The Effect of Green Tea Extract Supplementation in Bull Semen
Cryopreservation
Muhammed Enes İNANÇ
1,aBeste ÇİL
2,bDeniz YENİ
3,cFatih AVDATEK
3,dDurmuş ORAKÇI
4Pürhan Barbaros TUNCER
5,eRuhi TÜRKMEN
6,fUmut TAŞDEMİR
7,g
1 Department of Reproduction and Artificial Insemination, Faculty of Veterinary Medicine, Mehmet Akif Ersoy University, TR-15030 Burdur - TURKEY2 Department of Reproduction and Artificial Insemination, Faculty of Veterinary Medicine, Ankara University, TR-06110 Ankara - TURKEY
3 Department of Reproduction and Artificial Insemination, Faculty of Veterinary Medicine, Afyon Kocatepe University, TR-03200 Afyonkarahisar - TURKEY
4 Sultansuyu Agribusiness, Artificial Insemination Laboratory, TR- 44600 Malatya - TURKEY 5 Technical Sciences Vocational School, Mersin University, TR-33343 Mersin - TURKEY
6 Department of Pharmacology and Toxicology, Faculty of Veterinary Medicine, Afyon Kocatepe University, TR-03200 Afyonkarahisar - TURKEY
7 Technical Sciences Vocational School, Aksaray University, TR-68100 Aksaray - TURKEY
a ORCID: 0000-0001-6954-6309; b ORCID: 0000-0003-2822-1625; c ORCID: 0000-0002-9105-5677; d ORCID: 0000-0003-2345-8826 e ORCID: 0000-0002-9257-9544; f ORCID: 0000-0003-4726-3900; g ORCID: 0000-0003-2827-1286
Article ID: KVFD-2019-21702 Received: 08.01.2019 Accepted: 07.05.2019 Published Online: 07.05.2019 How to Cite This Article
İnanç ME, Çil B, Yeni D, Avdatek F, Orakçı D, Tuncer PB, Türkmen R, Taşdemir U:The effect of green tea extract supplementation in bull bemen cryopreservation. Kafkas Univ Vet Fak Derg, 25 (5): 703-708, 2019. DOI: 10.9775/kvfd.2018.21702
Abstract
This study aims to investigate the effect of catechin (CT), green tea extract, as a supplement to Tris extender on semen quality parameters in frozen-thawed of bull sperm. Ejaculates were taken with artificial vagina from Holstein bulls and divided equal five aliquots, diluted to containing different amounts of CT (5, 10, 25 and 50 μg/mL) and no-additive (control). All samples were equilibrated at 4°C for 4 h and were frozen using a digital freezing machine. Post-thawed sperm motility and kinetic parameters were determined using the sperm analyser system. Spermatozoa DNA integrity was evaluated with the single cell gel electrophoresis, abnormal spermatozoa rate was evaluated by fluid fixation test and lipid peroxidation status was evaluated colorimetrically. CT supplementation did not improve motility and kinetic parameters. However, the higher morphological integrity was detected in CT10, 25 and 50 groups compared to control (P<0.05). Regarding chromatin integrity, positive effects of catechin were observed in the treatment groups while in CT 50 group adverse effects were found (P<0.05). Although there was no improvement in malondialdehyde levels, the highest total antioxidant activity was seen in the CT50 group (P<0.05). In conclusion, CT supplementation could be used the protection of morphological and DNA integrity from cryodamage and it has increased the total antioxidant activity depending of the dose in bull semen.
