Enzyme inhibitory and antioxidant properties of six mushroom species
from the Agaricaceae family
I. Akata
a, G. Zengin
b,⁎
, C.M.N. Picot
c, M.F. Mahomoodally
ca
Ankara University, Science Faculty, Department of Biology, Ankara, Turkey
bSelcuk University, Science Faculty, Department of Biology, Campus, 42250, Konya, Turkey c
University of Mauritius, Faculty of Science, Department of Health Sciences, Réduit, Mauritius
a b s t r a c t
a r t i c l e i n f o
Available online 3 February 2018 Edited by J Van Staden
Mushrooms are excellent sources of nutraceuticals and have attracted much interest as functional foods. In this work the inhibitory capacity of six macrofungus (Coprinus comatus, Macrolepiota mastoidea, Agaricus campestris, Lycoperdon utriforme, Macrolepiota procera, and Leucoagaricus leucothites) against key enzymes related to diabetes type II (α-amylase, α-glucosidase), Alzheimer's disease [acetylcholinesterase (AChE) and butyrylcholinesterase (BChE)] was assessed for thefirst time. The antioxidant properties (total antioxidant capacity, metal chelating, ferric reducing antioxidant power (FRAP), cupric reducing antioxidant capacity (CUPRAC), 2,2-diphenyl-1-picrylhydrazyl (DPPH), and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) radical scavenging as-says) and phenolic contents of the selected mushroom species were also evaluated. We found that L. utriforme had the highest enzyme inhibitory activity (0.97 mg galantamine equivalents (GALAE)/g extract, 1.33 mg GALAE/g extract, 0.22 mmol acarbose equivalents (ACAE)/g extract, and 2.97 mmol ACAE/g extract against AChE, BChE, α-amylase, and α-glucosidase, respectively). A widely consumed mushroom species namely A. campestris pos-sessed the highest phenolic content (15.63 mg GAE/g extract) and the maximum antioxidant potential against DPPH, ABTS, FRAP, and CUPRAC. Experimental data collected in the present study support the use of these mushrooms specially L. utriforme and A. campestris as functional foods for the management and/or prevention of diabetes type II, Alzheimer's disease, and oxidative stress related complications.
© 2018 SAAB. Published by Elsevier B.V. All rights reserved.
Keywords: Macrofungus Alzheimer's disease Diabetes Glucosidase, cholinesterases Agaricaceae Phenolic compound 1. Introduction
For centuries, mushrooms have been appraised and extensively used for their medicinal properties (Muszyńska et al., 2017). In fact, several well-known pharmacophores, such as penicillin, ergot alkaloids, cyclosporine, and griseofulvin, are of fungal origin (Zengin et al., 2016a). Research over the past decades has revealed the role of mushrooms as a nutritional component of diet being an excellent source of riboflavin, selenium, copper, potassium, vitamin D, dietary fibre, chitin, andβ-glucans and having a very low fat content (Kalaras et al., 2017). Besides, their nutritious importance in diet, the repertoire of biologically active compounds present in mushrooms has attracted considerable attention for the development of functional formulations for the prevention and/or management of chronic complications.
In the present study six macrofungus were studied, namely Coprinus comatus (O.F.Mull.) Pers., Macrolepiota mastoidea (Fr.) Singer, Agaricus campestris L., Lycoperdon utriforme Bull., Macrolepiota procera (Scop.) Singer, and Leucoagaricus leucothites (Vittad.) Wasser. The selected
mushroom species belong to the Agaricaceae family, which contain spe-cies cultivated for culinary purposes and health promoting applications (Wasser and Didukh, 2003). C. comatus, also known as shaggy ink cap, is a common and edible mushroom, with multiple valuable medicinal functions such as hypolipidemic, immunomodulation, antibacterial, an-titumor, and hypoglycemic effects (Liu et al., 2013). Macrolepiota mastoidea, frequently found in hardwood forests, is widely consumed for its high nutritional value and is used for the management of stomach and heart complications (Kolcuoğlu et al., 2007). Agaricus campestris is a widely consumed mushroom which is used for the management of ul-cers, bed sores, scalds, and burns (Harding, 2008). Lycoperdon utriforme (Karun and Sridhar, 2017), showed potent antimicrobial activity against Bacillus subtilis, Escherichia coli, Klebsiella pneumonia, Pseudomonas aeruginosa, Salmonella typhimurium, Staphylococcus aureus, Streptococ-cus pyogenes, and Mycobacterium smegmatis (Dulger, 2005). Calcaelin, a bioactive compound isolated from L. utriforme (De Silva et al., 2012a) was reported as an anti-cancerous agent (Prasad et al., 2015). M. procera, also called the parasol mushroom, was identified as an edible species of the Macrolepiota genus, having a large prominent parasol-like fruiting body (Ge et al., 2010). Leucoagaricus leucothites, which grows among grass in gardens (Kaya, 2009), is an edible macrofungus previ-ously reported to exhibit strong antimicrobial activity against some
⁎ Corresponding author.
