Contents lists available atScienceDirect
Industrial Crops & Products
journal homepage:www.elsevier.com/locate/indcropMetabolomics-based pro
filing with chemometric approach to delineate the
bio-pharmaceutical properties of fruit extracts from Ligustrum vulgare L
Gabriele Rocchetti
a,1, Biancamaria Senizza
a,1, Gokhan Zengin
b,⁎, Ismail Senkardes
c,
Nabeelah Bibi Sadeer
d, Mohamad Fawzi Mahomoodally
d, Luigi Lucini
a,⁎⁎aDepartment for Sustainable Food Process, Università Cattolica del Sacro Cuore, Via Emilia Parmense 84, 29122, Piacenza, Italy bDepartment of Biology, Faculty of Science, Selcuk University, Campus, Konya, Turkey
cDepartment of Pharmaceutical Botany, Faculty of Pharmacy, Marmara University, Istanbul, Turkey dDepartment of Health Sciences, Faculty of Science, University of Mauritius, 230 Réduit, Mauritius
A R T I C L E I N F O Keywords: Ligustrum Bioactive compounds Metabolomics Multivariate statistics Antioxidant Enzyme inhibition A B S T R A C T
Ligustrum vulgare L. (Family: Oleaceae) is an evergreen shrub native to North-west Africa, Europe and Western Asia. In the present work, infusion (water), ethyl acetate, methanol and n-hexane extracts of the fruits of L. vulgare L. were studied for their in vitro antioxidant and enzyme inhibitory properties along with their un-targeted polyphenolic profile (using an ultra-high-performance liquid chromatography-quadrupole time-of-flight (UHPLC-QTOF) mass spectrometry approach). Untargeted metabolomics coupled to multivariate statistics showed that Ligustrum fruit extracts were very abundant in polyphenols, such asflavonoids, tyrosols and lignans, with ethyl acetate allowing the highest recovery among the extraction solvents tested. The methanolic extract inhibited the highest acetylcholinesterase (AChE), tyrosinase and glucosidase activity (4.23 mg galatamine equivalent (GALAE)/g, 132.85 mg kojic acid equivalent (KAE)/g and 1.94 mmol acarbose equivalent (ACAE)/g respectively) while the ethyl acetate extract inhibited the highest butyrylcholinesterase (BChE) and amylase activity (4.92 mg GALAE/g and 0.73 mmol ACAE/g, respectively). However, the infusion showed the weakest activity against all the enzymes studied. However, the extract showed highest antioxidant property in the an-tioxidant assays (except for phosphomolybdenum). The present paper is thefirst comprehensive report on L. vulgare fruits and thus ourfindings are of considerable interest to design new bioproducts for the pharmaceutical and cosmeceutical industry in the future.
1. Introduction
Plants have played a fundamental role in human life over the past few decades and are still considered as imperative to assuage human suffering. For instance, the usage of plants has a multifaceted involve-ment in our daily life in terms of foods, fodder crops, building materials, crafts and most importantly as medicines (Teixidor-Toneu et al., 2018). Modern science has adopted the ancient or traditional belief on the curative nature of plants in our contemporary life based on their proven healing abilities on several occasions (Petrovska, 2012). Thus, screening medicinal plants for their therapeutic properties have in-creased greatly with the aim to find new treatment against various existing and emerging diseases. Accordingly, several plant species are being scrutinized for possible bio-pharmaceutical properties and for
bioproducts development.
In the present study, a poorly explored plant, Ligustrum vulgare (Family: Oleaceae), is being investigated for its biological properties. This plant is commonly known as common privet, golden privet, wild privet or European privet. Ligustrum vulgare is native to North-west Africa (Morocco), Europe (Ireland, United Kingdom, Belgium), Western Asia (Turkey, Armenia, Georgia) and widely naturalized in Southern Australia (Australia, 2016). The wild privets are known under different names, for instance as Liguster in Germany, Ligustro in Italy, Alfena in Portugal and Raisin de chien or Troène vulgaire in France (USDA-ARS, 2018). The genus Ligustrum consists of 200 species names as reported in The Plant List; of these 43 are accepted species names, 150 synonyms and 7 unassessed (List, 2013).
Morphologically, L. vulgare L. is an evergreen shrub reaching a
https://doi.org/10.1016/j.indcrop.2019.111635
Received 23 April 2019; Received in revised form 30 July 2019; Accepted 31 July 2019 ⁎Corresponding author.
⁎⁎Corresponding author.
E-mail addresses:gokhanzengin@selcuk.edu.tr(G. Zengin),luigi.lucini@unicatt.it(L. Lucini). 1These authors contributed equally to this work and are the co-first authors.
Available online 03 August 2019
0926-6690/ © 2019 Elsevier B.V. All rights reserved.
height of 3–6 m with shiny, green and ovate leaves of 2–6 cm long, 0.5–1.5 cm broad attached to a stiffed, erected and grey-brown bark stem. The plant produced small glossy black berries of 6–8 mm in dia-meter containing 1–4 seeds (CABI, 2018). The berries can be poisonous and may cause unpleasant effects if consumed (CABI, 2018; Natural Medicine Facts, 2015). A few studies have reported its toxicity and that the berries may cause vomiting and diarrhea or even persisted gastro-enteritis for 48–72 h (Quattrocchi, 2016).
It is known that Ligustrum species are widely used as a folkloric medicine. The wide traditional usage of this plant is attributed to the anti-inflammatory, antidiabetic, antioxidant and pro-apoptotic effects of the aerial part (Ćurčić et al., 2012;Gao et al., 2009). In earlier stu-dies, the Ligustrum species exhibited significant pharmacological effects such as neuroprotective, mutagenic, hepatoprotective and anti-oxidative effects (Lin et al., 2007;Wu et al., 2011;Zhu et al., 2009). For instance, Yu et al., 2015projected that the methanolic extract of L. robustum (Roxb.) exhibited significant antioxidant activity and re-markable inhibitory effects of α-amylase and α-glucosidase. Ad-ditionally, in 2002 a study explored the hepatoprotective effects of the leaf of L. robustum in CCl4-intoxicated livers of male rats. Eventually, results showed that the curative effect of the extract has significant effect to protect liver functions and attend in detoxifying processes in the body and it was concluded that these results partially supported the traditional belief of this Ligustrum species (Lau et al., 2002).