Keywords: Bull semen freezing, Catechin, DNA Integrity, Lipid peroxidation
Yeşil Çay Ekstraktı İlavesinin Boğa Sperması Dondurulmasına Etkisi
Öz
Bu çalışmanın amacı yeşil çay ekstraktı olan kateşinin (CT) tris sulandırıcısına eklenerek boğa sperma dondurulmasında dondurma çözdürme sonucu sperma kalitesinin incelenmesidir. Ejakülatlar Holstein boğalardan alınarak eşit beş kısma ayrıldı ve farklı oranlarda (5, 10, 25 and 50 μg/mL) CT içeren ve içermeyen (kontrol) sulandırıcı ile sulandırıldı. Bütün örnekler 4°C’de 4 saat ekilibrasyona bırakıldı ve daha sonra otomatik dondurma makinası ile donduruldu. Çözüm sonu spermatozoa motilitesi ve kinetik parametreler sperma analiz sistemi ile değerlendirildi. DNA bütünlüğü tek hücre jel elektroforezi, anormal spermatozoa oranı sıvı fiksasyon testi ile, lipit peroksidasyon seviyesi ise kolorimetrik olarak ölçüldü. CT ilavesinin motilite ve kinetik parametreleri olumlu olarak etkilemediği görüldü. Fakat CT10 ve 25 ve 50 gruplarında kontrol grubuna göre daha yüksek morfolojik bütünlük tespit edildi (P<0.05). DNA bütünlüğü açısından CT’nin olumlu etkisi görülürken; CT 50 grubunda olumsuz etki tespit edildi (P<0.05). Malondialdehid seviyesi açısından herhangi bir iyileşme tespit edilememesine rağmen en yüksek total antioksidan kapasite CT50 grubunda görüldü. CT ilavesinin morfolojik ve DNA bütünlüğünü total antioksidan kapasitesini yükselterek soğuktan koruduğu tespit edildiği için boğa spermasında doza bağlı olarak kullanılabileceği sonucuna varıldı.
Anahtar sözcükler: Boğa sperma dondurulması, Kateşin, DNA bütünlüğü, Lipid peroksidasyon
İletişim (Correspondence)
+90 535 6654375INTRODUCTION
Sperm cryopreservation is essential for preserving the genetic diversity, conservation of wild and domestic species, world-wide dissemination of genetic progress and livestock management [1]. However, it causes detrimental effects on
sperm quality parameters, such as motility, morphology, viability and DNA integrity through the cryoinjury and it may also lead to the production of reactive oxygen species (ROS) [2]. Notably, the cold shock and atmospheric oxygen
exposition during the semen collection and freezing/ thawing procedures render the semen vulnerable for lipid peroxidation and lead to further damage to spermatozoa [3].
The primary sources of ROS in semen are the immature spermatozoa and leucocytes. Although adequate levels of ROS are required for some cellular processes as the capacitation, hyperactivation and binding of spermatozoa to the zona pellucida, excess amounts of ROS can adversely affect the motility, morphology and concentration of sperm as well as it can cause DNA damage and lipid peroxidation in the sperm [4]. Thus, to prevent the damage caused by
ROS on spermatozoa, there has been a growing interest in the use of plant-based substances as antioxidants in assisted reproductive technologies [5]. Up to now, many
plant-derived compounds, notably including carotenoids and flavonoids, have been studied for their antioxidant capacity to improve the fertility as components of in
vitro culture media and through their intake as dietary
supplements [6]. Some polyphenols have been found to
exhibit higher antioxidant activity and lower toxicity than synthetic antioxidants [7].As one of the natural antioxidants,
green tea polyphenols are water-soluble, phytochemical flavonoids and include epigallocatechin gallate, epicatechin gallate, epicatechin and epigallocatechin [8]. They are
found in high density in a variety of plant-based beverages and foods such as apricots, strawberries, black grapes and broad beans [3,9]. Consumption of catechin (CT) has
been associated with the increased plasma antioxidant activity (the ability of plasma to scavenge free radicals), the resistance of LDL to oxidation and fat oxidation, while decreasing the plasma lipid peroxide and malondialdehyde (MDA) concentrations [10]. Recently, various effects of CT
buck, ram and boar semen [11,12] have been presented by
several research groups however, there were very few studies regarding its effect on the cryopreservation process in bulls [13]. Thus, the current study aims to investigate the
effect of CT addition into Tris extender on sperm quality parameters following the cryopreservation of bull sperm.
