Effects of continuous theta burst transcranial magnetic stimulation on
cortical excitability in patients with idiopathic generalized epilepsy
Guray Koc
⁎
, Zeki Gokcil
1, Semai Bek
2, Tayfun Kasikci
3, Erdal Eroglu
4, Zeki Odabasi
5 Department Of Neurology, Gulhane Training and Research Hospital, Ankara, Turkeya b s t r a c t
a r t i c l e i n f o
Article history: Received 2 May 2017 Revised 12 September 2017 Accepted 16 September 2017 Available online 23 October 2017Introduction: Transcranial magnetic stimulation (TMS) is a noninvasive technique for investigating cortical phys-iologic functions in the brain. In this study, the effects of continuous theta burst stimulation (cTBS) on motor evoked potential (MEP) parameters in patients with idiopathic generalized epilepsy (IGE) were investigated. Materials and methods: Fifteen patients with IGE were included. Motor threshold (MT) and cortical silent period (CSP) were determined before cTBS application. Next, cTBS was applied to the dominant (left) hemisphere M1 hand area as thefirst application. After 1 day, cTBS was applied first to the left M1 hand area and then to the right lateral cerebellar area as the second application. Parameters were again determined after the applications. Results: There was no difference in resting MT values before and after cTBS application (pN 0.05). Although CSP increased after stimulation (pb 0.05), it was not significantly different between applications (p N 0.05). Conclusion: For patients with epilepsy, cTBS is a safe technique when applied at a low intensity. The inhibitory effect of cTBS, a noninvasive technique, on cortical excitability in patients with IGE was determined using MEP parameters. The effect lasted at least 1 h. To our knowledge, this is thefirst study to assess the effect of cTBS on cortical excitability in patients with IGE. Ourfindings indicate that cTBS decreases cortical excitability in pa-tients with IGE.
© 2017 Elsevier Inc. All rights reserved.
Keywords: Epilepsy
Continuous theta burst stimulation Transcranial magnetic stimulation Cortical excitability
1. Introduction
Epilepsy is defined as a clinical condition that is characterized by two or more recurrent and unprovoked epileptic seizures. An epileptic seizure is a temporary clinical condition that emerges because of uncon-trolled, excessive, and abnormal discharges of cortical neurons, which is caused by increased, rapid, and local electrical discharges in the gray matter. In neurological practice, epilepsy is the second most frequently observed chronic disease after cerebrovascular diseases, and its etiopathogenesis has not been completely understood. Its prevalence is 0.5%–1%[1].
Epilepsy is divided into subgroups on the basis of whether the seizures originate from a focus or occur in a generalized manner. Most generalized epilepsies are idiopathic generalized epilepsies (IGEs)
[2]. In general, two primary characteristics describe the epileptiform
activity: hyperexcitability and hypersynchrony of multiple neurons. Diffuse cortical hyperexcitability has been hypothesized to be the primary pathophysiological mechanism in IGE[3].
Transcranial magnetic stimulation (TMS) has been used for investi-gating cortical excitability hypothesis in patients with epilepsy[4]. Theta burst stimulation (TBS) is a complex stimulation known as con-tinuous TBS (cTBS) or intermittent TBS depending on the interval dura-tion between successive stimuladura-tions[5]; cTBS application to the motor cortex of healthy individuals reduced M1 excitability, and because the cerebellum plays a role in sensorimotor adaptation, cerebellar cTBS application decreased M1 excitability in healthy individuals[6,7].
Motor evoked potential (MEP) is an indicator of the integrity of the corticospinal tract and normal excitability of alpha motor neurons by neurons in the motor cortex[4]; MEP is the best-studied parameter for assessing the excitatory effect of TMS[8]. Descending corticomotor neurons are stimulated transsynaptically because of the activation of excitatory cortical interneurons with TMS[9].
The motor MEP amplitude is a measure of cortical excitability. Stimulus intensity is considerably affected by variables such as volun-tary muscle contraction before the stimulus and magnetic coil diameter
[10]. Motor threshold (MT) is one of the most frequently used TMS parameters in cortical excitability. It represents the excitability of the motor system at the cortical or spinal level. The threshold value varies from person to person, and when parameters such as the cortical silent period (CSP) are studied, stimulus intensity must be standardized
Epilepsy & Behavior 77 (2017) 26–29
⁎ Corresponding author.
E-mail address:guray.koc@saglik.gov.tr(G. Koc).
1
Department of Neurology, Eastern Mediterranean University, Faculty of Health Sciences, Physiotherapy and Rehabilitation, Cyprus.