However, as far as the literature search could establish, there has been no attempt to evaluate the inhibitory activity L. vulgare on key enzymes linked to human ailments namely diabetes and Alzheimer’s disease. Consequently, to shed more light on the biological potential of this plant, the present study which is second-to-none provides a deeper and broader understanding on the possible inhibitory action on α-amylase, α-glucosidase, tyrosinase, acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) and also on the antioxidative potential of the aqueous, n-hexane, ethyl acetate and methanolic extracts of the fruits of L. vulgare. Besides, considering the potential of untargeted metabolomics to comprehensively investigate the (poly)-phenolic pro-file of plant matrices (Rocchetti et al., 2018a), a complete screening of polyphenols characterizing Ligustrum fruits was done for thefirst time by using this omics approach. Taken together, the presented results couldfill the gap on the biological propensities and chemical profile of L. vulgare that could subsequently open new research avenues, parti-cularly in therapeutic bioproducts development.
2. Materials and methods 2.1. Plant material
The plant materials (Ligustrum vulgare fruits) were collected from Nevsehir (Turkey) in August 2018. The scientific identification was performed by Dr. Ismail Senkardes (botanist at Marmara University, Department of Pharmaceutical Botany, Istanbul, Turkey). Then, vou-cher specimen was deposited in the herbarium of Marmara University (voucher number: MARE 20217). The fruits were kept at the room temperature for drying (about ten days). After that, a laboratory mill was used to powder the dried fruits.
2.2. Extraction methods
Methanolic, ethyl acetate and n-hexane were selected as extraction solvents in the maceration techniques (5 g of plant sample was mixed with 100 ml of each solvents for 24 h.). After that, the extracts were filtered and evaporated in vacuo at 40 °C. Water extract was prepared as traditional infusion (5 g plant sample was kept 100 ml of boiling water for 20 min). The infusion wasfiltered and then dried (by lyophilizer). All extracts were stored at +4 °C protected from the light until analysis.
2.3. Profiling of bioactive compounds
By referring to our previous paper (Uysal et al., 2017), the flavo-noids (TFC) and total phenolic (TPC) contents were determined on the basis of AlCl3and standard Folin-Ciocalteu assay respectively. The re-sults were expressed as equivalent of rutin (mg RE/g) for TFC and gallic acid equivalent (mg GAE/g) for TPC.
Afterwards, the untargeted phenolic profiling of the different Ligustrum extracts was depicted by using ultra-high-pressure liquid chromatography (Agilent 1290 HPLC liquid chromatograph) coupled to quadrupole-time-of-flight mass spectrometer (Agilent 6550 iFunnel), via a JetStream dual electrospray ionization system. In this regard, the experimental conditions for the analysis of plant extracts by means of untargeted metabolomics were optimized based on previously works (Rocchetti et al., 2019a,b). The mass spectrometer acquired ions in the range 50–1200 m/z in positive scan mode. The database Phenol-Ex-plorer (http://phenol-explorer.eu) was then used for annotation pur-poses, exploiting the complete isotopic profile (i.e. combining mono-isotopic accurate mass, mono-isotopic ratios and spacing). Therefore, the approach used was based on a Level 2 of identification (i.e. putatively annotated compounds), as set out by the COSMOS Metabolomics Standards Initiative. The Agilent Profinder B.06 software was then used for post-acquisition data filtering (Rocchetti et al., 2019a; Rocchetti et al., 2019b). Finally, the phenolic compounds ascribed into classes and subclasses were then quantified using standard solutions (80/20, v/ v methanol/water) of pure standard phenolic compounds analyzed with the same method (Rocchetti et al., 2019a). The following phenolic classes were targeted: anthocyanins (quantified as cyanidin equiva-lents),flavones (quantified as luteolin equivalents), flavonols and fla-vanols (quantified as catechin equivalents), lignans (quantified as se-samin equivalents), alkylphenols (quantified as 5-Pentadecylresorcinol equivalents), stilbenes (quantified as resveratrol equivalents), low-mo-lecular-weight phenolics (quantified as tyrosol equivalents) and phe-nolic acids (quantified as ferulic acid equivalents).
2.4. Determination of enzyme inhibitory and antioxidant effects
The in vitro enzyme inhibitory effects of extracts on four enzymes, that areα-amylase, α-glucosidase, cholinesterases, and tyrosinase were evaluated, as previously reported (Uysal et al., 2017). The enzyme in-hibitory actions of extracts were assessed as equivalents of kojic acid (KAE) for tyrosinase, galantamine for acetyl cholinesterase (AChE) and butyryl cholinesterase (BChE), and acarbose forα-amylase and α-glu-cosidase.
Regarding antioxidant capacity of the extracts, different spectro-photometric experiments as ferrous ion chelating, phosphomo-lybdenum and radicals scavenging tests (ferric reducing antioxidant power (FRAP), 2,2′-azino-bis(3-ethylbenzothiazoline)-6-sulfonic acid (ABTS), cupric reducing antioxidant capacity (CUPRAC) and 1,1-di-phenyl-2-picrylhydrazyl (DPPH)) were performed as previously re-ported. Thefindings were given as standard compounds equivalents of EDTA or Trolox (mg EDTAE/g and mg TE/g). The assay methods were given in our earlier work (Uysal et al., 2017).