MATERIAL and METHODS
Animal Experiments and Semen Collection
Semen from five bulls (Holstein breed) with proven fertility, aged 3-5 years, from Sultansuyu Agribusiness (Sultansuyu, Malatya, Turkey) was used for this study. Ejaculates taken by an artificial vagina once a week, the ejaculates were
pooled to eliminate variability among the evaluated samples. This trial was replicated ten times for each group. All samples were kept in 37°C water bath for further evaluation of motility, concentration and progressive motility. All experiments were carried out in accordance with the approval of the Animal Care Committee of Afyon Kocatepe University Veterinary Medicine Faculty regarding ethics, with the authorisation number 49533702/29. Semen Processing and Freezing
Semen volume was determined via a graded collection tube, and concentration was calculated with a photometer (Minitube GmbH, Tiefenbach, Germany). Samples were showing a minimum of 80% progressive motile and of 80% morphologically normal spermatozoa were used. A Tris- based extender was used to the primary medium in this study [5]. Extracted CT (10 mg) was diluted with 1 mL ethanol
(Merck, 99%) to create the CT stock solution. Ejaculates were divided into five aliquots and extended 15x106 spermatozoa/
straw with the Tris extender containing no-additive (control) and CT (5, 10, 25 and 50 μg/mL), and subsequently, sperm was loaded into mini straws. The experiment semen samples were cooled (4°C) and equilibrated for 4 h. After, every group was frozen with controlled semen freezing machine (SY LAB Gerate GmbH, Neupurkersdorf, Austria) with Avdatek et al.[5] protocol. Finally, the straws were
immersed in liquid nitrogen at -196°C. Frozen straws were thawed individually at 37°C for 30 s in a water bath for post-thawed spermological evaluations.
Assessment of Sperm Motility
Spermatozoa motilities were assessed using Computer-Assisted Sperm Analysis (CASA) system (Microptic S.L., Barcelona, Spain). A 5 μL diluted semen sample was put onto a slide (pre-warmed) put on to cover slide and percentages of progressive and non-progressive motility, as well as total motility, were recorded. Besides, motility kinetic parameters (curvilinear velocity μm/s (VCL), Straight linear velocity μm/s (VSL), average path velocity μm/s (VAP), amplitude of lateral head displacement, μm (ALH), Wobble (WOB, [VAP/VCL] × 100), beat cross frequency (BCF), Linearity (LIN, [VSL/VCL] ×100) and Strainess (STR, [VSL/VAP] ×100) and were determined. The spermatozoa motilities were calculated set as static, slow >20 μm/s, medium >60 μm/s and fast >80 μm/s protocols. For each assessment, between 220 and 370 spermatozoa were analysed in six different fields in microscope [5].
Evaluation of Sperm Morphology
Spermatozoa morphologies were evaluated Schafer and Holzmann [14] protocols. Hancock solution (500 mL
double-distilled water with 150 mL buffer solution, 150 mL saline solution and 62.5 mL formalin 37%) was used. 10 μL sample was added to 1000 μL Hancock solution to examine spermatozoa morphological integrity. 5 μL mix was put on a slide and mounted with a cover slide. The morphological
integrity (tail, acrosome, head and total abnormality) were evaluated under phase-contrast microscopy (1.000×) by evaluated minimum 200 spermatozoa.
Evaluation of DNA Integrity
Spermatozoa DNA integrity was evaluated by the comet (single cell gel electrophoresis) assay kit using a (Trevigen, Gaithersburg, MD, USA). Slides were examined under fluorescent microscopy (Olympus CX31, Tokyo, Japan), and images were reflected for following scoring analysis with TriTek Comet Score software (V. 1.5). On each sample, a total of 100 spermatozoa cells from five different fields were evaluated for analysis [15].
Assessment of Oxidative Stress
Total antioxidant (TA) status was evaluated by using a colourimetrically commercial kit (RelAssay®, Gaziantep, Turkey). Glutathione peroxidase (GPx) activity was identified using a GPx assay (OxisResearch™, Bioxytech® GPx-340™, Portland, USA). Levels of lipid peroxidation, which depends on MDA, were measured using a commercial kit (MDA-586; OxisResearch, Portland, USA). The results were indicated in μmol/mL [16].
Statistical Analysis
One-way analysis of variance (ANOVA) and Duncan’s post hoc test were used to state the differences among
the treatment groups in terms all spermatological and biochemical parameters. Data are presented as a mean ± standard error of means (SEM). The degree of significance was set at P<0.05. SPSS/PC (Version 10.0; SPSS, Chicago, IL) software package program was used for all analysis.