E-mail address:gokhanzengin@selcuk.edu.tr(G. Zengin).
https://doi.org/10.1016/j.sajb.2018.01.008
0254-6299/© 2018 SAAB. Published by Elsevier B.V. All rights reserved.
Contents lists available atScienceDirect
South African Journal of Botany
foodborne and spoilage bacteria and is rich in catechin (Aslim and Ozturk, 2011).
The current study aimed at assessing the phenolic content and the antioxidant potential of the selected macrofungus using standard in vitro bio-assays. The inhibitory action of those species on key enzymes linked to diabetes type II and Alzheimer's disease (AD), namely,α-amylase, α-glucosidase, acetylcholinesterase (AChE), and butyrylcholinesterase (BChE) was also evaluated. It is anticipated that this study will establish valuable baseline data on the possible use of the selected mushrooms in the development of novel functional foods and pharmaceutical agents.
2. Materials and methods 2.1. Chemicals and reagents
Folin-Ciocalteu's reagent and methanol were purchased from Merck (Darmstadt, Germany). 2,2-Diphenyl-1-picrylhydrazyl (DPPH), 2,2 ′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), CuCl2, ammonium acetate, neocuproine, ferric chloride, ferrous sulphate, ferrozine, ammonium molybdate, H2SO4, trolox, EDTA,α-amylase (ex-porcine pancreas, EC 3.2.1.1),α-glucosidase solution (from Saccharomyces cerevisiae, EC 3.2.1.20), DTNB (5,5-dithio-bis(2-nitrobenzoic) acid), AChE (Electric ell acetylcholinesterase, Type-VI-S, EC 3.1.1.7), BChE (horse serum butyrylcholinesterase, EC 3.1.1.8), acetylthiocholine iodide (ATCI), butyrylthiocholine chloride (BTCl), galantamine, tyrosinase (from mushroom, EC 1.14.18.1), 3,4-dihydroxy-L-phenylalanine (L-DOPA), kojic acid, potato starch, potassium iodide, sodium carbonate, 4-N-trophenyl-α-D-glucopyranoside (PNPG) and acarbose were purchased from Sigma Chemical Co. (Sigma–Aldrich GmbH, Sternheim, Germany). All other chemicals used were of analytical grade.
2.2. Mushroom materials and preparation of extracts
Fruiting bodies of mushrooms species were collected at full maturity from different regions of Turkey (Table 1) and based on their micro-scopic and macromicro-scopic characteristics were authenticated by Dr. Ilgaz Akata (Department of Biology, Ankara University, Ankara, Turkey). The fruiting bodies were air-dried in an oven for 48 h at 40 °C before analysis. Samples were stored at Ankara University Herbarium (ANK) (in air-tight plastic bags, at room temperature). Mushroom names, localities, and voucher numbers are given inTable 1.
For organic extract, the dried powder mushroom materials (10 g) were macerated for 24 h with 200 mL of methanol at room temperature, and then the organic solvent was removed with rotary evaporator at 40 °C. All extracts were stored at +4 °C until further analysis.
2.3. Total phenolics content
The total phenolics content was determined by the Folin-Ciocalteu method (Slinkard and Singleton, 1977; Zengin et al., 2016b) with slight modification and expressed as gallic acid equivalents (GAEs/g extract). 2.4. Biological activities evaluation
Antioxidant [radical scavenging (ABTS and DPPH), reducing power (CUPRAC and FRAP), phosphomolybdenum, metal chelating (ferrozine method)] and enzyme inhibitory activities (cholinesterase -Elmann's method-, amylase -iodine/potassium iodide method-, and α-glucosidase -chromogenic PNPG method) were determined as de-scribed byGrochowski et al. (2017). The antioxidant abilities were expressed as equivalents of trolox (EDTA was used as a standard for evaluating metal chelating activity). The enzyme inhibitory activities of the extracts were obtained as equivalents of standard drugs per gram of the mushroom extract (galantamine for AChE and BChE, and acarbose forα-amylase and α-glucosidase assays).