2.5. Statistical analysis
A one-way analysis of the variance (ANOVA) was performed con-sidering data from each assay and using the software PASW Statistics 25.0 (SPSS Inc.), followed by a Duncan’s post hoc test (p > 0.05). Besides, Pearson’s correlation coefficients (p = 0.01, two-tailed) were inspected using the same statistical software. An unsupervised hier-archical cluster analysis (HCA) was then performed in order to group each extract according to intrinsic similarities in their measurements (Rocchetti et al., 2019a,b). Afterwards, the metabolomics-based da-taset exported from Mass Profiler Profession B.12.06 (Agilent Tech-nologies) was elaborated into a second software, namely SIMCA 13
(Umetrics, Malmo, Sweden) for supervised OPLS-DA model, according to previously reported works (Rocchetti et al., 2019a,b). Finally, the importance of each variable into the OPLS-DA model in discriminating the different extraction techniques was assessed by using the VIP se-lection method (i.e. variables’ importance in projection). Phenolic compounds possessing a VIP score > 1.2 werefinally retained. 3. Results and discussion
3.1. Phenolic profile by UHPLC-QTOF mass spectrometry and multivariate statistics
Total phenolic content (TPC) and totalflavonoid content (TFC) of L. vulgare fruit extracts were detected and the results have been reported in Supplementary Table 1. Among the secondary metabolites present in plants, polyphenols are considered as the major group associated with numerous pharmacological properties namely antioxidant, anti-microbial, anti-inflammatory, anti-allergic and anti-carcinogenic (Mohammedi and Atik, 2011). Besides, polyphenols are the main an-tioxidants present in human diets. It has been acknowledged by various studies that there is a positive correlation between total phenolics and antioxidant activities of plant extracts (Dutta and Ray, 2018). In our study, the highest TPC value was reported with the aqueous fruit ex-tract (65.96 mg GAE/g) and the highest TFC value with methanolic fruit extract (12.74 mg RE/g). The n-hexane extract showed the lowest TPC (13.66 mg GAE/g) and TFC (3.17 mg RE/g) values.
However, considering the limitations recently reported by (Granato et al., 2018) when spectrophotometric methods are used to assess TPC and TFC, a following high-resolution and untargeted UHPLC-QTOF mass spectrometric approach was exploited to evaluate more accurately the phenolic composition of each Ligustrum fruit extract. The experi-mental plan designed allowed to putatively annotate 65 anthocyanins, 78flavones, 81 flavonols and flavanols, 29 lignans, 17 alkylphenols, 61 tyrosols, 99 phenolic acids and 9 stilbenes. For a more detailed de-scription, polyphenols identified are listed into Supplementary Table 2, together with annotation scores and composite mass spectra. The un-targeted metabolomics-based approach allowed to identify a wide set of compounds, thus demonstrating that this plant is particularly rich in polyphenols, above allflavonoids. In particular, when considering the phenolic composition of Ligustrum fruit, the untargeted approach (Supplementary Table 2) showed that this matrix was very abundant in anthocyanins, such as delphinidin 3-O-sambubioside, vitisin A, pe-largonidin 3-O-rutinoside and glycosidic forms of cyanidin. When considering other flavonoids, glycosidic forms of luteolin, apigenin, quercetin and kaempferol were main components in the tested extracts. (Supplementary Table 2).
Afterwards, by using a semi-quantitative analysis from the UHPLC-QTOF data, all phenolic compounds identified were classified and ex-pressed according to a standard per class as mg/g dry extract. The re-sults of the semi-quantitative analysis are reported in Table 1. Ac-cording to this analysis, we found that ethyl acetate fruit extracts were characterized by the highest content of polyphenols (72.2 mg/g). In this regard, aqueous and methanolic extracts showed similar phenolic content (51.9 and 46.2 mg/g, respectively), while n-hexane fruit showed the lowest TPC (22.8 mg/g) thus corroborating the
spectrophotometric results. In particular, looking to the specific dis-tribution of the main phenolic classes (Table 1), anthocyanins were better extracted by using ethyl acetate and water as extraction solvents, on average 15.8 mg/g, whileflavonols recorded the highest values in ethyl acetate fruits extracts (25.8 mg/g), followed by water (19.3 mg/g) and methanolic extracts (16.4 mg/g). Ethyl acetate was again the best solvent in promoting the extraction of phenolic acids (10.1 mg/g) fol-lowed by methanol (9.9 mg/g). Interestingly, stilbenes were better ex-tracted by using methanol and water, recording 2.2 and 1.3 mg/g, re-spectively, while ethyl acetate was found to be extremely selective in determining the extraction of tyrosol equivalents (i.e. 8.0 mg/g) when compared with the other extraction solvents used (on average 1.3 mg/ g). In an earlier study, the secoiridoid glucosides, coumarin and lignans have been reported for the members of the genus Ligustrum (Czerwińska et al., 2018). Also, phenylpropanoids and hydroxycinnamates including echinacoside, verbascoside, or p-coumaric acid have been found in their study (Czerwińska et al., 2018). In our experimental conditions, a clear abundance of lignans (such as sesamin derivatives), ligstroside, oleur-opein and other tyrosol equivalents (Supplementary Table 2) was found, and above all when considering ethyl acetate as extraction sol-vent, thus corroborating the results previously reported in literature. To the best of our knowledge, there is a scarcity of information regarding a robust characterization of the polyphenolic composition in Ligustrum vulgare fruit extracts. In fact, most of research available in literature is focused on assessing the potential health-promoting effects of Ligus-trum fruit and leaf extracts (Ćurčić et al., 2012;Czerwińska et al., 2018; Kiss et al., 2008) without performing a chromatography or mass spec-trometric characterization of the main compounds responsible of this bioactive activity. Therefore, our results could be very useful in pro-viding a better understanding about the phytochemical composition of these fruit extracts.
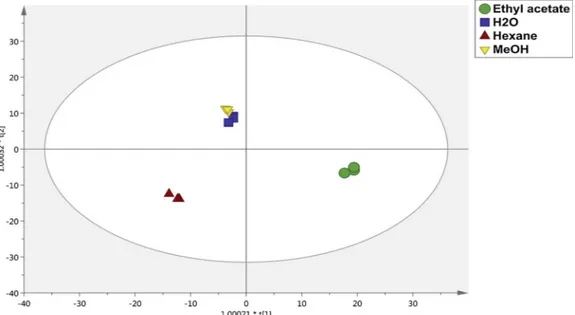
Subsequently, multivariate statistics was used to highlight di ffer-ences in the extraction efficiency, when considering each solvent used (i.e. n-hexane, ethyl acetate, water and methanol). The differential extraction efficiencies were confirmed by both unsupervised hier-archical clustering (HCA; in supplementary Table 2) and principal component analysis (PCA) carried out from the metabolomics-based data. In fact, looking at the HCA heat map produced from the fold-change distribution, n-hexane fruit extracts were included in a more distinct group, clearly indicating a differential abundance in some cluster of compounds. Accordingly, methanolic and water extracts were included in the same cluster, thus confirming a similar extraction effi-ciency promoted by these two extraction solvents used. Besides, the most differential profile was obtained when considering ethyl-acetate fruit extracts, clearly indicating the presence of an exclusive up-regu-lated cluster of compounds (as resulted from the HCA heat-map; in supplementary Table 2). Overall, using a class membership criterium in supervised and multivariate statistical approaches represents a valid strategy to maximize differences into the score plot hyperspace. Therefore, an OPLS-DA model was built considering each different ex-traction condition. The output of this analysis isfinally provided in Fig. 1. Notably, the discriminant model parameters were characterized by high values, being R2Y = 0.99 and Q2Y = 0.93. Besides, the OPLS-DA model was cross-validated (in supplementary Table 2), thus con-firming the robustness of the OPLS model built.