RESULTS
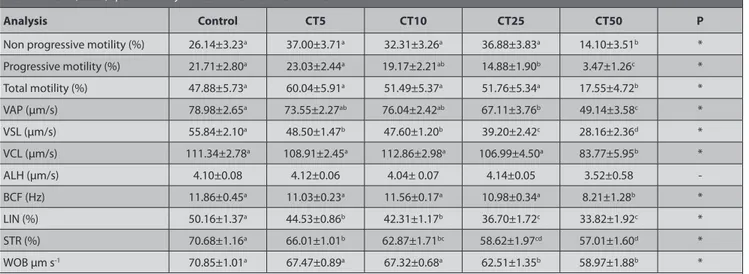
As presented in Table 1, CT supplementation did not enhance the motility or the kinetic parameters of sperm. In other respects, CT50 had led to a significant decrease in motility and sperm motion characteristics (P<0.05). CT10, 25 and 50 concentrations have shown lower total abnormalities compared the control (Table 2; P<0.05) however, CT50 has produced unfavorable results regarding the chromatin integrity (Table 3; P<0.05). The other treatment groups had shown the lowest tail moment values, indicating the minimal DNA damage (Table 3; P<0.05). As shown in Table 4, the increased antioxidant activity of the CT was determined to start from the CT10 concentration (P<0.05). A dose-depending positive effect was observed regarding the GPx and total antioxidant capacity values however, MDA values were increased as well within the higher dose groups.
DISCUSSION
As various authors well documented, freezing and thawing steps of cryopreservation reduce the motility
Table 1. Mean (±SEM) sperm motility values in frozen thawed bull semen
Analysis Control CT5 CT10 CT25 CT50 P
Non progressive motility (%) 26.14±3.23a 37.00±3.71a 32.31±3.26a 36.88±3.83a 14.10±3.51b *
Progressive motility (%) 21.71±2.80a 23.03±2.44a 19.17±2.21ab 14.88±1.90b 3.47±1.26c * Total motility (%) 47.88±5.73a 60.04±5.91a 51.49±5.37a 51.76±5.34a 17.55±4.72b * VAP (µm/s) 78.98±2.65a 73.55±2.27ab 76.04±2.42ab 67.11±3.76b 49.14±3.58c * VSL (µm/s) 55.84±2.10a 48.50±1.47b 47.60±1.20b 39.20±2.42c 28.16±2.36d * VCL (µm/s) 111.34±2.78a 108.91±2.45a 112.86±2.98a 106.99±4.50a 83.77±5.95b * ALH (µm/s) 4.10±0.08 4.12±0.06 4.04± 0.07 4.14±0.05 3.52±0.58 -BCF (Hz) 11.86±0.45a 11.03±0.23a 11.56±0.17a 10.98±0.34a 8.21±1.28b * LIN (%) 50.16±1.37a 44.53±0.86b 42.31±1.17b 36.70±1.72c 33.82±1.92c * STR (%) 70.68±1.16a 66.01±1.01b 62.87±1.71bc 58.62±1.97cd 57.01±1.60d * WOB µm s-1 70.85±1.01a 67.47±0.89a 67.32±0.68a 62.51±1.35b 58.97±1.88b *
a b,c,d Different superscripts within the same row demonstrate significant differences; *P<0.05; - No significant difference (P>0.05)
Table 2. Mean (±SEM) sperm abnormality values in frozen thawed bull semen
Analysis Control CT5 CT10 CT25 CT50 P
Head abnormalities (%) 2.95±2.83 3.86±2.41 1.75±1.77 2.59±2.08 2.44±1.73 -Mid-piece abnormalities (%) 7.34±4.86a 5.63±1.47ab 3.89±3.51b 4.34±3.39ab 2.65±1.83b *
Tail abnormalities (%) 6.21±4.35a 4.03±4.16ab 6.01±4.95a 3.62±2.61ab 1.97±0.78b *
Total abnormalities (%) 16.50±5.85a 13.53±6.18ab 11.65±5.96abc 10.56±4.65bc 7.07±3.96c *
and fertilizing ability of spermatozoa leads to damage of plasma membrane [17], induce premature capacitation
and nuclear decondensation [18]. ROS production during
these cycles is considered to be one of the major cause that is accountable for the emerging impairment in the functionality of the sperm cell [19]. Whereas the problem is
well-being pointed, the solution to prevent these damage is still being researched by many scientific groups. In recent years, numerous antioxidants have been introduced to the cryopreservation process on account of enhancing the post-thawed quality of sperm. Due to lack of carbonyl group in the epicatechin molecules, it is considered as a less potent antioxidant than other flavonoids such as quercetin or naringenin [12,20]. In the meantime, the emerging use
of plant-based scavengers has resulted in controversial effects [7,21,22]. In the present study, CT supplementation
did not enhance the post-thawed motility or kinetic parameters, but rather, adverse effects were observed in the highest dose group (P<0.05). In accordance with the delivered study, Gale et al.[23], the addition of green tea
extract to the cryo-medium of boar semen extender did not produce any beneficial effects on motility, viability, acrosome integrity or membrane integrity. Another boar sperm study, in which, the toxicity of green tea extract on chilled spermatozoa was evaluated. Although no toxic effect was observed, sperm quality parameters did not differ between the control and different concentrations of green tea extract supplementation [11]. Additionally, in
several studies on different species, the inclusion of natural antioxidants did not produce any positive effects [24-26].