2 Department of Neurology, Mugla Sitki Kocman University, Faculty of Medicine, Mugla,
Turkey.
3
Department of Neurology, Canakkale City Hospital, Canakkale, Turkey.
4
Department of Neurology, TOBB University of Economics And Technology, Faculty of Medicine Ankara, Turkey.
5
Department of Neurology, Ufuk University, Faculty of Medicine, Ankara, Turkey.
https://doi.org/10.1016/j.yebeh.2017.09.011
1525-5050/© 2017 Elsevier Inc. All rights reserved.
Contents lists available atScienceDirect
Epilepsy & Behavior
according to the threshold value. When the motor cortex is stimulated while the target muscle is contracted, a silent period is observed in the trace after the MEP activity, and then electromyography (EMG) activity continues. This period is called CSP. The duration of CSP increases with an increase in the stimulus intensity[11–13].
In this study, the effect of cTBS application to the left (dominant) hemisphere M1 area and then to the right cerebellar area on MT and CSP amplitudes were examined to assess cortical excitability in patients with IGE.
2. Methods and materials 2.1. Participants
Right-handed patients diagnosed with IGE were examined. The exclusion criteria were the presence of intracranial lesions that can cause epilepsy, another accompanying neurological disease, abnormal findings in the neurological examination, pregnancy, use of antiepileptic drugs (AEDs) or any drug that affects the central nervous system in the previous month, presence of a cardiac pacemaker, presence of an intra-cranial metal implant, nonepileptic case suspicion, and not wanting to participate in the study. The detailed history of the patients was obtained, and neurologic and systemic examinations were performed. Cerebral imaging (brain magnetic resonance imaging) studies were conducted. The study was approved by the local ethics committee, and informed consent for experimentation was obtained from all patients.
2.2. TMS and electromyographic techniques
Stimulation was performed using the Magstim Rapid (Magstim Company Ltd. Whitland, Dyfed, UK) TMS device. Recordings were ob-tained using the Medelec (VIASYS Healthcare Madison WI, USA) EMG device. Micromed 10-mm waterproof gold-plated disc electrodes were used for recording. The stimulator was connected to the eight-shaped coil (outer wing with 9 cm in diameter). The coil was placed in an opti-mal position at a 45-degree angle to the left hemisphere to obtain MEP from the contralateral abductor pollicis brevis (APB) muscle. Wefirst defined the hand motor area of M1, where stimulation evoked the largest MEP from the contralateral APB muscle[14]. The best location to stimulate the hand area (M1 hand) was identified.
Initially, the resting MT (rMT) was detected. The lowest stimulus in-tensity that provides an acquisition of 50-microvolt MEP in 5 of 10 stim-ulations was accepted to be rMT. The lowest stimulus intensity that provides an acquisition of 200-microvolt MEP in 5 of 10 stimulations, ensuring that patients contracted the APB muscle at 20%–30% of maxi-mum voluntary muscle contraction, was accepted to be active MT (aMT). Next, the patients were asked to contract their ABP muscle at the maximum level, and CSP was obtained by stimulating the M1 hand area at an intensity of 150% of rMT. The stimulus intensity was kept constant to ensure standardization. Cortical silent period was ob-tained three times, and the average CSP duration was calculated[15,16]. 2.3. Experimental design
After the above evaluations, cTBS was applied to the left M1 hand area of the patients. In total, 600 stimulations were performed. As de-scribed by Huang, cTBS comprises three-beat bursts at a frequency of 50 Hz that was repeated at 200-ms intervals, representing 80% of aMT
[5]. After 1 h, the rMT value and three CSPs were obtained. The interval between each stimulation was 5 s.
Because previousfindings indicated that TBS-related behavioral changes might last for up to 10 h, after 1 day (24 h), cTBS wasfirst ap-plied to the left M1 hand area and then to the right lateral cerebellar (1 cm below and 3 cm lateral of the inion) area as the second application
[17]. The rMT value and three CSPs were again obtained. Intervals between each stimulation were 5 s. Thus, three values, namely before application, afterfirst application, and after second application, were obtained for comparison.
2.4. Statistical analysis
The variables were investigated using visual (histograms) and analyt-ical methods (Kolmogorov–Smirnov) to determine normal distribution. Table 1
Demographic features of the patients.