Table 1
Extraction yields (%) and quantification per classes of phenolics identified from UHPLC-QTOF-MS in the different Ligustrum fruit extractsa.
Solvents Extraction yields (%) Anthocyanins Flavones Flavanols Lignans Alkylphenols Tyrosols Phenolic acids Stilbenes
n-Hexane 4.84 2.0 ± 1.3a 1.2 ± 0.3a 9.8 ± 1.5a 1.0 ± 0.2a 1.2 ± 0.2a 0.9 ± 0.1a 6.0 ± 0.5a 0.7 ± 0.4a
Ethyl acetate 6.10 16.3 ± 4.6c 6.7 ± 0.6c 25.8 ± 5.1c 2.3 ± 0.1a 2.4 ± 0.2b 8.0 ± 1.4b 10.1 ± 1.2b 0.6 ± 0.1a
MeOH 35.64 7.8 ± 1.8b 5.9 ± 0.5c 16.4 ± 1.2b 2.6 ± 1.7a 0.9 ± 0.4a 1.2 ± 0.4a 9.9 ± 2.6b 2.2 ± 0.2b
Water 26.67 15.3 ± 2.8c 4.8 ± 0.4b 19.3 ± 2.6b 1.9 ± 1.7a 0.8 ± 0.3a 1.7 ± 0.9a 6.8 ± 0.8a 1.35 ± 0.7ab
a Results are expressed as mean values (mg/g) ± standard deviation (n = 3). Superscript letters within each column indicate homogeneous sub-classes as resulted from ANOVA (p < 0.01; Duncan’s post-hoc test).
Finally, the variable selection method VIP (variable importance in projections) was used in combination with a Fold-Change analysis in order to highlight those compounds most affected by the extraction solvent used. The results of these two different statistical approaches are reported inTable 2. Overall, 52 compounds were found to possess a VIP score higher than 1.2, with a great abundance offlavonoids among the VIP markers was depicted (32 compounds), followed by phenolic acids (11 compounds) and lignans (6 compounds). Overall, the list of VIP markers showed that each solvent was able to promote the selective extraction of some compounds. In this regard, the VIP approach al-lowed to confirm that ethyl acetate and water were the best extraction solvents in promoting the recovery of polyphenols. However, water was found to promote a higher recovery when compared with ethyl acetate, and considering the number of discriminant compounds observed. This finding was confirmed by inspecting the LogFC values when con-sidering the comparison ethyl acetate vs water (Table 2); in fact, an overall down-regulation was observed for all the discriminant markers, except for Quercetin 3-O-(6”-acetyl-galactoside) and isomeric forms of hydroxybenzoic acids. In addition, water promoted the best recovery of procyanidin trimers (belonging toflavanols) when considering all the pairwise comparisons carried out (Table 2). Interestingly, methanol determined the highest recovery of lignans, such as syringaresinol (VIP score = 1.33), sesamin (VIP score = 1.27) and sesamolinol (VIP score = 1.26). Finally, looking to the phenolic acids class, isomeric forms of caffeoylquinic and coumaroylquinic acids were found to be the most affected by the different extraction solvents used (Table 2). 3.2. In vitro antioxidant activity of Ligustrum fruit extracts antioxidant activity of Ligustrum fruit extracts
The in vitro antioxidant capacity of L. vulgare was determined by a series of assays including radical scavenging (DPPH and ABTS), redu-cing power (FRAP and CUPRAC), total antioxidant capacity (phospho-molybdenum) and metal chelating ability. There is a thin line between the activity of antioxidants and their chemical structure. Compounds such as flavonoids, phenolics, coumarins, limonoids are reported to have a good structure-activity relationship (Zou et al., 2016). Subse-quently, statistical analysis was done to determine any correlation be-tween different compounds and tested activities. Data showed that no positive correlation existed between radical scavenging capacities (DPPH, ABTS), reducing powers (FRAP, CUPRAC) and metal chelating.
On the other hand, phosphomolybdenum assay presented positive correlation withflavones (p = 0.93), anthocyanins (p = 0.72) and fla-vanols (p = 0.79) however, no significant correlation was reported with stilbenes, lignans, alkylphenols and tyrosols (Supplemental Table 3).
Table 3shows the antioxidant capacities that the berries of L. vul-gare hold. The best DPPH scavenging properties were exhibited by the aqueous fruit extracts. For instance, the strongest DPPH scavenging activity was observed by the aqueous fruit extract (181.16 mg TE/g) while the lowest activity was observed by the n-hexane extract (3.00 mg TE/g). Similarly, the highest ABTS scavenging potential was displayed by the aqueous fruit extract (237.05 mg TE/g) and the weakest ABTS scavenger was found to be the n-hexane extract (2.73 mg TE/g). The total antioxidant activity of the extracts was also evaluated using phosphomolybdenum assay involving an electron-transfer reaction based on the reduction of molybdenum (VI) to molybdenum (V) by the antioxidant (Meshkini, 2016). In this study, the highest antioxidant activity was exhibited by the ethyl acetate fruit extract (2.60 mg TE/g) and the least active extract was n-hexane (1.52 mg TE/g).