On the other hand, several previous studies in different species generated results inconsistent to those found in current study, namely in chilled dog semen, the addition of green tea polyphenols into the extender has shown a significant protective effect on the motility and viability parameters up to four weeks of semen [8]. Also on boar
semen [11] and at low concentration on human sperm [27]
motility can be improved by green tea extract. Khan et
al.[28] have cryopreserved the bull semen with different
rates of (0.25; 0.5; 0.75; 1.0%) green tea extract and evaluated
in vitro spermological parameters (motility, viability and
membrane integrity). They found the highest progress in 0.75% green tea groups. Besides, in bull semen cryo-preservation, in which motility and membrane integrity were improved with the supplementation of green tea extract [13]. Considering our results, we can hypothesize that
the discrepancy in the results may be due to the density of the other substances (Tris, egg yolk) used in the extender. Since catechins are unstable molecules, ROS formation can occur due to auto-oxidation. The stability of these molecules can be altered depending on the environmental temperature, pH or oxygen level [29]. When the antioxidant
capacity of the samples was evaluated, decreased lipid peroxidation level was observed in CT concentrations starting from the 10 μg/mL (P<0.05). A dose-dependent positive effect was detected regarding the GPx and total antioxidant capacity; however, MDA values were increased as well within the higher dose groups. El- Seadawy et al. [30]
have found that addition of pomegranate peel methanolic extract enriched with CT into chilled rabbit semen has decreased the lipid peroxidation level and increased the antioxidant activity. Similar results were observed in a rat [31], ram [12] and stallion [32] semen in which, were extended
by CT supplementations. Also, Sugiyama et al.[33] reported
that epigallocatechin gallate promotes protection against testicular ischemia-reperfusion injury due to its antioxidant activity since epigallocatechin gallate is the major CT compound of green tea extract with a 52% ratio of the total CT content [34]. The results demonstrated that polyphenols
might interact with components of the spermatozoa and would have decreased the lipid peroxidation induced by free radicals [35]. On the contrary with the present study,
Moretti et al.[20] supplemented swim-up selected human
semen with 200 µM epicatechin and they could not find any improvement regarding the antioxidant capacity. This
Table 3. Mean (±SEM) chromatin integrity values in frozen thawed bull semen
Analysis Control CT5 CT10 CT25 CT50 P
Tail length (µm/s) 19.27±3.18b 14.26±3.12bc 11.71±3.97bc 8.75±2.61c 37.60±4.07a *
Tail DNA (%) 19.11±3.06b 12.36±1.59b 23.04±5.58b 17.85±1.77b 45.10±4.90a *
Tail moment (µm/s) 29.01±4.06a 12.59±2.06b 11.16±2.04b 7.43±2.77b 22.30±3.17a *
a,b,c Different superscripts within the same row demonstrate significant differences; * P<0.05; - No significant difference (P>0.05)
Table 4. Mean (±SEM) glutathione peroxidase (GPx), malondialdehyde (MDA) and total antioxidant (TA) activities in frozen thawed bull semen
Analysis Control CT5 CT10 CT25 CT50 P
GPx (mU/mL) 12.53±0.12d 12.91±0.15cd 13.22±0.20bc 13.69±0.29b 15.83±0.14a *
MDA (µmol/mL) 2.51±0.02d 2.57±0.02d 2.67±0.03c 2.82±0.02b 3.12±0.02a *
Total antioxidant activities
(mmol/trolox/mL-109 cell/mL) 0.14±0.01d 0.15±0.02d 0.16±0.02c 0.24±0.01b 0.27±0.01a *
result might have seen due to the chemical structure of epicatechin which does not contain the carbonyl group. The COMET assay is widely used for analysing DNA damages in multiple cell [36]. It is a suitable cell evaluation method while
maintaining the integrity of genetic material in biological evaluations [37]. Zini et al.[38] reported that DNA damage of
spermatozoa has a high impact on the fertilization rate, embryo quality, and the rate of miscarriages [39]. Green
tea extract might also have a promoting effect on in vitro maturation and embryo development [40-42]. In the current
study highest dose of CT had adversely affected the DNA integrity; however, the lower doses were able to protect the DNA integrity compared to control (P<0.05). Besides CT10, 25 and 50 concentrations have shown lower total abnormalities. This results might be due to phenolic compounds of CT that improve morphological and DNA integrity if an appropriate amount is used. Various research supports the present study with obtained improvement on morphological integrity in different animal models [11,32]
in which, the effects were observed with the supplementation of different rates green tea extracts. On the contrary of our results, Bucci et al.[42] did not find any improvement
in supplementing the thawing medium of boar semen with epigallocatechin gallate (50 µM). On the basis of our results, we might hypothesize that the diferences among our results may be associated with the amounts and types of antioxidants that were used.