2.5. Statistical analysis
All the assays were carried out in triplicate. The results are expressed as mean values and standard deviation (SD). Statistical difference be-tween the activity of the different extracts was analysed using one-way analysis of variance (ANOVA) followed by Tukey's post hoc test (α = 0.05). Statistical analysis was carried out using SPSS v. 14.0 program.
3. Results and discussion
Consumption and production of mushrooms have substantially in-creased these past years and this was associated with the uncovering of the multiple therapeutic actions of these macrofungus (Nakajima et al., 2018). Indeed, mushrooms are highly praised functional food with antioxidant, antitumor, anticancer, antimicrobial, antidiabetic, and hypotensive properties (Zengin et al., 2017a). In addition to their good protein content, mushrooms are rich in other nutrients like phosphorus, riboflavin, ascorbic acid, ergosterol, iron, thiamine, niacin, and secondary metabolites like terpenoids, polyphenols, lactones, sterols, sesquiterpenes, and alkaloids (Kumar, 2015).
AD existed long before 1907 as described by the observations made by Alois Alzheimer on the brain of a 55-year old woman who succumbed after 4 year suffering from dementia (Jellinger, 2007). The increase in life expectancy in the 20th century has made AD a common disorder of late life (Selkoe, 2015). This neurodegenerative disease, characterised by memory and cognitive impairment, accounts for 50 to 75% of all dementia cases (Niu et al., 2017). Cholinesterase inhibitors increase synaptic plasticity, thereby facilitating learning and memory (Parsons et al., 2013). Interestingly, mushrooms were defined as a rev-olutionary agent to combat neurodegenerative disorders (Sabaratnam et al., 2013). Indeed, several lines of evidence highlight the neuroprotec-tive properties of mushrooms to improve neuronal health (Phan et al., 2013, 2015; Seow et al., 2013). As illustrated inTable 2, the selected mushroom species exhibited variable inhibitory actions (reported as galantamine equivalents) against AChE (0.83–0.97 mg GALAE/g extract) and BChE (0.86–1.33 mg GALAE/g extract). Lycoperdon utriforme showed the highest cholinesterases inhibitory capacity (0.97 and 1.33 mg GALAE/g extract, for AChE and BChE, respectively).De Silva et al. (2012a)reported the presence of peptides in L. utriforme. Peptides have been found to inhibit amyloid-β aggregation and their binding to such aggregations was employed in in vivo imaging methods for early diagnosis of AD (Aileen Funke and Willbold, 2012). It was also noted that galantamine equivalent values were highest against BChE as compared to AChE (Table 2). The level of this enzyme was found to increase significantly during the late stage of AD (Greig et al., 2002).
Table 1
The studied mushroom species and theirs locations and voucher numbers.
Mushroom species Localities Voucher number Agaricus campestris L Ankara-Tandogan, in meadow,
N 39°56′-E 32°49′, 860 m
Akata 6700 Coprinus comatus
(O. F. Müll.) Pers.
İstanbul-Belgrad Forest, near oak forest, N 41°10′-E 28°56′, 60 m Akata 6181 Leucoagaricus leucothites (Vittad.) Wasser: Ankara-Tandogan, in meadow, N 39°56′-E 32°49′, 860 m Akata 6701 Lycoperdon utriforme Bull. Kocaeli-İnönü Plateu, in meadow,
40°34′ K-30°00′ D, 1170 m
Akata 6605 Macrolepiota mastoidea
(Fr.) Singer:
Kömürcü Bent, in beech forest, N 41°12′-E 28°57′, 110 m
Akata 6320 Macrolepiota procera
(Scop.) Singer:
İstanbul-Begrad Forest, in beech forest, N 41°12′-E 28°56′, 95 m
Indeed, in the human brain, BChE is expressed in specific regions (white matter and glia) of the brain, important in cognition and behaviour functions, which are greatly compromised in AD (Darvesh, 2016). BChE knockout in mouse model was associated with diminished amyloid-β deposits (Reid and Darvesh, 2015) (Table 3).
