1 (1), 2007, 17-25
©BEYKENT UNIVERSITY
SOLID/LIQUID EXTRACTION OF COPPER (Cu)
FROM MUNICIPAL SOLID WASTE COMPOST
Cuma BAYAT*, Emine ELMASLAR OZBAS**, Tuncer
CELIK*
*Beykent University, Beylikduzu, Buyukcekmece, Istanbul;
TURKEY
e-mail: cbayat@beykent.edu.tr
Istanbul University, Faculty of Engineering, Environmental
Eng. Dept., Avcilar, Istanbul;TURKEY
e-mail: elmaslar@istanbul.edu.tr
ABSTRACT
Compost can be used as a soil conditioner as it contains major plant nutrients, such as N, P, and K, microplant nutrients such as Cu, Fe, and Zn, and organic matter, which improves the physical properties in order to have a better soil aeration and water holding capacity. The presence of organic and inorganic contaminants in compost may, however, constitute a danger to the environment. It is the heavy metal content which is the main factor leading to restricted agricultural use of compost. Literature on the effect of compost use on heavy metal levels in the environment shows it to vary according to soil type, plant species and compost quality.
This paper deals with the removal of copper from Municipal Solid Waste (MSW) compost using Na2EDTA solution and a mixture of Na2S2O5
(sodium metabisulfite) solution and Na2EDTA solution in the batch mode. At the end of extraction studies, for 3 hours at 1:25 solid:liquid ratio by using 0.05 M Na2EDTA solution, 100% removal yield was obtained for Cu. 100% heavy
metal removal yield was obtained with 0.01 M Na2EDTA and 0.1 M N a 2 S 2 O 5 , at 1:6 solid:liquid ratio, for Cu in this study.
Key Words: Municipal solid waste compost, Copper (Cu), Extraction.
ÖZET
Kompost toprak özelliklerini iyileştirmesi, bitkiler için gerekli nütrientleri içermesi gibi özellikleri nedeniyle toprak şartlandırıcısı olarak kullanılmaktadır. Bununla birlikte bazı inorganik ve organik toksik maddeler içerdiği takdirde çevre için bir tehlike oluşturması söz konusudur. Bu nedenle ağır metal içeriği kompostun tarımsal amaçlı kullanımında önemli bir
sınırlayıcı faktördür ve kompostun ağır metal içeriği ile ilgili yasal düzenlemeler mevcuttur.
Bu çalışmada evsel katı atıktan elde edilen kompost içerisinden bakır (Cu)'ın ekstraksiyon ile giderilebilirliği incelenmiştir. Deneyler Na2EDTA
çözeltisinin tek başına veya Na2S2O5 (sodyum metabisülfit) ve Na2EDTA çözeltilerinin karışımının kullanılmasıyla kesikli olarak gerçekleştirilmiştir. Ekstaksiyon deneyleri sonucunda 3 saatlik bekletme süresinde 1:25 katı:sıvı oranında 0.05 M Na2EDTA çözeltisi kullanılarak Cu için %100 giderim
verimi elde edildiği görülmüştür. 0.01 M Na2EDTA ve 0.1 M Na2S2O5
çözeltileri karışımı kullanıldığında ise 1:6 katı:sıvı oranında 3 saatlik çalkalama süresinde aynı giderim veriminin eldedildiği görülmüştür.
Anahtar kelimeler: Evsel katı atık kompostu, Bakır (Cu), Ekstraksiyon.
1. INTRODUCTION
Compost can be used as a soil conditioner as it contains major plant nutrients, such as N, P, and K, microplant nutrients such as Cu, Fe, and Zn, and organic matter, which improves the physical properties in order to have a better soil aeration and water holding capacity [1]. The presence of organic and inorganic contaminants in compost may, however, constitute a danger to the environment. It is the heavy metal content which is the main factor leading to restricted agricultural use of compost. Literature on the effect of compost use on heavy metal levels in the environment shows it to vary according to soil type, plant species and compost quality [2-4].
The presence of heavy metal ions in the environment has been of great concern because their toxic nature and other adverse effects on many life forms. For instance, excessive intake of copper results in an accumulation in the liver and it is also toxic to organisms [5].
Technologies available for treating metal contaminated sludge and soils include solidification/stabilization and vitrification. Recently, researchers try to develop soil washing techniques where soil-bound contaminants are transferred to the liquid phase by desorption and solubilization. Several washing solutions have been investigated such as water, acids, bases, chelating agents, alcohol and other additives. In practice, acid washing and chelator soil washing are two most prevalent removal methods [6]. EDTA is effective in removing metals from contaminated soils but the cost of EDTA is very high. Na2S2O5 is an inexpensive reducing agent, which has been extensively used to treat different metal contaminated waste. Abumaizar and Smith [7] studied on the batch and column washing of metals contaminated silky sand. They found that a mixture of 0.1 M Na2S2Os and 0.01 M Na2EDTA provides an economically optimum solution for cadmium and zinc removal.