In conclusion, CT supplementation has provided the protection of morphological and DNA integrity from cryo-damage and it has increased the total antioxidant activity depending of the dose in bull semen. Addition of 25 μg/mL CT concentration in Tris extender can be beneficial when the overall parameters are considered. Further research is required to understand the cellular mechanisms involved in antioxidant activity.
A
cknowledgementThis study was supported by General Directorate of Agribusiness, Ankara, Turkey.
c
onflıctofı
nterestThe authors confirm that they have no conflict of interest to declare
REFERENCES
1. Ari UC, Kulaksiz R, Ozturkler Y, Lehimcioglu NC, Yildiz S: Effect of N-Acetylcysteine (NAC) on post-thaw semen qualityof Turkish rams. Kafkas
Univ Vet Fak Derg, 22 (6): 883-887, 2016. DOI: 10.9775/kvfd.2016.15558
2. OzkavukcuS, Erdemli E, Isik A, Oztuna D, Karahuseyinoglu S: Effects of cryopreservation on sperm parameters and ultrastructural morphology of human spermatozoa. J Assist Reprod Genet, 25 (8): 403-411, 2008. DOI: 10.1007/s10815-008-9232-3
3. Seddiki Y, Moreira da Silva F: Antioxidant properties of polyphenols and their potential use in improvement of male fertility: A review. Biomed
J Sci Tech Res, 1 (3): 612-617, 2017. DOI: 10.26717/BJSTR.2017.01.000259
4. Ko EY, Sabanegh ES, Agarwal A: Male infertility testing: Reactive oxygen species and antioxidant capacity. Fertil Steril, 102, 1518-1527, 2014. DOI: 10.1016/j.fertnstert.2014.10.020
5. Avdatek F, Yeni D, İnanç ME, Çil B, Tuncer PB, Türkmen R, Taşdemir U: Supplementation of quarcetin for advanced DNA integrity in bull semen cryopreservation. Andrologia, 50:e12975, 2018. DOI: 10.1111/ and.12975
6. Moretti E, Mazzi L, Bonechi C, Salvatici MC, Iacoponi F, Rossi C, Collodel G: Effect of quercetin-loaded liposomes on induced oxidative stress in human spermatozoa. Reprod Toxicol, 60, 140-147, 2016. DOI: 10.1016/j.reprotox.2016.02.012
7. Zhong R, Zhou D: Oxidative stress and role of natural plant derived antioxidants in animal reproduction. J Integr Agric, 12 (10): 1826-1838, 2013. DOI: 10.1016/S2095-3119(13)60412-8
8. Wittayarat M, Ito A, Kimura T, Namula Z, Luu VV, Do LT, Sato Y, Taniguchi M, Otoi T: Effects of green tea polyphenol on the quality of canine semen after long-term storage at 5°C. Reprod Biol, 13 (3): 251-254, 2013. DOI: 10.1016/j.repbio.2013.07.006
9. Williamson G, Manach C: Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am J Clin Nutr, 81, 243S-255S, 2005. DOI: 10.1093/ajcn/81.1.243S
10. Scalbert A, Williamson, G: Dietary intake and bioavailability of polyphenols. J Nutr, 130, 2073S-2085S, 2000. DOI: 10.1093/jn/130.8.2073S 11. Park SH, Yu IJ: Evaluation of toxicity of green tea extract in chilled boar spermatozoa. J Anim Reprod Biotechnol, 30, 1-6, 2015. DOI: 10.12750/ JET.2015.30.1.1
12. Mehdipour M, Kia HD, Najafi A, Dodaran HV, García-Álvarez O: Effect of green tea (Camellia sinensis) extract and pre-freezing equilibration time on the post-thawing quality of ram semen cryopreserved in a soybean lecithin-based extender. Cryobiology, 73 (3): 297-303, 2016. DOI: 10.1016/j.cryobiol.2016.10.008
13. Ali H, Riaz A, Ghafoor A, Javeed A, Ashraf M, Satter A: Antioxidative protection by Strawberry and green tea extracts during cryopreservation of Sahiwal bull semen. Pak J Life Soc Sci, 12, 97-100, 2014.