Patient number Gender Age Age of the onset Seizure frequency (n/year)
1 Male 22 19 12 2 Male 21 14 12 3 Male 21 15 2 4 Male 23 18 12 5 Male 23 20 24 6 Male 22 10 18 7 Male 20 15 6 8 Male 24 17 18 9 Male 20 14 2 10 Male 24 19 18 11 Male 23 16 12 12 Female 18 11 48 13 Male 22 17 2 14 Male 24 20 6 15 Male 23 17 18
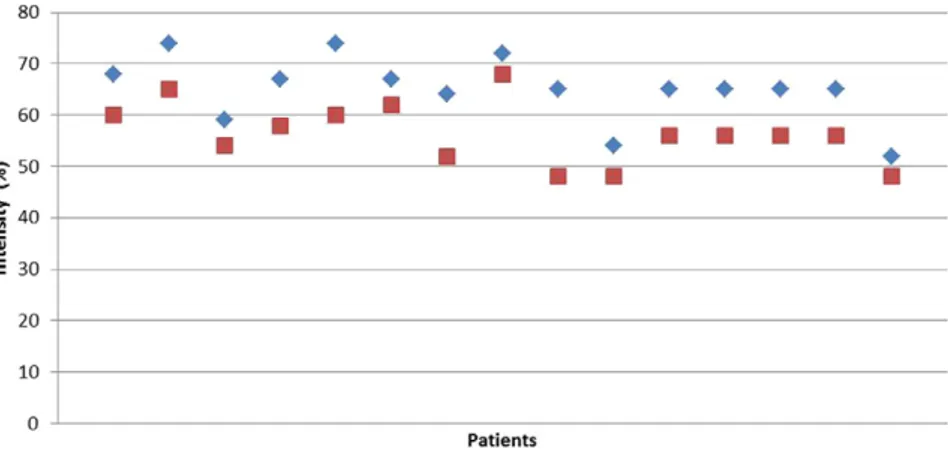
Fig. 1. rMT and aMT values before application.
27 G. Koc et al. / Epilepsy & Behavior 77 (2017) 26–29
Data were evaluated using analysis of variance for recurrent measure-ments. Data are represented as mean ± standard deviation. The software Statistical Package for the Social Sciences v.15.0 was used. A p value of b0.05 was accepted to be statistically significant.
3. Results
3.1. Demographic and clinical data
Fifteen patients (1 female and 14 males) were included in this study. Their average age was 22 ± 1.73 years, and the average age of the onset of epilepsy was determined to be 16.13 ± 3.02 years. Family history was positive in two patients, and all patients were evaluated using the IGE classification. Age, age at epilepsy onset, and seizure frequency data of the patients are presented inTable 1.
3.2. MEP parameters
The average rMT value in the patients before TBS application was 65.06% ± 6.29%, average aMT value was 56.46% ± 6.01%, and average CSP value was 124.89 ± 50.12 ms.
The average rMT value obtained 1 h after thefirst TBS application was 65.93% ± 7.21%, and the average CSP value was 139.57 ± 43.61 ms.
The average rMT value obtained 1 h after the second TBS application was 65.33% ± 7.61%, and the average CSP value was 150.02 ± 47.38 ms. No adverse effects were detected as a complaint or symptom in the patients.
The intensity values at which rMT and aMT were obtained before cTBS application in the patients are presented inFig. 1. No significant difference was found between rMT values in the patients before applica-tion and after thefirst and second applications (p N 0.05) (Fig. 2). A sig-nificant difference was found between CSP values in the patients before application and after thefirst application (p b 0.05). A significant differ-ence was also found between CSP values before application and after the second application (pb 0.05). No significant difference was found between CSP values after thefirst and second applications (p N 0.05;
Fig. 3).
4. Discussion
In this study, the effect of cTBS application to the motor cortex and cerebellar area on cortical excitability in patients with IGE was examined. The cTBS application to the dominant hemisphere M1 hand area decreased cortical excitability. Thesefindings are consistent with studies that report that cTBS application in healthy individuals de-creases cortical excitability[5,18]. The cTBS application to the right cerebellar area subsequent to the left M1 hand area did not provide additional inhibition. To our knowledge, this is thefirst study to assess the effects of cTBS application in patients with IGE. We also found that cTBS applied to the right cerebellar area subsequent to the left primary motor stimulation did not provide additional inhibition.