The aim of the study was to practise soil washing techniques for removing Cu from M S W compost using the different concentrations of Na2EDTA solutions and the different concentration of mixture of Na2S2O5
(reducing reagent) and Na2EDTA (chelating reagent). The experiments were
done in batch mode.
2. MATERIALS AND METHODS
Compost samples
The compost samples were taken from Istanbul Solid Waste Recycling and Composting Plant in Kisirmandra, Istanbul. Fresh compost sample was dried at 105 °C for total Cu analysis and ground to yield 0.25 mm-sized particles. An approximately 1-2 g of this sample was weighed into an erlenmeyer. Digestion was performed by heating with a 10 mL-mixture of 1:1 (v/v) deionized water and nitric acid. After cooling, hydrogen peroxide, concentrated hydrochloric acid, and deonized water were added to the sample, and heated again. The contents were allowed to cool to room temperature, after which they were diluted with deionized water and passed through a 0.45 |im filter, and processed on an atomic absorption spectrometer (UNICAM 929 AA spectrometer) for total Cu concentration determination [8].
Total Cu content in the sample (Y) was calculated as mg/kg (dry weight) with the following equation:
Y= (CxV)/ m,
where C is Cu concentration (mg/l), V is the final volume of the sample (liter), and m is the mass of the sample (kg).
Total Cu amount in the compost used in the study is 348.6 mg/kg-dry compost. The maximum acceptable value of Cu in German standarts is 150 mg/kg-dry compost [8]. In this context, 57 % Cu removal yields should be obtained. Heavy metal compounds in compost were determined with Rigaku D/Max-2200/PC XRD spectrometer.
Batch washing
Five grams of dry sample was placed into a volumetric flask. Varying volumes and concentrations of washing solution were added into the sample. The sample was shaken horizontally at room temperature for 3 h at 160 rpm. After mixing, the aliquot was filtered through a 0.45-^m membrane filter using vacuum filtration. Following the filtration, the filtrate was acidified to pH <2.0 with 1:1 HNO3 for heavy metal analysis. Precision was established by
preparing a replicate for each test. It was assumed that the metal concentration of the filtrate represents the released from the compost. Removal efficiencies were determined by dividing the heavy metal release quantities by the initial quantity in the compost. Heavy metal analyses were performed using atomic absorption spectrometer (AAS) (UNICAM 929 AA spectrometer).
3. RESULTS AND DISCUSSION
Heavy metal compounds in compost
XRD spectrometer results are shown in Figure 1 and Table 1. As shown in Table 1 and Figure 1, Cu is C u20 (cuprit) form in compost sample.
Two téta angles (degree) Figurel. XRD results of compost sample Table 1. Heavy metal compounds in compost sample
Compounds names Chemical Formula
Cadmium lead oxide CdPbOs
Cuprit CU2O
Gypsum Ca(SO4)(H2O)2
Magnesium calsit (MgO.6CaO.94)(CO3)
Quartz SÍO2
Zinc Oxide ZnO
Extractions with NaiEDTA
Determination of the optimum Na2EDTA concentration
Five grams of dry sample was placed into a volumetric flask. 30 mL of Na2EDTA solutions with varying concentrations were added into the sample.
These samples were analyzed to determine the optimum Na2EDTA
concentration. Results are shown in Table 2. Heavy metal removal efficiencies (%) for different concentrations of Na2EDTA are shown in Figure 2.
Table 2. Cu amounts removed from the compost (mg/kg-dry compost) and removal efficiencies obtained after extraction (%)
Concentrations (M) Cu(mg/kg-dry compost) Removal efficiencies (%)
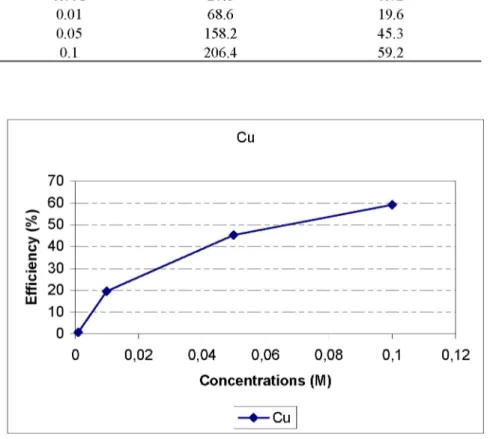
0.001 20.8 0.72 0.01 68.6 19.6 0.05 158.2 45.3 0.1 206.4 59.2 Cu oN 0) Ü £ LU 0 0,02 0,04 0,06 0,08 0,1 0,12 Concentrations (M) - • -C u
Figure 2. Cu removal efficiencies (%) obtained for different Na2EDTA concentrations
Upon examination of Figure 2, it was observed that increased removal yields for Cu were obtained with increased molarity of Na2EDTA solution.
