RESEARCH PAPER
Selective chiral recognition of alanine enantiomers by chiral calix[4]
arene coated quartz crystal microbalance sensors
Farabi Temel1,2&Serkan Erdemir3&Begum Tabakci3&Merve Akpinar1,2&Mustafa Tabakci1,2
Received: 22 December 2018 / Revised: 31 January 2019 / Accepted: 21 February 2019 / Published online: 1 April 2019 # Springer-Verlag GmbH Germany, part of Springer Nature 2019
Abstract
We describe the synthesis of new chiral calix[4]arene derivatives having (R)-1-phenylethylamine, (S)-1-phenylethylamine, (R)-2-phenylglycinol, and (S)-2-phenylglycinol moieties, and chiral recognition studies for enantiomers of some selected α-amino acid derivatives such as alanine, phenylalanine, serine, and tryptophan using a quartz crystal microbalance (QCM). Initial experiments indicated that the highest selective chiral recognition factor was 1.42 for alanine enantiomers. The sensitivity, limit of detection, and time constant forL-alanine were calculated as 0.028 Hz/μM, 60.9 μM, and 36.2 s, respectively. The results indicated that real-time, sensitive, selective, and effective chiral recognition of alanine enantiomers was achieved with a QCM sensor coated with a chiral calix[4]arene derivative having (R)-2-phenylglycinol moieties.
Keywords Amino acid . Calixarene . Alanine . Chiral recognition . Quartz crystal microbalance sensor
Introduction
Selective chiral recognition and enantiomeric separation are an important issue for areas such as drug development, chem-ical applications, and biologchem-ical applications [1–4]. The chiral recognition of amino acids, which are the key elements of proteins, is indispensable for new drug applications and dis-coveries [5]. Therefore, designing an efficient analytical meth-od with practically sufficient sensitivity and selectivity to rec-ognize amino acid enantiomers is an essential task for the screening, diagnosis, and treatment of metabolic diseases [6]. For the recognition of amino acids, analytical methods such as high-performance liquid chromatography [6,7], fluid chromatography [8], mass spectrometry [9], UV–vis
spectros-copy [10, 11], fluorescence spectroscopy [12], 1H NMR
spectroscopy [13], and potentiometric measurements [14] have been used and developed. However, use of these methods may have some disadvantages, such as high time consumption, expensive analysis, high investment cost, and the requirement for skilled personnel. Therefore, sensor de-vices and methods such as those involving acoustic systems have attracted attention for the determination and sensing of sensor material–analyte interaction in sensor applications [15]. In acoustic systems, a quartz crystal microbalance (QCM) is useful because of the easy, rapid, and cheap detec-tion of analytes in both dry and aqueous condidetec-tions at low concentrations [16–22]. In the literature, there are many stud-ies on the applications of a QCM for the sensing of biological analytes, such as carbohydrates, antibiotics, bacteria, and DNA [23–26]. In the QCM system, the relation between the mass of the adsorbed analyte and the frequency shift of quartz oscillation can be expressed by the Sauerbrey equation, which is defined as [27]
Δf ¼ −cfΔm; ð1Þ
whereΔf is the observed frequency change in hertz, Δm is the change in mass per unit area in grams per square centimeter on quartz, andcfis the sensitivity factor for the crystal (56.6 Hz μ-1
cm2for a 5-MHz AT-cut quartz crystal at room tempera-ture). The QCM sensing system can allow measurement of other physical parameters, such as motional resistance (ΔR), which is determined by the physical properties of the mass Electronic supplementary material The online version of this article
(https://doi.org/10.1007/s00216-019-01705-5) contains supplementary material, which is available to authorized users.
* Mustafa Tabakci
mtabakci@ktun.edu.tr; mtabakci@selcuk.edu.tr
1
Department of Chemical Engineering, Konya Technical University, 42130 Konya, Turkey
2
Department of Chemical Engineering, Selçuk University, 42130 Konya, Turkey
3 Department of Chemistry, Selçuk University, 42130 Konya, Turkey https://doi.org/10.1007/s00216-019-01705-5
layer, and frequency change (ΔF). ΔR can be used to deter-mine the dissipation energy or damping process, which is closely related to the damping medium's physical properties in the system [28–31]. To determine the change of visco-elasticity of a deposited film, measurement ofΔR is a good technique.ΔR and ΔF will be zero and linearly proportion-al to the mass change, respectively, for an elastic mass change [28, 32]. In a liquid medium, a large decrease of ΔF corresponds to a proportional increase of ΔR, indicating the viscoelastic character of the interfacial layer [29]. Therefore, measurements of ΔF and ΔR simultaneously lead to the distinguishing of an elastic mass effect from viscosity-induced effects [32].