Previous TMS studies conducted in patients with IGE not undergoing treatment focused on the differences in MT between untreated patients and controls. Reutens and Berkovic found that rMT was lower in patients with IGE who were not undergoing treatment. Thisfinding supports the fact that membrane excitability is increased in patients with epilepsy[19]. However, Lee et al. found no difference in rMT values obtained from the dominant hemisphere when they compared patients with IGE who were not undergoing treatment and healthy volunteers
[13]. Because AEDs such as carbamazepine and lamotrigine affect corti-cal excitability by increasing the threshold value, patients who do not use drugs for at least 1 month were selected in this study[20]. Gianelli et al. found that compared with controls, the threshold value was high in a small group of patients with absence seizures who were on or off medical treatment. Thesefindings suggest that cortical membrane ex-citability can be different in different types of generalized epilepsies
[21]. Thus, there are a limited number of studies that considers the effect of rTMS application on rMT in patients with epilepsy and healthy volunteers. In our study, no difference was found in rMT values before application and after thefirst and second applications. Lezzi et al. applied cTBS to 17 healthy individuals at 80% aMT intensity, similar to our study, and found no difference in rMT before and after the applica-tion[22]. Our study also found that cTBS in patients with IGE did not have an effect on rMT, similar to that observed in healthy individuals. Fig. 2. rMT values before application and after thefirst and second applications.
Fig. 3. Mean CSP values before application and after thefirst and second applications.
Thesefindings indicate that the effect of cTBS on cortical excitability is not based on rMT.
Longer CSP was found in untreated patients in studies conducted among patients with generalized epilepsy. Increased CSP activity may be an indicator of a compensatory interictal mechanism that prevents the transition of hyperactivity of the inhibitory neuronal network from the interictal to the ictal condition[13,23]. However, an increase in the cortical inhibitory mechanisms and an increase in cortical hyper-excitability were indicated by the above studies. Based on TMS parame-ters, an imbalance in cortical excitatory and inhibitory mechanisms was found in patients with IGE[13]. In contrast, decreased CSP was detected in a small group of patients with IGE, indicating possible deterioration in inhibitory mechanisms, possibly playing a role in epileptogenesis. These contradictoryfindings have been interpreted as different pathophysio-logical mechanisms that play roles in different patients with IGE[24].
In this study, we found that CSP values increased after thefirst and second cTBS applications, showing that cTBS decreases cortical excitability. However, the fact that there was no difference between the two applications implies that cTBS applied to the right cerebellar hemisphere subsequent to the left M1 hand area does not provide additional inhibition. The second part of the silent period (N75 ms) is probably attributable to cortical inhibitory mechanisms related to GABA-B receptor activation. Increase of CSP because of cTBS application may be an indicator of the increase in cortical inhibitory mechanisms and a mechanism which can prevent seizure formation. As in the study by Lee et al., we also could notfind an association between rMT and CSP[13].
When compared with low-frequency rTMS, the main advantage of cTBS is that the stimulation is performed in a shorter time and at a lower intensity. Therefore, stimulation can be provided in a more com-fortable and reliable manner[5]. In our study, cTBS was applied to patients with IGE who were not undergoing treatment because it is a noninvasive and comfortable technique that lasts a short period and it provides stimulation at a low intensity. The effects of cTBS on cortical excitability were then examined.
The limitations of this study were that intracortical facilitation and inhibition parameters could not be assessed because there was no healthy control group and no opportunity for double stimulation. The lack of a sham arm to demonstrate superiority over a potential placebo effect was also a limitation. However, we found that cTBS decreased cor-tical excitability and its effect lasted for 1 h in patients with IGE, which is similar to that previously observed in healthy volunteers. We found that cerebellar stimulation did not have an additional inhibitory effect. We determined that this application could be safely performed in patients with IGE because no adverse effects were observed either as a complaint or as a symptom during or after the procedure.
5. Conclusion
For patients with epilepsy, cTBS is a safe technique when applied at a low intensity. Because cTBS only lasts for a short period, it is advanta-geous both for the practitioner and patient compared with conventional rTMS techniques. To our knowledge, this is thefirst study to assess the effect of cTBS on cortical excitability in patients with IGE. Ourfindings indicate that cTBS decreases cortical excitability in patients with IGE and provide a rational basis for studies on preventing transition from the interictal period to the ictal period by promoting inhibitory mechanisms.
Conflicts of interest
Authors do not have any conflict of interest.
Acknowledgments None.
Funding
This research did not receive any specific grant from funding agen-cies in the public, commercial, or not-for-profit sectors.
References
[1]Sander JW, Shorvon SD. Epidemiology of the epilepsies. J Neurol Neurosurg Psychiatry 1996;61:433–43.
[2]Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol 2015;126: 1071–107.