Diabetes type II is the leading cause of morbidity and mortality worldwide, projected to incur a treatment cost amounting to 642 mil-lion by 2040 (Ogurtsova et al., 2017). Evidences of the burden of diabe-tes type II render its prevention and management of paramount importance. Finding new alternatives to existing treatments, which have shown low success rates, is the new goal of researchers (Mocan et al., 2016, 2017; Zengin et al., 2017b). Several lines of evidences dis-course the role of carbohydrate hydrolysing enzymes in the manage-ment of diabetes type II. Developing and promoting mushrooms as a functional food for the management and/or prevention of diabetes type II reveals to be an interesting approach. Several studies have re-ported in vitro and in vivo antidiabetic potential of mushrooms and their secondary metabolites and support further investigation geared towards the development of lead candidates for the management of di-abetes type II (De Silva et al., 2012b; Ravi et al., 2013; Wu and Xu, 2015). A comprehensive review published byFriedman (2016)discussed the numerous benefits of mushrooms, including their hypoglycemic effects, and related this observation to the presence of dietary-polysaccharides. Our results showed that the selected mushroom species were good in-hibitors ofα-amylase and α-glucosidase. The order of inhibition against α-amylase was as follows L. utriforme N M. mastoidea N C. comatus N M. proceraN A. campestris and L. leucothites. The latter species was the most active species againstα-glucosidase (2.97 mmol ACAE/g extract). Moreover, the presentfinding tends to justify the use of C. comatus as hypoglycaemic agent. Additionally,Yamac et al. (2009)also reported the drop (42.78%) in serum glucose level in streptozotocin-induced di-abetic rats following oral administration of C. comatus. On the other hand, A. campestris administered to streptozocin-induced diabetic mice induces hypoglycaemic action by stimulating 2-deoxyglucose transport, glucose oxidation, and incorporation of glucose in glycogen in the mouse abdominal muscles (De Silva et al., 2012b). It was noted that the selected mushroom species were more potent inhibitors ofα-glucosidase compared to α-amylase. A marked inhibition of α-amylase by currently used hypoglycaemic therapies was associated with gastrointestinal discomforts due to undigested carbohydrates
(Zengin et al., 2016a). Thus, the development of hypoglycaemic agents with mildamylase inhibition and pronounced inhibition against α-glucosidase is an important requirement in the development of novel hypoglycaemic agents.
Oxidative stress has been increasingly recognised as a contributing factor in the pathogenesis of multiple complications including AD and diabetes type II (Maritim et al., 2003; Zhao and Zhao, 2013; Asmat et al., 2016; Huang et al., 2016). This state essentially arises as a conse-quence of abnormal production of reactive oxygen species coupled to poor innate antioxidant defense mechanism (Hybertson et al., 2011). This advocates the need for external sources of antioxidants. Apart from being highly nutritious, mushrooms contain a wide range of sec-ondary metabolites which confer them high therapeutic value including antioxidant properties (Akata et al., 2012; Kosanić et al., 2012; Kozarski et al., 2015). In the present study, various antioxidant assays were employed to evaluate the antioxidant capacity of the selected mushroom species. The phenolic content of the mushrooms was also determined.
As shown inFig. 1, A. campestris (15.63 mg GAE/g extract) had the highest phenolic content. This widely consumed mushroom also showed
Table 2
Enzyme inhibitory effects of the mushroom extracts. Mushrooms AChE inhibition
(mg GALAE/g extract)
BChE inhibition (mg GALAE/g extract)
Amylase inhibition (mmol ACAE/g extract)
Glucosidase inhibition (mmol ACAE/g extract) Lycoperdon utriforme 0.97 ± 0.03a 1.33 ± 0.06 0.22 ± 0.01 2.97 ± 0.14 Macrolepiota mastoidea 0.94 ± 0.03 1.28 ± 0.01 0.21 ± 0.01 2.74 ± 0.53 Agaricus campestris 0.83 ± 0.09 0.92 ± 0.10 0.16 ± 0.02 0.92 ± 0.09 Macrolepiota procera 0.83 ± 0.03 0.86 ± 0.06 0.17 ± 0.02 0.24 ± 0.10 Leucoagaricus leucothites 0.95 ± 0.01 1.27 ± 0.01 0.16 ± 0.01 1.80 ± 0.56 Coprinus comatus 0.88 ± 0.02 1.15 ± 0.04 0.18 ± 0.01 1.99 ± 0.75
GALAE: galantamine equivalents; ACAE: acarbose equivalents.
aData from three repetitions, with mean ± standard deviation.