In sensor applications, some chemical compounds, such as macromolecules, polymers, and gold nanoparticles, that are produced synthetically are widely used as sensor materials for the sensing of biological molecules [33–36]. Among mac-romolecules, calix[n]arenes (n is especially 4, 6, or 8), which are cyclic polyphenols, are attractive excellent receptors for charged and neutral molecules because they have unique three-dimensional structures with many derivatization possi-bilities [37]. They can be obtained synthetically on a large scale by condensation ofp-tert-butylphenol with formalde-hyde in basic conditions [38,39]. They have been used as promising materials for sensor applications because of their outstanding sensing properties [40–42].
To best of our knowledge, use of calixarenes as sensor materials for the chiral recognition of amino acid enantio-mers by a QCM system has never been studied before. On the other hand, it is known that amino acid functionalized calixarenes have interesting structural possibilities [37]. Consequently, we attached amino acid groups to the calixarene skeleton to facilitate chiral recognition. In this study, we primarily synthesized new chiral calix[4]arene building blocks bearing chiral amine and amino alcohol moieties on their upper rim and a disulfide moiety on the lower rim (Scheme 1). Subsequently, we coated QCM quartz crystals with them to obtain chiral calix[4]arene coated QCM sensors. Attachment of calixarenes to the gold QCM surface can be achieved through sulfide groups. After coating the sensor surface with the calixarenes, we investigated the chiral recognition abilities of the new QCM sensors for variousα-amino acid enantiomers, such asD/L-alanine,D/L-serine,D/L-phenylalanine, andD/L -tryp-tophan methyl esters (Fig.1).
Initial experiments clearly demonstrated that the QCM sen-sors with the chiral calix[4]arenes (5a, 5b, 5c, and 5d) exhib-ited good selective chiral recognition of alanine enantiomers, whereas they did not show good selective chiral recognition of the other amino acid enantiomers. Accordingly, the next stud-ies were performed to elucidate this behavior of the sensors. The chiral discrimination factors (α) [43] of the sensors were specified by the evaluation of the sensor responses [44]. The
limit of detection (LOD) [45], sensitivity (S) [46], and time constant (τ) [47,48] were calculated from analyte concentra-tion studies. The effects of mass loading and the deposiconcentra-tion technique on chiral recognition were studied in detail. Furthermore, the chiral discrimination studies were optimized by response surface methodology (RSM) with central com-posite design (CCD) for alanine racemic mixtures. A series of statistical analysis such as analysis of variance (ANOVA) was examined to check the accuracy of the fitted model for alanine racemic mixtures [49].
Materials and methods
Reagents and instrumentation
A Stuart SMP3 melting point apparatus and a sealed capillary were used to determine the melting points of the synthesized calix[4]arene compounds. Structure determinations of chiral calix[4]arene compounds were performed with a Varian 400-MHz NMR spectrometer and a PerkinElmer 100 Fourier transform IR spectrometer, and elemental analysis was per-formed with a Leco CHNS-932 analyzer. Analytical thin-layer chromatography was used to monitor the reactions with use of precoated silica gel plates (SiO2Merck F254). All re-agents used for the preparation of compounds and the sensing applications were of standard analytical grade from Merck, Sigma-Aldrich, or Fluka and were used without further purification.