Figure 2 shows that the maximum removal efficiency for 0.1 M Na2EDTA is
59% for Cu.
Determination of the optimum solid:liquid ratio
The optimum ratio was determined by addition of different amounts of 0.05 M Na2EDTA solution (1:2.5, 1:5, 1:12.5, 1:25). The samples prepared
before were analyzed with this manner and the results are shown in Table 3. In Figure 3, heavy metal removal yields (%), obtained from different solid:liquid ratios, were shown.
Table 3. Cu amounts removed from the compost (mg/kg-dry compost) and removal efficiencies obtained after extraction (%)
solid:liquid ratios (g/mL) Cu(mg/kg-dry compost) Removal efficiencies (%)
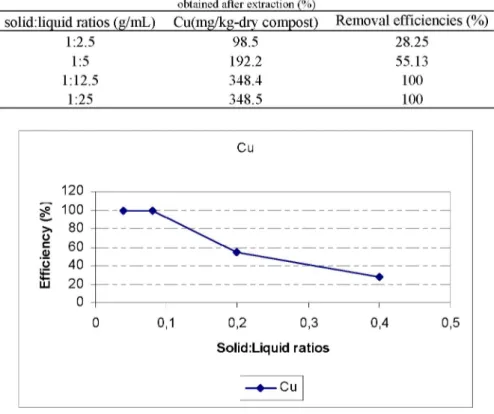
1:2.5 98.5 28.25 1:5 192.2 55.13 1:12.5 348.4 100 1:25 348.5 100 Cu 0s V o it LU 0 0,1 0,2 0,3 0,4 0,5 Solid:Liquid ratios —•—Cu
Figure 3. Cu removal efficiencies (%) obtained for different solid:liquid ratios
As stated before, the increasing amount of Na2EDTA solution, which necessitates that the solid:liquid ratio goes lower, gives higher removal yields. Table 3 and Figure 3 show that the 100% removal yield is reached. The highest yield is present with a ratio of 1:12.5.
Studies performed with Na2EDTA and Na2S2Os mixture
Determination of the optimum solid:liquid ratio
Experiments were carried out with 0.01 M Na2EDTA and 0.1 M
Na2S2O5 mixture, with several solid:liquid ratios. To prepare 0.01 M
Na2EDTA and 0.1 M Na2S2O5 mixture, 19.01 gr Na2S2O5 and 3.7224 gr Na2EDTA were added into 1 liter of distilled water. To 5 g of compost,
several different volumes of this mixture were added and the samples were prepared. They were analyzed to determine the optimum solid:liquid (g/mL) ratio. Results are shown in Table 4. Figure 4 indicates Cu removal efficiencies (%), obtained for different solid:liquid ratios.
Table 4. Cu amounts removed from the compost (mg/kg-dry compost) and Cu removal efficiencies (%) obtained after extraction
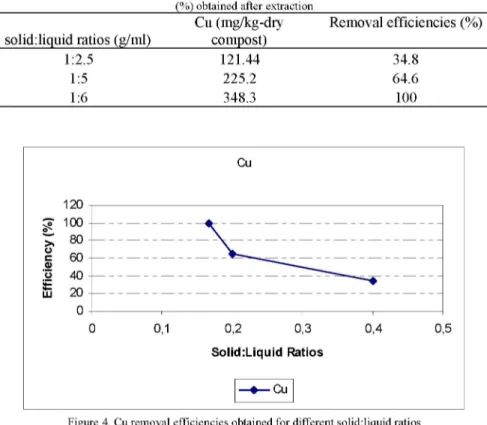
Cu (mg/kg-dry Removal efficiencies (%) solid:liquid ratios (g/ml) compost)
1:2.5 121.44 34.8 1:5 225.2 64.6 1:6 348.3 100 Cu Solid:Liquid Ratios —•— Cu
Figure 4. Cu removal efficiencies obtained for different solid:liquid ratios
After the experiments, it is seen that decreasing the solid:liquid ratio increases Cu removal efficiencies. The highest removal efficiency was obtained with 1:6 solid:liquid ratio (Cu: %100) (Fig. 4).
Determination of the optimum concentration of the Na2EDTA and Na2S2O5 mixture
The results of experiments to find the optimum concentration are presented in Table 5. In Figure 5, Cu removal efficiencies (%) for different concentrations are shown. To prepare Solution 1, 1.901 gr Na2S2O5 and 3.7224
gr Na2EDTA were added into 1 liter of distilled water. To prepare Solution 2,
19.01 gr Na2S2O5 and 1.8612 gr Na2EDTA were added into 1 liter of distilled water. To prepare Solution 3, 19.01 gr Na2S2O5 and 3.7224 gr Na2EDTA were
added into 1 liter of distilled water.