[3]Macdonell RA, Curatolo JM, Berkovic SF. Transcranial magnetic stimulation and epilepsy. J Clin Neurophysiol 2002;19:294–306.
[4]Ziemann U, Steinhoff BJ, Tergau F, Paulus W. Transcranial magnetic stimulation: its current role in epilepsy research. Epilepsy Res 1998;30:11–30.
[5]Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron 2005;45:201–6.
[6]Hordacre B, Goldsworthy MR, Vallence AM, Darvishi S, Moezzi B, Hamada M, et al. Variability in neural excitability and plasticity induction in the human cortex: a brain stimulation study. Brain Stimul 2017;10:588–95.
[7]Bologna M, Paparella G, Fabbrini A, Leodori G, Rocchi L, Hallett M, et al. Effects of cerebellar theta-burst stimulation on arm and neck movement kinematics in patients with focal dystonia. Clin Neurophysiol 2016;127:3472–9.
[8]Cracco RQ, Cracco JB, Maccabee PJ, Amassian VE. Cerebral function revealed by trans-cranial magnetic stimulation. J Neurosci Methods 1999;86:209–19.
[9]Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H. Intracortical facilitation and inhibi-tion after transcranial magnetic stimulainhibi-tion in conscious humans. J Physiol 1997; 498:817–23.
[10]Kiers L, Fernando B, Tomkins D. Facilitatory effect of thinking about movement on magnetic motor-evoked potentials. Electroencephalogr Clin Neurophysiol 1997; 105:262–8.
[11]Boniface SJ, Mills KR, Schubert M. Responses of single spinal motoneurons to mag-netic brain stimulation in healthy subjects and patients with multiple sclerosis. Brain 1991;114:643–62.
[12]Taylor JL, Gandevia SC. Transcranial magnetic stimulation and human muscle fatigue. Muscle Nerve 2001;24:18–29.
[13]Lee JH, Joo EY, Seo DW, Hong SB. Lateralizing cortical excitability in drug naïve patients with generalized or focal epilepsy. J Epilepsy Res 2015;5:75–83.
[14]Mochizuki H, Franca M, Huang YZ, Rothwell JC. The role of dorsal premotor area in reaction task: comparing the“virtual lesion” effect of paired pulse or theta burst transcranial magnetic stimulation. Exp Brain Res 2005;167:414–21.
[15]Joo EY, Kim SH, Seo DW, Hong SB. Zonisamide decreases cortical excitability in patients with idiopathic generalized epilepsy. Clin Neurophysiol 2008;119: 1385–92.
[16]Badawy RA, Curatolo JM, Newton M, Berkovic SF, Macdonell RA. Changes in cortical excitability differentiate generalized and focal epilepsy. Ann Neurol 2007;61: 324–31.
[17]Nyffeler T, Wurtz P, Lüscher HR, Hess CW, Senn W, Pflugshaupt T, et al. Extending lifetime of plastic changes in the human brain. Eur J Neurosci 2006;24:2961–6.
[18]Suppa A, Ortu E, Zafar N, Deriu F, Paulus W, Berardelli A, et al. Theta burst stimula-tion induces after-effects on contralateral primary motor cortex excitability in humans. J Physiol 2008;586:4489–500.
[19]Reutens DC, Berkovic SF. Increased cortical excitability in generalised epilepsy demonstrated with transcranial magnetic stimulation. Lancet 1992;339:362–3.
[20]Ziemann U, Lönnecker S, Steinhoff BJ, Paulus W. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann Neurol 1996;40:367–78.
[21]Gianelli M, Cantello R, Civardi C, Naldi P, Bettucci D, Schiavella MP, et al. Idiopathic generalized epilepsy: magnetic stimulation of motor cortex time-locked and unlocked to 3-Hz spike-and-wave discharges. Epilepsia 1994;35:53–60.
[22]Iezzi E, Suppa A, Conte A, Agostino R, Nardella A, Berardelli A. Theta-burst stimula-tion over primary motor cortex degrades early motor learning. Eur J Neurosci 2010;31:585–92.
[23]Ertaş NK, Gül G, Altunhalka A, Kirbas D. Cortical silent period following transcranial magnetic stimulation in epileptic patients. Epileptic Disord 2000;2:137–40.
[24]Cincotta M, Giovannelli F, Borgheresi A, Tramacere L, Viggiano MP, Zaccara G. A meta-analysis of the cortical silent period in epilepsies. Brain Stimul 2015;8: 693–701.
29 G. Koc et al. / Epilepsy & Behavior 77 (2017) 26–29