A QCM200 QCM was purchased from Stanford Research Systems (Sunnyvale, CA, USA) to measure the frequency change of quartz crystals between gold electrodes. At the sur-face of a crystal with a 5-MHz fundamental frequency, a net change of 1 Hz means approximately 24 ng of adsorbed or desorbed material. QCM crystals were cleaned with an Isolab ultrasonic bath. The chiral calix[4]arene coated QCM sensor surfaces were characterized with an NTEGRA Spectra atomic force microscope (AFM) from NT-MDT (Moscow, Russia). Standard 125-μm-long NSG30 silicon cantilevers (NT-MDT) with a force constant of 22–100 N m-1
were used. The typical curvature radius of the tip-cantilever was approximately 10 nm. Topographic images were captured in semicontact mode with a resonance frequency of 240–440 kHz. The scanning speed was 255 × 255 pixels at 1 Hz. To determine the root-mean-square roughness, AFM images were processed with the software program Nova RC (NT-MDT). QCM sensor sur-faces were also characterized by contact angle measurements with a DSA25 drop shape analyzer from Krüss (Hamburg, Germany). For the spin-coating technique, the QCM crystal surface was coated with chiral calix[4]arene receptors with use of a Laurell Technologies spin coater (model WS-400BZ-6NPP/LITE). An ISM940E peristaltic pump from Ismatech (Wertheim, Germany) was used to transfer the solutions of
α-amino acid enantiomers onto the sensor surface. All of the sensing experiments were performed in a 5220120 glove box unit (Labconco, Kansas City, MO, USA).
The experimental procedures forα-amino acid racemic mixtures were optimized by RSM with CCD. All statistic analysis was performed with Design-Expert 10.0.3 trial ver-sion (Stat-Ease, Minneapolis, MN, USA).
Synthesis
p-tert-Butylcalix[4]arene (1) and its derivative 2 were synthe-sized according to previously published procedures [38,50].
Other calix[4]arene compounds (3, 4) and chiral calix[4]arene receptors (5a, 5b, 5c, and 5d) were synthesized for the first time with use of known procedures (see the electronic supplementary materialfor their synthesis procedures).
Coating of QCM crystals with chiral receptors
The solutions of chiral calix[4]arene receptors (5a, 5b, 5c, and 5d) were prepared as 1.0 mM solutions in chloroform. The surfaces of the QCM crystals were cleaned in an ultrasonic bath with chloroform and distilled water. After every cleaning, crystals were subjected to ultrapure nitrogen to dry them. The Scheme 1 Synthesis route of the chiral compounds 5a, 5b, 5c, and 5d. HMTA hexamethylenetetramine, TFA trifluoroacetic acid
baseline frequencies of the sensors were measured before coating with chiral receptors. Then the QCM crystal surfaces were coated with 3μL of chiral receptor solutions in 3 mL of chloroform in a beaker by a soaking method [51] and the solvent was evaporated overnight. After evaporation of chlo-roform, chiral calix[4]arene coated QCM sensors were obtain-ed as a result of self-assemby of the molecules on the QCM surface. The frequencies of the new QCM sensors were mea-sured to determine the mass loading of the deposited chiral receptors on the crystal surface in every deposition process. For the evaluation of the effect of coating methods on the chiral recognition of amino acid enantiomers, spin-coating and drop-casting methods were used as well as the soaking method. For the fabrication of QCM sensors by the spin-coating method, 100μL of the chiral calix[4]arene solutions (1 mM in chloroform) was applied to the QCM surface at 1000 rpm for 30 s. In the case of the drop-casting method, 20μL of the chiral calix[4]arene solutions (1 mM in chloro-form) was applied to the QCM surface, and then the solvent was removed by evaporation at 60 °C for 30 min in a drying oven.
Chiral discrimination abilities of chiral receptors
The chiral discrimination studies were performed according to the following procedure. The chiral calix[4]arene coated QCM sensors were placed in a QCM holder that was mounted in a QCM flow cell. Distilled water was circulated in the QCM
flow cell by a peristaltic pump to keep the frequency response at zero for stabilization of the sensors. Then 1000μM amino acid solutions were circulated in the QCM flow cell by a peristaltic pump.ΔF and ΔR of the QCM sensors were mon-itored as a function of time continuously during the circulation of the amino acid solutions in the sensing system. A home-made sensing system (see Fig.S1) was used in this study.ΔF decreased andΔR increased because of an increase of surface mass by the adsorption of amino acid molecules on QCM sensor surfaces. After the sensing processes, the sensor sur-faces were subjected to distilled water to break down interac-tions between chiral calix[4]arene and amino acid molecules. The completion of the desorption process was understood by the return of the sensor response to the initial frequency.