Table 5. Cu amounts removed from the compost (mg/kg-dry compost) and removal efficiencies (%)
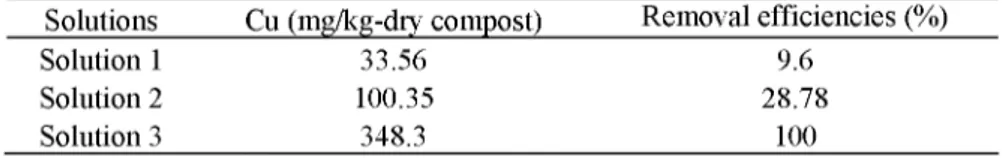
Solutions Cu (mg/kg-dry compost) Removal efficiencies (%)
Solution 1 33.56 9.6 Solution 2 100.35 28.78 Solution 3 348.3 100 Solution 1 Solution 2 Solution 3
0.001 M Na2EDTA and 0.1 M Na2S2O5 mixture
0.01 M Na2EDTA and 0.05 M Na2S2O5 mixture
0.01 M Na2EDTA and 0.1 M Na2S2O5 mixture
After the experiments, the highest removal yield was obtained with the mixture of 0.01 M Na2EDTA and 0.1 M Na2S2Ü5. Cu removal yields for this
mixture is 100% (Table 5).
4. CONCLUSIONS
Cu removal efficiencies increase with increasing chelator concentration. Further, as the solid:liquid ratio decreases, higher removal efficiencies are reached. After agitation for 3 hours at 1:25 solid:liquid ratio by using 0.05 M Na2EDTA, 100% removal yield was obtained. Literature suggests that sole
chelator usage proves more costly, unless Na2S2O5 is included as the reducing
reagent. Therefore, the optimum removal yield was obtained with 0.01 M Na2EDTA and 0.1 M Na2S2O5 mixture. 0.01 M Na2EDTA and 0.1 M Na2S2O5
provided more efficient removal than sole Na2EDTA usage at 0.05 M, which
indicates that the solid:liquid ratio of the former is higher (i.e., less solution volume). 100% Cu removal yield was obtained with 0.01 M Na2EDTA and 0.1
M Na2S2O5, at 1:6 solid:liquid ratio. But in German standarts, the maximum
acceptable value of Cu is 150 mg/kg-dry compost, for this reason 57 % Cu removal yields should be obtained. It can thus be concluded that the mixture of 0.01 M Na2EDTA and 0.1 M Na2S2O5, at 1:5 solid:liquid ratio can
REFERENCES
1. Zorpas A. A., Vassilis I., Loizidou M. and Grigoropoulou H.; Particle size effects on uptake of heavy metals from sewage sludge compost using natiral zeolite clinoptilolite, J Colloid Interf Sci, 250 (2002), 1-4. 2. Zorpas A. A., Constantinides T., Vlyssides A. G., Haralambous I. and
Loizidou M.; Heavy metal uptake by natural zeolite and metals partitioning in sewage sludge compost, Bioresource Technol, 72 (2000)
113-119.
3. Horn Andreas L., Düring R-A, Gath S.; Comparison of decision support systems for an optimised application of compost and sewage sludge on agricultural land based heavy metal accumulation in soil, Sci Total Environ, 311 (2003) 35-48.
4. Ciba J., Zolotajkin M., Kluczka J., Loska K., Cebula J.; Comparison of methods for leaching heavy metals from composts, Waste Manage, 23 (2003) 897-905.
5. Kasgoz H., Sahin U., Temelli T. Y., Kasgoz A. and Bayat C.; The hydrogels with acid groups for removel of copper (II) and lead (II) ions, Polymer-Plastics Tech & Eng (PPTE), 45 (2006) 117-124.
6. Chaiyaraksa C. and Sriwiriyanuphap N.; Batch washing of cadmium from soil and sludge by a mixture of Na2S2O5 and Na2EDTA,
Chemosphere, 56 (2004) 1129-1135.
7. Abumaizar R. J. and Smith E. H.; Heavy metal contaminants removal by soil washing, J Hazard Mater, B70 (1999) 71-86.
8. Demir A., Tosun 1., Ozkaya B., Bilgili M. S., Gunay A., Avsar F. and Karaaslan Y.; Aerobic Composting of municipal solid wastes in Istanbul: Start-up and operational experiences, ISWA 2002 World Congress&Exhibition, Appropriate Environmental and Solid Waste Management and Technologies for Developing Countries, Istanbul,