In terms of the reducing powers of the different extracts studied, CUPRAC and FRAP were conducted. According to the radical scaven-ging assays, results showed that aqueous fruit extract have the most significant reducing potential on CUPRAC and FRAP (391.92 and 440.25 mg TE/g). On the other hand, the aqueous fruit extract exerted the highest metal chelating effect (15.75 mg EDTAE/g) and the least chelating effect was observed with the methanolic fruit extract (4.45 mg EDTAE/g) agreeing with its corresponding TFC value (12.74 mg RE/g). In summary, the aqueous fruit extract showed the highest and most significant antioxidant activities in all assays conducted except with phosphomolybdenum. It can be extrapolated that the aqueous berry extract can be seen as a promising antioxidant source. Nowadays, there is a current trend towards replacing synthetic ones with“natural” an-tioxidants. As compared with other berries, L. vulgare berries exhibited significant antioxidant potentials. For example, Mocan et al. (2018) reported that the antioxidant potentials of two Goji berries cultivars (8.79–9.35 mg TE/g in DPPH; 24.86–25.12 mg TE/g in ABTS; 26.91–35.41 mg TE/g in CUPRAC and 16.91–19.52 mg TE/g in FRAP) which were lower than results recorded in the present study for L. vulgare (especially ethyl acetate, methanol and water extracts). Con-sistent with our results,Delgado et al. (2018)also found the strongest antioxidant abilities for water extracts of Ligustrum lucidum as compared ethanolic extracts. Taken together, it can be argued that the berries of L. Fig. 1. Multivariate and supervised OPLS-DA modelling considering the extraction solvent as class membership criterium. Individual replications (n = 3) are pro-vided into the OPLS-DA score plot.
vulgare can be as an important bioproducts for the food industry, due to their antioxidant activity and the presence of phenolic compounds. 3.3. Inhibitory activity on key enzymes
The enzyme inhibitory potential of L. vulgare fruit extracts was tested against several enzymes. Thefindings are represented inTable 4. Alzheimer’s disease (AD) existed long ago and has now become a common disorder among elder people. The World Health Organisation (WHO) reported more than 50 million cases of AD annually across the
globe (WHO, 2017). Cholinesterase inhibitors enhance synaptic plasti-city, thus improving learning and memory (Parsons et al., 2013). In this study the different fruit extracts prepared are screened for cholines-terase activity. The strongest AChE and BChE inhibition were observed methanolic and ethyl acetate extracts; 4.23 and 4.92 mg GALAE/g re-spectively while the weakest inhibition was observed with aqueous extract (0.31 and 0.67 mg GALAE/g, respectively). No activity was observed with the n-hexane extract for AChE inhibition property. In terms of statistical evaluation, a positive Pearson’s correlation of 0.81 and 0.67 was observed with phenolic acids for both AChE and BChE Table 2
Discriminant polyphenols identified by VIP (variable importance in projection) selection method following multivariate and supervised OPLS-DA modelling as function of the different extraction solvents. Compounds are provided together with VIP score and LogFC.
VIP marker Subclass VIP score LogFC EtAc vs H2O LogFC EtAc vs n-hexane LogFC EtAc vs MeOH LogFC H20 vs n-hexane LogFC H20 vs MeOH LogFC n-hexane vs MeOH
Peonidin 3-O-galactoside Anthocyanins 1.36 ± 0.58 −2.05 19.13 20.62 21.18 22.67 1.48 Delphinidin 3-O-sambubioside Anthocyanins 1.25 ± 0.50 −2.49 5.42 10.67 7.92 13.17 5.25 Cyanidin 3-O-(6”-acetyl-glucoside) Anthocyanins 1.24 ± 0.29 −2.67 −1.92 −3.38 0.75 −0.70 −1.45 Pelargonidin 3-O-glucosyl-rutinoside Anthocyanins 1.23 ± 0.71 −4.67 0.77 −2.85 5.44 1.81 −3.62 Cyanidin glucoside/ Petunidin
arabinoside/ Peonidin 3-O-arabinoside
Anthocyanins 1.21 ± 0.26 −8.66 10.98 −9.41 19.64 −0.75 −20.40 Peonidin glucoside/ Malvidin
3-O-arabinoside
Anthocyanins 1.21 ± 0.13 −2.05 19.13 −0.96 21.18 1.08 −20.09 Procyanidin trimer C1/C2 Flavanols 1.34 ± 0.76 −18.07 −2.20 −0.71 15.86 17.35 1.48 Procyanidin trimer EEC/ Procyanidin
trimer T2
Flavanols 1.34 ± 0.76 −18.07 −2.20 −0.71 15.86 17.35 1.48 Cinnamtannin A2 Flavanols 1.33 ± 0.81 −19.37 −2.20 −0.71 17.16 18.65 1.48 (+)-Catechin 3-O-gallate/ (-)-Epicatechin
3-O-gallate
Flavanols 1.31 ± 0.43 −0.63 13.66 −2.70 14.29 −2.07 −16.36 Theaflavin 3-O-gallate Flavanols 1.22 ± 0.21 −19.06 −2.20 −19.68 16.86 −0.61 −17.47 Naringin 4'-O-glucoside Flavanones 1.32 ± 0.52 −21.11 −22.07 −26.45 −0.96 −5.34 −4.38 Naringenin 7-O-glucoside Flavanones 1.32 ± 0.52 −21.11 −15.17 −26.45 5.93 −5.34 −11.28 Tangeretin Flavones 1.26 ± 0.33 −1.61 8.70 8.09 10.32 9.71 −0.60 Luteolin 7-O-glucuronide Flavones 1.25 ± 0.34 −8.28 12.11 −9.44 20.39 −1.15 −21.55 Apigenin
7-O-(6”-malonyl-apiosyl-glucoside)
Flavones 1.24 ± 0.23 −1.11 18.47 −2.31 19.58 −1.19 −20.78 6-Hydroxyluteolin Flavones 1.20 ± 0.23 −13.80 −2.20 −20.67 11.59 −6.86 −18.46 Scutellarein/ Luteolin Flavones 1.20 ± 0.16 −2.07 −0.51 −2.15 1.56 −0.08 −1.65 Quercetin xyloside/ Quercetin
3-O-arabinoside
Flavonols 1.25 ± 0.45 −20.60 −2.20 −12.96 18.40 7.64 −10.75 Quercetin 3-O-(6”-acetyl-galactoside)
7-O-rhamnoside
Flavonols 1.25 ± 0.24 6.16 18.61 −1.52 12.45 −7.68 −20.13 Kaempferol 3-O-glucuronide Flavonols 1.24 ± 0.34 −8.28 12.11 −9.44 20.39 −1.15 −21.55 Morin/ Quercetin Flavonols 1.20 ± 0.23 −13.80 −2.20 −20.66 11.59 −6.86 −18.46 Kaempferol Flavonols 1.20 ± 0.16 −2.07 −0.51 −2.15 1.56 −0.08 −1.65 Syringaresinol Lignans 1.33 ± 0.57 −6.40 −8.53 −11.50 −2.12 −5.09 −2.97 Episesamin/Sesamin Lignans 1.27 ± 0.54 −16.22 −20.89 −19.90 −4.67 −3.68 0.98 7-Oxomatairesinol Lignans 1.26 ± 0.32 −1.61 8.70 8.09 10.32 9.71 −0.60 Sesamolinol/Sesamol Lignans 1.26 ± 0.33 −1.36 −7.77 −9.18 −6.40 −7.81 −1.40 4-Ethylphenol Alkylphenols 1.34 ± 0.79 −28.07 −2.20 −15.31 25.87 12.75 −13.11 Catechol Others 1.25 ± 0.26 −1.50 15.51 −2.56 17.02 −1.05 −18.07 Protocatechuic aldehyde Hydroxybenzaldehydes 1.23 ± 0.49 −1.36 −7.77 −9.18 −6.40 −7.81 −1.40 2/4-Hydroxybenzoic acid Hydroxybenzoic acids 1.32 ± 0.69 12.40 −7.77 −9.18 −20.17 −21.58 −1.40 3/4/5-Caffeoylquinic acid Hydroxycinnamic acids 1.27 ± 0.31 −1.08 −5.63 −4.08 −4.54 −3.00 1.54 3/4/5-p-Coumaroylquinic acid Hydroxycinnamic acids 1.26 ± 0.39 −3.16 −1.30 −1.61 1.86 1.55 −0.30 Coumaric acid 4-O-glucoside/
p-Coumaroyl glucose
Hydroxycinnamic acids 1.23 ± 0.80 −12.26 −8.52 −9.73 3.74 2.52 −1.22
Table 3
In vitro antioxidant properties of the plant extractsa.