Table 3
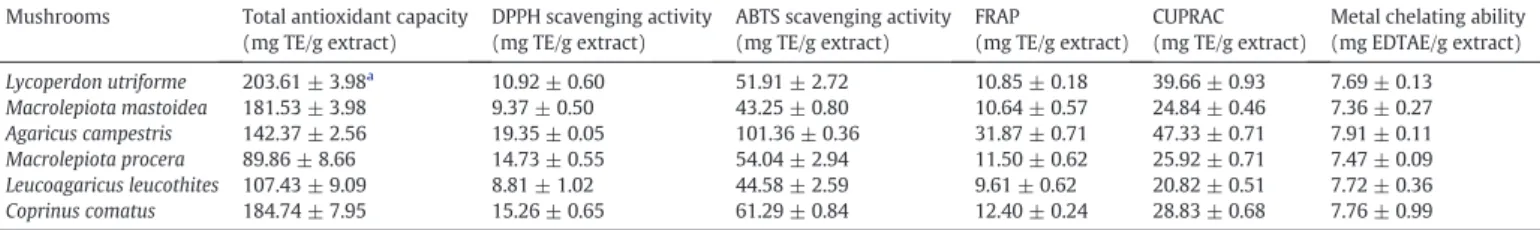
Antioxidant properties of the mushroom extracts. Mushrooms Total antioxidant capacity
(mg TE/g extract)
DPPH scavenging activity (mg TE/g extract)
ABTS scavenging activity (mg TE/g extract)
FRAP
(mg TE/g extract) CUPRAC (mg TE/g extract)
Metal chelating ability (mg EDTAE/g extract) Lycoperdon utriforme 203.61 ± 3.98a 10.92 ± 0.60 51.91 ± 2.72 10.85 ± 0.18 39.66 ± 0.93 7.69 ± 0.13 Macrolepiota mastoidea 181.53 ± 3.98 9.37 ± 0.50 43.25 ± 0.80 10.64 ± 0.57 24.84 ± 0.46 7.36 ± 0.27 Agaricus campestris 142.37 ± 2.56 19.35 ± 0.05 101.36 ± 0.36 31.87 ± 0.71 47.33 ± 0.71 7.91 ± 0.11 Macrolepiota procera 89.86 ± 8.66 14.73 ± 0.55 54.04 ± 2.94 11.50 ± 0.62 25.92 ± 0.71 7.47 ± 0.09 Leucoagaricus leucothites 107.43 ± 9.09 8.81 ± 1.02 44.58 ± 2.59 9.61 ± 0.62 20.82 ± 0.51 7.72 ± 0.36 Coprinus comatus 184.74 ± 7.95 15.26 ± 0.65 61.29 ± 0.84 12.40 ± 0.24 28.83 ± 0.68 7.76 ± 0.99 TE: Trolox equivalents; EDTAE: EDTA equivalents.
aData from three repetitions, with mean ± standard deviation.
L U MM AC MP L L CC 0 5 1 0 1 5 2 0 mgGAE/g
Fig. 1. Total phenolic content of the studied mushrooms (LU: Lycoperdon utriforme; MM: Macrolepiota mastoidea; AC: Agaricus campestris; MP: Macrolepiota procera; LL: Leucoagaricus leucothites; CC: Coprinus comatus; GAE: gallic acid equivalents).
the maximum DPPH radical (19.35 mg TE/g extract) and ABTS radical scavenging activities (101.36 mg TE/g extract), and also FRAP (31.87 mg TE/g extract) and CUPRAC (47.33 mg TE/g extract) activities. Findings from this study suggest the consumption of A. campestris as a functional food with potential antioxidant assets. Furthermore, recentfindings corroborate our results and reported high levels of ergosterol in A. campestris (Gąsecka et al., 2017). We reported the metal chelating abilities (7.91–7.36 mg EDTAE/g extract) of the selected mushrooms extracts. Mounting evidence highlight the role played by metals in amyloid-β fibrils formation and neurodegeneration (Huang et al., 2016) in AD. Metal-mediated formation of free radicals was associated with several conditions including diabetes type II (Matough et al., 2012).