14. Schafer S, Holzmann A: The use of transmigration and spermac stain to evaluate epididymal cat spermatozoa. Anim Reprod Sci, 59, 201-211, 2000. DOI: 10.1016/S0378-4320(00)00073-7
15. Gundogan M, Yeni D, Avdatek F, Fidas AF: Influence of sperm concentration on the motility, morphology, membrane and DNA integrity along with oxidative stress parameters of ram sperm during liquid storage. Anim Reprod Sci, 122, 200-207. 2010. DOI: 10.1016/j. anireprosci.2010.08.012
16. Kasimanickam R, Pelzer KD, Kasimanickam V, Swecker WS, Thatcher CD: Association of classical semen parameters, sperm DNA fragmentation index, lipid peroxidation and antioxidant enzymatic activity of semen in ram-lambs. Theriogenology, 65, 1407-1421, 2006. DOI: 10.1016/j.theriogenology.2005.05.056
17. Hammerstedt RH, Graham JK, Nolan JP: Cryopreservation of mammalian sperm: What we ask them to survive? J Androl, 11, 73-88, 1990. 18. Cormier N, Sirard MA, Beiley JL: Premature capacitation of bovine spermatozoa is initiated by cryopreservation. J Androl, 18 (4): 461-468, 1997. DOI: 10.1002/j.1939-4640.1997.tb01953.x
19. Chatterjee S, Gagnon C: Production of reactive oxygen species by spermatozoa undergoing cooling, freezing, and thawing. Mol Reprod Dev, 59 (4): 451-458, 2001. DOI: 10.1002/mrd.1052
20. Moretti E, Mazzi L, Terzuoli G, Bonechi C, Iacoponi F, Martini S, Rossi C, Collodel G: Effect of quercetin, rutin, naringenin and epicatechin on lipid peroxidation induced in human sperm. Reprod Toxicol, 34 (4): 651-657, 2012. DOI: 10.1016/j.reprotox.2012.10.002
21. Middleton E, Kandaswami C, Theoharides TC: The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol Rev, 52, 673-751, 2000
22. Purdy PH, Ericsson SA, Dodson RE, Sternes KL Garner DL: Effects of the flavonoids, silibinin and catechin, on the motility of extended cooled caprine sperm. Small Ruminant Res, 55, 239-243, 2004. DOI: 10.1016/j. smallrumres.2004.02.005
23. Gale I, Gil L, Malo C, González N, Martínez F: Effect of Camellia
sinensis supplementation and increasing holding time on quality of
cryopreserved boar semen. Andrologia, 47 (5): 505-512, 2015. DOI: 10.1111/and.12293
24. Gonzalez N, Gil L, Martinez F, Malo C, Cano R, Mur P, Espinosa E: Effect of natural antioxidant rosemary in canine soya freezing extender.
Reprod Domest Anim, 45: 88, 2010
25. Camara DR, Silva SV, Almeida FC, Nunes JF, Guerra MMP: Effects of antioxidants and duration of pre‐freezing equilibration on frozen thawed ram semen. Theriogenology, 76, 342-350, 2011. DOI: 10.1016/j. theriogenology.2011.02.013
26. Gadea J, Molla M, Selles E, Marco MA, Garcia-Vazquez FA, Gardon JC: Reduced glutathione content in human sperm is decreased after cryopreservation: Effect of the addition of reduced glutathione to the freezing and thawing extenders. Cryobiology, 62, 40-46, 2011. DOI: 10.1016/j.cryobiol.2010.12.001
27. De Amicis F, Santoro M, Guido C, Russo A, Aquila S: Epigallocatechin gallate affects survival and metabolism of human sperm. Mol Nutr Food
Res, 56, 1655-1664, 2012. DOI: 10.1002/mnfr.201200190
28. Khan H, Khan M, Qureshi MS, Ahmad S, Gohar A, Ullah H, Ullah F, Hussain A, Khatri P, Shah SSA, Rehman H, Khan A: Effect of green tea extract (Camellia sinensis) on fertility indicators of post-thawed bull spermatozoa. Pak J Zool, 49, 1243-1243, 2017. DOI: 10.17582/journal. pjz/2017.49.4.1243.1249
29. Sang S, Hou Z, Lambert JD, Yang CS: Redox properties of tea polyphenols and related biological activities. Antioxid Redox Signal, 7, 1704-1714, 2005
30. El-Sayed El-Seadawy I, Aziza SAH, El-Senosy YA, El-Nattat WS, El-Tohamy MM, Hussein AS: Effect of pomegranate peel methanolic extract on oxidative/antioxidant status of chilled diluted rabbit semen.