Results and discussion
Synthesis and characterization
T h e s y n t h e s is o f n e w S c h i ff - b a s e - f u n c t i o n a l i z e d calix[4]arene-based receptors (5a, 5b, 5c, 5d) with chiral binding sites (Scheme1), such as amines or amino alcohols, was performed to study their chiral recognition abilities to-ward some α-amino acid enantiomers (Fig. 1) through the QCM method. Their structures were characterized by a com-bination of Fourier transform IR spectroscopy,1H NMR spec-troscopy, and13C NMR spectroscopy. The synthesis of 1 and Fig. 1 The molecular structures
of the chiralα-amino acid methyl ester derivatives
2 was performed according to previous literature methods [50,
38]. Detailed spectroscopic information for all synthesized compounds is given in theelectronic supplementary material.
Chiral discrimination assays
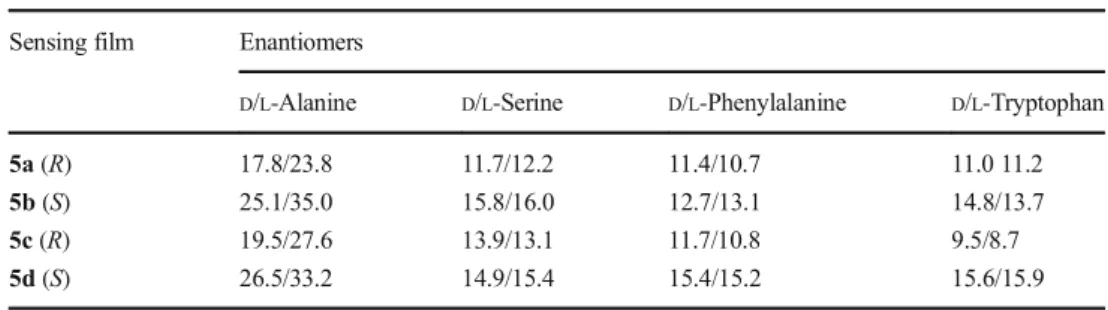
A QCM gold surface was coated with the new chiral calix[4]arene based receptors (5a, 5b, 5c, and 5d) to obtain four chiral calix[4]arene coated QCM sensors by the soaking method. In this step, disulfide moieties of 5a, 5b, 5c, and 5d can be strongly adsorbed on the gold surface to afford stable and ordered layers because of the formation of the covalent bond between gold and sulfur [43,52]. These QCM sensors were used in recognition studies ofα-amino acid enantiomers to determine the chiral calix[4]arene receptors that are effec-tive and seleceffec-tive sensing material. The frequency changes (indicating the response of the QCM sensor) and the chiral discrimination factors (α) of 5a-, 5b-, 5c-, and 5d-coated QCM sensors toward amino acid enantiomers are given in Tables1and2, respectively. As seen in Table1, remarkable frequency shifts were observed for alanine enantiomers with all QCM sensors, whereas there were no substantial differ-ences for the other amino acid enantiomers. This indicates that all the chiral QCM sensors prepared in this study can be chiral selectors for alanine enantiomers.
The chiral discrimination factors (Table2) were calculated through the resonance frequency shifts (Table1) for each en-antiomer by Eq. (2):
α ¼ Δfl Δfd
; ð2Þ
where ΔfL and ΔfDare the frequency changes of the QCM
sensors toward the amino acid enantiomers.