4. Conclusion
Collected scientific evidence supports the use of the selected mushrooms in the management of diseases. In this work L. utriforme had potent inhibitory action against key enzymes linked to diabetes type II and AD, which are diseases afflicting millions of people world-wide and causing severe societal and economic burden. Additionally, current therapies for the management of diabetes type II and AD fail to normalise the patients' conditions and are responsible for undesired side effects. Besides, several pathologies are related to oxidative stress. Data from this study showed that A. campestris exhibited high antioxi-dant potential. The present study provides the rationale for further identification and characterisation of biologically active constituents for the development of new functional food formulations for the man-agement of these health problems.
References
Aileen Funke, S., Willbold, D., 2012.Peptides for therapy and diagnosis of Alzheimer's dis-ease. Current Pharmaceutical Design 18, 755–767.
Akata, I., Ergonul, B., Kalyoncu, F., 2012.Chemical compositions and antioxidant activities of 16 wild edible mushroom species grown in Anatolia. International Journal of Pharmacology 8, 134–138.
Aslim, B., Ozturk, S., 2011.Phenolic composition and antimicrobial and antioxidant activities of Leucoagaricus leucothites (Vittad.) Wasser. Journal of Medicinal Food 14, 1419–1424.
Asmat, U., Abad, K., Ismail, K., 2016.Diabetes mellitus and oxidative stress-a concise review. Saudi Pharmaceutical Journal 24, 547–553.
Darvesh, S., 2016.Butyrylcholinesterase as a diagnostic and therapeutic target for Alzheimer's disease. Current Alzheimer Research 13, 1173–1177.
De Silva, D.D., Rapior, S., Fons, F., Bahkali, A.H., Hyde, K.D., 2012a.Medicinal mushrooms in supportive cancer therapies: an approach to anti-cancer effects and putative mechanisms of action. Fungal Diversity 55, 1–35.
De Silva, D.D., Rapior, S., Hyde, K.D., Bahkali, A.H., 2012b.Medicinal mushrooms in prevention and control of diabetes mellitus. Fungal Diversity 56, 1–29.
Dulger, B., 2005.Antimicrobial activity of ten Lycoperdaceae. Fitoterapia 76, 352–354.
Friedman, M., 2016.Mushroom polysaccharides: chemistry and antiobesity, antidiabetes, anticancer, and antibiotic properties in cells, rodents, and humans. Food 5, 80.
Gąsecka, M., Magdziak, Z., Siwulski, M., Mleczek, M., 2017.Profile of phenolic and organic acids, antioxidant properties and ergosterol content in cultivated and wild growing species of Agaricus. European Food Research and Technology 1–10.
Ge, Z., Yang, Z.L., Vellinga, E.C., 2010.The genus Macrolepiota (Agaricaceae, Basidiomycota) in China. Fungal Diversity 45, 81–98.
Greig, N.H., Lahiri, D.K., Sambamurti, K., 2002.Butyrylcholinesterase: an important new target in Alzheimer's disease therapy. International Psychogeriatrics 14, 77–91.
Grochowski, D.M., Uysal, S., Aktumsek, A., Granica, S., Zengin, G., Ceylan, R., Locatelli, M., Tomczyk, M., 2017.In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochemistry Letters 20, 365–372.
Harding, P., 2008.Mushroom Miscellany. Collins 978-0-00-728464-1.
Huang, W.J., Zhang, X., Chen, W.W., 2016.Role of oxidative stress in Alzheimer's disease. Biomedical Reports 4, 519–522.
Hybertson, B.M., Gao, B., Bose, S.K., McCord, J.M., 2011.Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Molecular Aspects of Medicine 32, 234–246.
Jellinger, K.A., 2007.6 - Alzheimer's Disease A2 - Gilman, Sid, Neurobiology of Disease. Academic Press, Burlington, pp. 69–82.
Kalaras, M.D., Richie, J.P., Calcagnotto, A., Beelman, R.B., 2017.Mushrooms: a rich source of the antioxidants ergothioneine and glutathione. Food Chemistry 233, 429–433.