Solvents DPPH (mg TE/g) ABTS (mg TE/g) CUPRAC (mg TE/g) FRAP (mg TE/g) Phosphomolybdenum (mmol TE/g) Chelating (mg EDTAE/g)
n-hexane 3.00 ± 0.48a 2.73 ± 0.20a 42.70 ± 1.03a 17.03 ± 0.93a 1.52 ± 0.04a 13.82 ± 0.24c
Ethyl acetate 75.17 ± 0.78b 65.87 ± 1.66b 167.44 ± 6.07b 94.69 ± 0.99b 2.60 ± 0.05c naa
Methanol 96.14 ± 0.13c 123.16 ± 1.567c 258.22 ± 1.09c 156.22 ± 7.93c 2.41 ± 0.14b 4.45 ± 0.97b
Water 181.16 ± 4.87d 237.05 ± 4.26d 391.92 ± 2.10d 440.25 ± 0.87d 2.46 ± 0.11bc 15.75 ± 2.90c
a Values expressed are means ± S.D. of three parallel measurements. DPPH: 1,1-diphenyl-2-picrylhydrazyl; ABTS: 2,2 ′-azino-bis(3-ethylbenzothiazoline)-6-sul-fonic acid; CUPRAC: Cupric reducing antioxidant capacity; FRAP: Ferric reducing antioxidant power; TE: Trolox equivalent; EDTAE: EDTA equivalent. na = not active.
assays respectively. Consequently, the results can be justified based on the presence of phenolic acids observed in the tested extracts.
Tyrosinase is a copper containing enzyme responsible to produce a pigment called melanin. This pigment is secreted and produced by the melanocyte cells and this physiological process known as melanogen-esis. In human, melanin has great importance of in absorbing reactive oxygen species and also protecting the skin from harmful UV radiation (Mapunya et al., 2012;Zengin et al., 2019). Medicinal plants produced a panoply of phytochemicals among which some are appraised for their natural inhibition of tyrosinase. However, some compounds could also show adverse effects on the skin upon usage. For instance, hydro-quinone which was once widely used as topical treatment for skin whitening has been revoked by the FDA in 2006, due to skin irritation problems, mutagenicity, and cytotoxicity (Stratford et al., 2012). In this current study, the different extracts of L. vulgare were screened for their tyrosinase activity. As illustrated inTable 4, the methanolic fruit extract yielded the highest kojic acid equivalent values (132.85 mg KAE/g) and the lowest activity was observed with the aqueous extract (25.43 mg KAE/g). The following order of inhibition observed for L. vulgare was methanol extract > n-hexane extract > ethyl acetate extract > aqueous extract.
In addition to AD, diabetes mellitus (DM) has been one of the most burning topics among many scientists across the globe. At the time of writing, the WHO estimated that there are 422 million people of all age suffering from DM. It is reported that the etiology of this disease could be linked to smoking, lack of physical activity and unhealthy diet (Soni et al., 2018;WHO, 2018). Diabetic patients are vulnerable to strokes and the risk may be twice that of patients without diabetes ( Castilla-Guerra et al., 2018). DM is the most common endocrine disorder characterized by hyperglycemia resulting from defects in insulin se-cretion or insulin action (Nandini and Naik, 2019). Importantly, α-amylase and α-glucosidase are potential targets in producing lead compounds for the management of diabetes (Gulçin et al., 2018). In this study, the extracts of L. vulgare have been screened for their antidiabetic property. Results showed that the ethyl acetate extract inhibited the highest amylase activity (0.73 mmol ACAE/g) and the least inhibited extract was observed with the aqueous extract (0.12 mmol ACAE/g). Statistical analysis revealed that a positive correlation exists between amylase and alkylphenols (p = 0.73) and tyrosols (p = 0.61) respec-tively. However, the aforementioned compounds did not correlate with glucosidase activity, but instead phenolic acids presented a positive correlation of 0.73.
To the best of our knowledge, there is currently a paucity of in-formation on the scientific literature regarding the inhibitory effects of L. vulgare fruit extracts against the tested enzymes. However, the ob-tained results could be compared with some berries. For example, the enzyme inhibitory effects of two Goji berries cultivars (Mocan et al., 2018) have been reported lower than that of L. vulgare fruit extracts in the present study. In addition, the L. vulgare extracts exhibited stronger inhibitory abilities when compared to Schisandra chinensis fruit extracts (0.98 mg GALAE/g in AChE; 1.78 mg GALAE/g in BChE; 0.46 mmol ACAE/g in amylase; 0.31 mmol ACAE/g in glucosidase and 10.24 mg KAE/g in tyrosinase) (Mocan et al., 2016). In this perspective, given the global interest of natural enzyme inhibitors to manage common health
problems, preliminary date amassed in the present study could open new avenues for further research.
