.,AndreI.,G.,Parent,D.,VanVooren,J.P.,
Hermans,P.,Lunardi-yskandar,Y.,Lambert,C.,Dieye,T.,
Farber,C.M.,Liesnard,C.,Snoeck,R.,Heenen,M.,Boelaert,
J.R.,1998.Ironasapotentialco-factorinthepathogenesisof
Kaposi’ssarcoma?Int.J.Cancer78,720–726.
Song,S.,Christova,T.,Perusini,S.,Alizadeh,S.,Bao,R.Y.,
Miller,.B.W.,Hurren,R.,Jitkova,Y.,Gronda,M.,Isaac,M.,
Joseph,B.,Subramaniam,R.,Aman,A.,Chau,A.,Hogge,D.E.,
Weir,S.J.,Kasper,J.,Schimmer,A.D.,Al-awar,R.,Wrana,J.L.,
Attisano,L.,2011.Wntinhibitorscreenrevealsiron
dependenceof-cateninsignalingincancers.CancerRes.71, 7628–7639.
Speit,G.,1984.Considerationsonthemechanismofdifferential
giemsastainingofbromodeoxyuridine-substituted chromosomes.Hum.Genet.67,264–269.
Speit,G.,Hauper,S.,1985.Onthemechanismofdifferential
giemsastainingofBrdU-substitutedchromosomes.Hum. Genet.70,126–129.
Stinson,T.J.,Jaw,S.,Jeffery,E.H.,Plewa,M.J.,1992.The
relationshipbetweennickelchloride-inducedperoxidation andDNAstrandbreakageinratliver.Toxicol.Appl. Pharmacol.117,98–103.
Sugimoto,R.,Kumagai,Y.,Nakai,Y.,Ishii,T.,2005.
9,10-Phenanthraquinoneindieselexhaustparticles downregulatesCu,Zn-SODandHO-1inhumanpulmonary epithelialcells:intracellularironscavenger
1,10-phenanthrolineaffordsprotectionagainstapoptosis. FreeRadic.Biol.Med.38,388–395.
Topaktas,M.,Rencuzogullari,E.,Ila,H.B.,1996.Invivo
chromosomalaberrationsinbonemarrowcellsofratstreated withmarshal.Mutat.Res.371,259–264.
Wang,T.,Gao,C.,Chen,B.A.,2010.Deferasirox–aneworaliron
chelator–review.ZhongguoShiYanXueYeXueZaZhi18, 1359–1364.
Whittaker,P.,Seifried,H.E.,San,R.H.,Clarke,J.J.,Dunkel,V.C.,
2001.GenotoxicityofironchelatorsinL5178Ymouse
lymphomacells.Environ.Mol.Mutagen.38,347–356.
Witte,I.,Zhu,B.Z.,Lueken,A.,Magnani,D.,Stossberg,H.,
Chevion,M.,2000.Protectionbydesferrioxamineandother
hydroxamicacidsagainsttetrachlorohydroquinone-induced cyto-andgenotoxicityinhumanfibroblasts.FreeRadic.Biol. Med.28,693–700.
Zhang,L.,Bandy,B.,Davison,A.J.,1996.Effectsofmetals,ligands
andantioxidantsonthereactionofoxygenwith 1,2,4-benzenetriol.FreeRadic.Biol.Med.20, 495–505.