Karun, N.C., Sridhar, K.R., 2017.Edible wild mushrooms of the Western Ghats: data on the ethnic knowledge. Data in Brief 14, 320–328.
Kaya, A., 2009.Macrofungal diversity of Nemrut mount National Park and its environs (Adiyaman–Turkey). African Journal of Biotechnology 8, 2978–2983.
Kolcuoğlu, Y., Colak, A., Sesli, E., Yildirim, M., Saglam, N., 2007.Comparative characteriza-tion of monophenolase and diphenolase activities from a wild edible mushroom (Macrolepiota mastoidea). Food Chemistry 101, 778–785.
Kosanić, M., Ranković, B., Dašić, M., 2012.Mushrooms as possible antioxidant and antimicrobial agents. Iranian Journal of Pharmaceutical Research: IJPR 11, 1095–1102.
Kozarski, M., Klaus, A., Jakovljevic, D., Todorovic, N., Vunduk, J., Petrović, P., Niksic, M., Vrvic, M.M., Van Griensven, L., 2015.Antioxidants of edible mushrooms. Molecules 20, 19489–19525.
Kumar, K., 2015.Role of edible mushrooms as functional foods—a review. South Asian Journal of Food Technology and Environment 1, 211–218.
Liu, Y., Zhao, Y., Yang, Y., Tang, Q., Zhou, S., Wu, D., Zhang, J., 2013.Structural characteris-tics and hypoglycemic activity of polysaccharides from Coprinus comatus. Bioactive Carbohydrates and Dietary Fibre 2, 164–169.
Maritim, A., Sanders, A., Watkins III, J., 2003.Diabetes, oxidative stress, and antioxidants: a review. Journal of Biochemical and Molecular Toxicology 17, 24–38.
Matough, F.A., Budin, S.B., Hamid, Z.A., Alwahaibi, N., Mohamed, J., 2012.The role of oxidative stress and antioxidants in diabetic complications. Sultan Qaboos University Medical Journal 12, 5.
Mocan, A., Zengin, G., Uysal, A., Gunes, E., Mollica, A., Degirmenci, N.S., Alpsoy, L., Aktumsek, A., 2016.Biological and chemical insights of Morina persica L.: a source of bioactive compounds with multifunctional properties. Journal of Functional Foods 25, 94–109.
Mocan, A., Zengin, G., Simirgiotis, M., Schafberg, M., Mollica, A., Vodnar, D.C., Crişan, G., Rohn, S., 2017.Functional constituents of wild and cultivated Goji (L. barbarum L.) leaves: phytochemical characterization, biological profile, and computational studies. Journal of Enzyme Inhibition and Medicinal Chemistry 32, 153–168.
Muszyńska, B., Grzywacz-Kisielewska, A., Kała, K., Gdula-Argasińska, J., 2017. Anti-inflammatory properties of edible mushrooms: a review. Food Chemistry 243, 373–381.
Nakajima, V.M., de Freitas Soares, F.E., de Queiroz, J.H., 2018.Screening and decolorizing potential of enzymes from spent mushroom composts of six different mushrooms. Biocatalysis and Agricultural Biotechnology 13, 58–61.
Niu, H., Álvarez-Álvarez, I., Guillén-Grima, F., Aguinaga-Ontoso, I., 2017.Prevalence and incidence of Alzheimer's disease in Europe: a meta-analysis. Neurología (English Edition) 32, 523–532.
Ogurtsova, K., da Rocha Fernandes, J.D., Huang, Y., Linnenkamp, U., Guariguata, L., Cho, N.H., Cavan, D., Shaw, J.E., Makaroff, L.E., 2017.IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Research and Clinical Practice 128, 40–50.
Parsons, C.G., Danysz, W., Dekundy, A., Pulte, I., 2013.Memantine and cholinesterase inhibitors: complementary mechanisms in the treatment of Alzheimer's disease. Neurotoxicity Research 24, 358–369.
Phan, C.-W., David, P., Naidu, M., Wong, K.-H., Sabaratnam, V., 2013.Neurite outgrowth stimulatory effects of culinary-medicinal mushrooms and their toxicity assessment
using differentiating Neuro-2a and embryonicfibroblast BALB/3T3. BMC
Comple-mentary and Alternative Medicine 13, 261.