4. Conclusions
This study reports for thefirst time a detailed investigation on the fruit of L. vulgare involving UHPLC-QTOF chemical profiling, anti-oxidant and enzymatic inhibitory activities. Findings presented herein revealed potential antioxidant and enzymatic activities which could be partially ascribed to the presence phenolic andflavonoid compounds. Using Pearson’s correlation coefficient, we could observe a positive satisfactory correlation between phenolic acids and acet-ylcholinesterase and glucosidase accordingly. In this regard, the fruit of L. vulgare could be considered as a future potent medicinal plant with an array of therapeutic properties. The methanolic extract exhibited the highest acetylcholinesterase and tyrosinase activities while the aqueous extracts exhibited the most significant antioxidant activities. To further support the preliminary data collected in the present study, further investigations should be designed to assess the cytotoxicity, bioavail-ability and in vivo studies to support the development of biomedicine from this plant.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.indcrop.2019.111635. References
Australia,W.O.,2016.https://keyserver.lucidcentral.org/weeds/data/media/Html/ ligustrum_vulgare.htm.
CABI, 2018.https://www.cabi.org/isc/datasheet/30764.
Castilla-Guerra, L., Fernandez-Moreno, Md.C., Leon-Jimenez, D., Carmona-Nimo, E., 2018. Antidiabetic drugs and stroke risk. Current evidence. Eur. J. Intern. Med. 48, 1–5.
Ćurčić, M.G., Stanković, M.S., Mrkalić, E.M., Matović, Z.D., Banković, D.D., Cvetković, D.M.,Đačić, D.S., Marković, S.D., 2012. Antiproliferative and proapoptotic activities of methanolic extracts from Ligustrum vulgare L. As an individual treatment and in combination with palladium complex. Int. J. Mol. Sci. 13, 2521–2534.
Czerwińska, M.E., Gąsińska, E., Leśniak, A., Krawczyk, P., Kiss, A.K., Naruszewicz, M., Bujalska-Zadrożny, M., 2018. Inhibitory effect of Ligustrum vulgare leaf extract on the development of neuropathic pain in a streptozotocin-induced rat model of diabetes. Phytomedicine 49, 75–82.
Delgado, T., Paula, V.B., Campos, M.G., Farinha, N., Caeiro, A., Estevinho, L.M., Anjos, O., 2018. Screening of biological activities of Ligustrum lucidum Berries: a comparative approach. Nat. Prod. Comm. 13, 1685–1690.
Dutta, S., Ray, S., 2018. Comparative assessment of total phenolic content and in vitro antioxidant activities of bark and leaf methanolic extracts of Manilkara hexandra (Roxb.) Dubard. J. King Saud Univ. - Sci.https://doi.org/10.1016/j.jksus.2018.09. 015.
Gao, D., Li, Q., Li, Y., Liu, Z., Fan, Y., Liu, Z., Zhao, H., Li, J., Han, Z., 2009. Antidiabetic and antioxidant effects of oleanolic acid from Ligustrum lucidum Ait in alloxan-in-duced diabetic rats. Phytother. Res. 23, 1257–1262.
Granato, D., Shahidi, F., Wrolstad, R., Kilmartin, P., Melton, L.D., Hidalgo, F.J., Miyashita, K., Camp, Jv., Alasalvar, C., Ismail, A.B., Elmore, S., Birch, G.G., Charalampopoulos, D., Astley, S.B., Pegg, R., Zhou, P., Finglas, P., 2018. Antioxidant activity, total phenolics andflavonoids contents: Should we ban in vitro screening methods? Food Chem. 264, 471–475.
Gulçin,İ., Taslimi, P., Aygün, A., Sadeghian, N., Bastem, E., Kufrevioglu, O.I., Turkan, F., Şen, F., 2018. Antidiabetic and antiparasitic potentials: inhibition effects of some natural antioxidant compounds onα-glycosidase, α-amylase and human glutathione Table 4
Enzyme inhibitory activity of the plant extractsa.
Solvents AChE inhibition (mg GALAE/g)
BChE inhibition (mg GALAE/g)
Tyrosinase inhibition (mg KAE/ g)
Amylase inhibition (mmol ACAE/g)
Glucosidase inhibition (mmol ACAE/g)
n-hexane naa 3.00 ± 0.05b 116.39 ± 3.64c 0.49 ± 0.03b 0.87 ± 0.01b
Ethyl acetate 4.09 ± 0.52b 4.92 ± 0.28d 63.01 ± 0.35b 0.73 ± 0.02d 1.67 ± 0.01c
Methanol 4.23 ± 0.08b 4.58 ± 0.16c 132.85 ± 0.35d 0.53 ± 0.03c 1.94 ± 0.01d
Water 0.31 ± 0.12a 0.67 ± 0.06a 25.43 ± 0.90a 0.12 ± 0.01a 0.34 ± 0.09a
a Values expressed are means ± S.D. of three parallel measurements. AChE: Acetylcholinesterase; BChE: Butrylcholinesterase; GALAE: Galatamine equivalent; KAE: Kojic acid equivalent; ACAE: Acarbose equivalent; na: not active.
S-transferase enzymes. Int. J. Biol. Macromol. 119, 741–746.
Kiss, A.K., Mańk, M., Melzig, M.F., 2008. Dual inhibition of metallopeptidases ACE and NEP by extracts, and iridoids from Ligustrum vulgare L. J. Ethnopharmacol. 120, 220–225.
Lau, K.-M., He, Z.-D., Dong, H., Fung, K.-P., But, P.P.-H., 2002. Anti-oxidative, anti-in-flammatory and hepato-protective effects of Ligustrum robustum. J. Ethnopharmacol. 83, 63–71.
Lin, H.M., Yen, F.L., Ng, L.T., Lin, C.C., 2007. Protective effects of Ligustrum lucidum fruit extract on acute butylated hydroxytoluene-induced oxidative stress in rats. J. Ethnopharmacol. 111, 129–136.
List, T.P., 2013. http://www.theplantlist.org/1.1/browse/A/Oleaceae/Ligustrum/.