Benha Vet Med J, 33, 1-8, 2017.
31. Dias TR, Alves MG, Tomás GD, Socorro S, Silva BM, Oliveira PF: White tea as a promising antioxidant medium additive for sperm storage at room temperature: A comparative study with green tea. J Agric Food
Chem, 62 (3): 608-617, 2014. DOI: 10.1021/jf4049462
32. Nouri H, Shojaeian K, Samadian F, Lee S, Kohram H, Lee JI: Using resveratrol and epigallocatechin-3-gallate to improve cryopreservation of stallion spermatozoa with low quality. J Equine Vet Sci, 70, 18-25, 2018.
DOI: 10.1016/j.jevs.2018.07.003
33. Sugiyama A, Chiba M, Nakagami T, Kawano S, Sanada Y, Tajiri T, Toki A: Beneficial effects of (−)-Epigallocatechin gallate on ischemia-reperfusion testicular injury in rats. J Pediatr Surg, 47 (7): 1427-1432, 2012. DOI: 10.1016/j.jpedsurg.2012.01.069
34. Hilal Y, Engelhardt U: Characterisation of white tea - Comparison to green and black tea. J Verbr Lebensm, 2, 414-421, 2007. DOI: 10.1007/ s00003-007-0250-3
35. Hyon SH: A non-frozen living tissue bank for allotransplantation using green tea polyphenols. Yonsei Med J, 45, 1025-1034, 2004. DOI: 10.3349/ymj.2004.45.6.1025
36. Ostling O, Johanson KJ: Microelectrophoretic study of radiation-induced DNA damages in individual mammalian cells. Biochem Biophys
Res Commun, 123 (1): 291-298, 1984. DOI: 10.1016/0006-291X(84)90411-X
37. Novotna B, Topinka J, Solansky I, Chvatalova I, Lnenickova Z, Sram RJ: Impact of air pollution and genotype variability on DNA damage in Prague policemen. Toxicol Lett, 172, 37-47, 2007. DOI: 10.1016/j. toxlet.2007.05.013
38. Zini A, Boman JM, Belzile E, Ciampi A: Sperm DNA damage is associated with an increased risk of pregnancy loss after IVF and ICSI: Systematic review and meta-analysis. Hum Reprod, 23 (12): 2663-2668, 2008. DOI: 10.1093/humrep/den321
39. Alcay S, Toker B, Ustuner B, Nur Z, Sagırkaya H, Soylu MK: Investigation of relationships between DNA integrity and fresh semen parameters in rams. Kafkas Univ Vet Fak Derg, 20 (5): 793-798, 2014. DOI: 10.9775/kvfd.2014.11144
40. Barakat IA, Al Himaidi AR, Rady AM: Antioxidant effect of green tea leaves extract on in vitro production of sheep embryos. Pak J Zool, 46, 167-175, 2014.
41. Gadani, B, Bucci D, Spinaci M, Tamanini C, Galeati G: Resveratrol and Epigallocatechin-3-gallate addition to thawed boar sperm improves
in vitro fertilization. Theriogenology, 90, 88-93, 2017. DOI: 10.1016/j.
theriogenology.2016.11.020
42. Bucci D, Spinaci M, Yeste M, Mislei B, Gadani B, Prieto Martinez N, Love C, Mari G, Tamanini C, Galeati G: Combined effects of resveratrol and epigallocatechin-3-gallate on post thaw boar sperm and IVF parameters. Theriogenology, 117, 16-25, 2018. DOI: 10.1016/j. theriogenology.2018.05.016