Phan, C.-W., David, P., Naidu, M., Wong, K.-H., Sabaratnam, V., 2015.Therapeutic potential of culinary-medicinal mushrooms for the management of neurodegenerative diseases: diversity, metabolite, and mechanism. Critical Reviews in Biotechnology 35, 355–368.
Prasad, S., Rathore, H., Sharma, S., Yadav, A., 2015.Medicinal mushrooms as a source of novel functional food. International Journal of Food Science, Nutrition and Dietetics 4, 221–225.
Ravi, B., Renitta, R.E., Prabha, M.L., Issac, R., Naidu, S., 2013.Evaluation of antidiabetic potential of oyster mushroom (Pleurotus ostreatus) in alloxan-induced diabetic mice. Immunopharmacology and Immunotoxicology 35, 101–109.
Reid, G.A., Darvesh, S., 2015.Butyrylcholinesterase-knockout reduces brain deposition of fibrillar β-amyloid in an Alzheimer mouse model. Neuroscience 298, 424–435.
Sabaratnam, V., Kah-Hui, W., Naidu, M., David, P.R., 2013.Neuronal health–can culinary and medicinal mushrooms help? Journal of Traditional and Complementary Medicine 3, 62–68.
Selkoe, D.J., 2015.Chapter 67 - Alzheimer Disease A2 - Rosenberg, Roger N. In: Pascual, J.M. (Ed.), Rosenberg's Molecular and Genetic Basis of Neurological and Psychiatric Disease, Fifth edition Academic Press, Boston, pp. 753–768.
Seow, S.L.-S., Naidu, M., David, P., Wong, K.-H., Sabaratnam, V., 2013.Potentiation of neuritogenic activity of medicinal mushrooms in rat pheochromocytoma cells. BMC Complementary and Alternative Medicine 13, 157.
Slinkard, K., Singleton, V.L., 1977.Total phenol analysis: automation and comparison with manual methods. American Journal of Enology and Viticulture 28, 49–55.
Wasser, S.P., Didukh, M.Y., 2003.Medicinal value of species of the family Agaricaceae Cohn (higher basidiomycetes): current stage of knowledge and future perspectives. International Journal of Medicinal Mushrooms 5.
Wu, T., Xu, B., 2015.Antidiabetic and antioxidant activities of eight medicinal mushroom species from China. International Journal of Medicinal Mushrooms 17, 129–140.
Yamac, M., Zeytinoglu, M., Kanbak, G., Bayramoglu, G., Senturk, H., 2009.Hypoglycemic effect of crude exopolysaccharides produced by Cerrena unicolor, Coprinus comatus, and Lenzites betulina isolates in streptozotocin-induced diabetic rats. Pharmaceutical Biology 47, 168–174.
Zengin, G., Karanfil, A., Uren, M.C., Kocak, M.S., Sarikurkcu, C., Gungor, H., Picot, C.M.N., Mahomoodally, M.F., 2016a.Phenolic content, antioxidant and enzyme inhibitory capacity of two Trametes species. RSC Advances 6, 73351–73357.
Zengin, G., Nithiyanantham, S., Locatelli, M., Ceylan, R., Uysal, S., Aktumsek, A., Selvi, P.K., Maskovic, P., 2016b.Screening of in vitro antioxidant and enzyme inhibitory activities of different extracts from two uninvestigated wild plants: Centranthus longiflorus subsp. longiflorus and Cerinthe minor subsp. auriculata. European Journal of Integrative Medicine 8, 286–292.
Zengin, G., Uren, M.C., Kocak, M.S., Gungor, H., Locatelli, M., Aktumsek, A., Sarikurkcu, C., 2017a.Antioxidant and enzyme inhibitory activities of extracts from wild mushroom species from Turkey. International Journal of Medicinal Mushrooms 19, 327–336.
Zengin, G., Uysal, A., Aktumsek, A., Mocan, A., Mollica, A., Locatelli, M., Custodio, L., Reng, N.N., Nogueria, J.M.F., Aumeeruddy-Elalfi, Z., Mahomoodally, M.F., 2017b.Euphorbia
denticulata Lam.: a promising source of phyto-pharmaceuticals for the development of novel functional formulations. Biomedicine & Pharmacotherapy 87, 27–36.
Zhao, Y., Zhao, B., 2013.Oxidative stress and the pathogenesis of Alzheimer's disease. Oxidative Medicine and Cellular Longevity 2013.