Mapunya, M.B., Nikolova, R.V., Lall, N., 2012. Melanogenesis and antityrosinase activity of selected South African plants. J. Evid. Complementary Altern. Med. 2012 (6), 1–6.
Meshkini, A., 2016. Acetone Extract of almond hulls provides protection against oxidative damage and membrane protein degradation. J. Acupunct. Meridian Stud. 9, 134–142.
Mocan, A., Zengin, G., Crişan, G., Mollica, A., 2016. Enzymatic assays and molecular modeling studies of Schisandra chinensis lignans and phenolics from fruit and leaf extracts. J. Enzym. Inhib. Med. Chem. 31, 200–210.
Mocan, A., Moldovan, C., Zengin, G., Bender, O., Locatelli, M., Simirgiotis, M., Atalay, A., Vodnar, D.C., Rohn, S., Crișan, G., 2018. UHPLC-QTOF-MS analysis of bioactive constituents from two Romanian Goji (Lycium barbarum L.) berries cultivars and their antioxidant, enzyme inhibitory, and real-time cytotoxicological evaluation. Food Chem. Toxicol. 115, 414–424.
Mohammedi, Z., Atik, F., 2011. Impact of solvent extraction type on total polyphenols content and biological activity from Tamarix aphylla (L.) Karst. Int. J. Pharma Bio Sci. 2, 609–615.
Nandini, H.S., Naik, P.R., 2019. Antidiabetic, antihyperlipidemic and antioxidant effect of Vincamine, in streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 843, 233–239. Natural Medicine Facts, 2015. Ligustrum Vulgare. http://www.naturalmedicinefacts.
info/plant/ligustrum-vulgare.html.
Parsons, C.G., Danysz, W., Dekundy, A., Pulte, I., 2013. Memantine and cholinesterase inhibitors: complementary mechanisms in the treatment of Alzheimer’s disease. Neurotox. Res. 24, 358–369.
Petrovska, B.B., 2012. Historical review of medicinal plants’ usage. Pharmacogn. Rev. 6, 1–5.
Quattrocchi, U., 2016. CRC World Dictionary of Medicinal and Poisonous Plants: Common Names, Scientific Names, Eponyms, Synonyms, and Etymology (Volume 5). CRC press, NW, USA.
Rocchetti, G., Giuberti, G., Lucini, L., 2018a. Gluten-free cereal-based food products: the potential of metabolomics to investigate changes in phenolics profile and their in vitro bioaccessibility. Curr. Opin. Food Sci. 22, 1–8.
Rocchetti, G., Blasi, F., Montesano, D., Ghisoni, S., Marcotullio, M.C., Sabatini, S.,
Cossignani, L., Lucini, L., 2019a. Impact of conventional/non-conventional extraction methods on the untargeted phenolic profile of Moringa oleifera leaves. Food Res. Int. 115, 319–327.
Rocchetti, G., Giuberti, G., Gallo, A., Bernardi, J., Marocco, A., Lucini, L., 2018b. Effect of dietary polyphenols on the in vitro starch digestibility of pigmented maize varieties under cooking conditions. Food Res. Int. 108, 183–191.
Rocchetti, G., Lucini, L., Rodriguez, J.M.L., Barba, F.J., Giuberti, G., 2019b. Gluten-free flours from cereals, pseudocereals and legumes: phenolic fingerprints and in vitro antioxidant properties. Food Chem. 271, 157–164.
Soni, L.K., Dobhal, M.P., Arya, D., Bhagour, K., Parasher, P., Gupta, R.S., 2018. In vitro and in vivo antidiabetic activity of isolated fraction of Prosopis cineraria against streptozotocin-induced experimental diabetes: a mechanistic study. Biomed. Pharmacother. 108, 1015–1021.
Stratford, M.R.L., Ramsden, C.A., Riley, P.A., 2012. The influence of hydroquinone on tyrosinase kinetics. Bioorg. Med. Chem. 20, 4364–4370.
Teixidor-Toneu, I., Jordan, F.M., Hawkins, J.A., 2018. Comparative phylogenetic methods and the cultural evolution of medicinal plant use. Nat. Plants 4, 754–761. USDA-ARS, 2018.https://npgsweb.ars-grin.gov/gringlobal/taxonomydetail.aspx?id=
22101.
Uysal, S., Zengin, G., Locatelli, M., Bahadori, M.B., Mocan, A., Bellagamba, G., De Luca, E., Mollica, A., Aktumsek, A., 2017. Cytotoxic and enzyme inhibitory potential of two Potentilla species (P. Speciosa L. And P. Reptans Willd.) and their chemical composi-tion. Front. Pharmacol. 8, 290.
WHO, 2017.https://www.who.int/news-room/fact-sheets/detail/dementia. WHO, 2018.https://www.who.int/news-room/fact-sheets/detail/diabetes.
Wu, C.-R., Lin, W.-H., Hseu, Y.-C., Lien, J.-C., Lin, Y.-T., Kuo, T.-P., Ching, H., 2011. Evaluation of the antioxidant activity offive endemic Ligustrum species leaves from Taiwanflora in vitro. Food Chem. 127, 564–571.
Yu, Z.-L., Gao, H.-X., Zhang, Z., He, Z., He, Q., Jia, L.-R., Zeng, W.-C., 2015. Inhibitory effects of Ligustrum robustum (Rxob.) Blume extract on α-amylase and α-glucosidase. J. Funct. Foods 19, 204–213.
Zengin, G., Mahomoodally, M.F., Picot-Allain, C.M.N., Cakmak, Y.S., Uysal, S., Aktumsek, A., 2019. In vitro tyrosinase inhibitory and antioxidant potential of Consolida or-ientalis, Onosma isauricum and Spartium junceum from Turkey. S. Afr. J. Bot. 120, 119–123.
Zhu, F., Cai, Y.I.Z., Sun, M., Ke, J., Lu, D., Corke, H., 2009. Comparison of major phenolic constituents and in vitro antioxidant activity of diverse kudingcha genotypes from Ilex kudingcha, Ilex cornuta, and Ligustrum robustum. J. Agric. Food Chem. 57, 6082–6089.
Zou, Z., Xi, W., Hu, Y., Nie, C., Zhou, Z., 2016. Antioxidant activity of Citrus fruits. Food Chem. 196, 885–896.