The frequency changes were measured versus time as one of the enantiomers was injected into the system to interact with chiral calix[4]arene based receptors 5a, 5b, 5c, and 5d on the QCM surface. As seen in Table2, remarkable chiral discriminations for only alanine enantiomers were observed with use of 5b- and 5c-coated QCM sensors [43]. It was ex-pected that one of the chiral selectors would show more affin-ity for one of the enantiomers and the other chiral selector would show more affinity for the other enantiomer under the same conditions [53]. However, although the results do not fully meet that expectation, they reveal a remarkable chiral selectivity for alanine enantiomers. The relatively low chiral selectivity may have resulted from strong hydrogen bonding between alanine and the calixarenes [48], and chiral discrim-ination was not observed for any of the other amino acids tested. This could result from phenyl and indole groups of phenylalanine and tryptophan, respectively. These groups might hinder the effect of chiral–NH2groups in amino acids in terms of their chiral discrimination. In the case of serine, hydrogen-bonding interaction between QCM sensor films and –OH groups of serine enantiomers might be more important than the effect of chiral–NH2groups in amino acids in terms of their chiral discrimination. Consequently, the difference in the recognition ability of the QCM sensors for amino acid enantiomers may be explained by the size-fit concept, the three-dimensional structures of the molecules, steric effects, and complexation interaction between moieties of the sensible film layer and analyte molecules such as hydrogen-bonding interactions [54,55]. Moreover, the results in Table1indicate that all the sensors exhibited higher chiral recognition forL -alanine than for D-alanine. The 5c-coated QCM sensor was used in the next experiments as it was the best sensor in terms of chiral discrimination. This sensor was tested in alanine solutions of different concentrations (50, 100, 250, 500, and 1000μM). ΔF and ΔR for each alanine solution are given in Figs.S11andS12, respectively. As seen in Figs.S11andS12, ΔF decreased and ΔR increased gradually as expected when Table 1 Frequency changes
(−ΔHz) of the 5a-, 5b-, 5c-, and 5d-coated quartz crystal microbalance sensors for α-amino acid enantiomers
Sensing film Enantiomers
D/L-Alanine D/L-Serine D/L-Phenylalanine D/L-Tryptophan
5a (R) 17.8/23.8 11.7/12.2 11.4/10.7 11.0 11.2
5b (S) 25.1/35.0 15.8/16.0 12.7/13.1 14.8/13.7
5c (R) 19.5/27.6 13.9/13.1 11.7/10.8 9.5/8.7
5d (S) 26.5/33.2 14.9/15.4 15.4/15.2 15.6/15.9
Amino acid concentration 1000μM
Table 2 Chiral discrimination factors (α) of the 5a-, 5b-, 5c-, and 5d-coated quartz crystal microbalance sensors forα-amino acid enantiomers Sensing film Enantiomers
Alanine Serine Phenylalanine Tryptophan
5a (R) 1.33 1.04 0.94 1.02
5b (S) 1.39 1.01 1.03 0.93
5c (R) 1.42 0.94 0.92 0.92
5d (S) 1.25 1.03 0.99 1.02
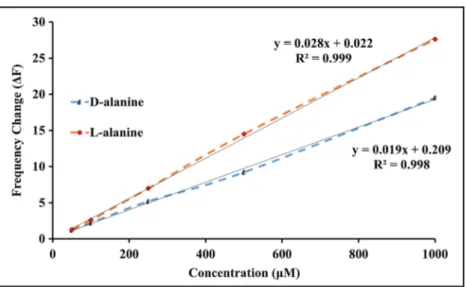
the concentration of the alanine enantiomer solutions in-creased. Additionally, the calibration curves of the frequency changes for each alanine solution are given in Fig.2. The responses of the QCM sensor and the chiral discrimination factors both increased linearly with increase of the alanine concentration (R2
= 0.998 forD-alanine andR2= 0.999 for L-alanine, Fig. 2). Furthermore, the QCM sensor was also tested with other amino acid solutions with different concen-trations, but no chiral discrimination was observed.
The sensor sensitivity (S) can be easily determined from the slope of calibration curves that are drawn as the frequency change of the sensor versus alanine solution concentration [46]. The LOD of the QCM sensor for alanine enantiomers was cal-culated with the calibration curves in Fig.2and Eq.3[45]:
LOD¼3xSbl
m ; ð3Þ
whereSblis the standard deviation of the response andm is the slope of the calibration curve. Time constants (τ) were also cal-culated for adsorption and desorption of alanine enantiomers from the frequency changes of the QCM sensor toward enantio-mers (1000μM) during the adsorption and desorption (Figs.
S13, andS14, respectively) [47,48]. All calculated sensing pa-rameters (sensitivity, LOD, and time constant) are given in Table3.
To evidence deposition of 5c onto QCM sensors, contact angle measurements were performed. As seen in Fig.S15, the contact angles of the bare crystal surface and the surfaces of 5c-coated QCM sensors obtained by the soaking, spin-coat-ing, and drop-casting methods were 68°, 91.8°, 82.7°, and 77.6°, respectively. As is well known, the surface can be clas-sified as high wettability (θ ≪ 90°) or low wettability (θ ≫ 90°) according to the contact angle [56]. Hence, the increase in contact angle due to the hydrophobic moieties of calix[4]arene molecules confirmed that molecules of 5c were successfully deposited onto the QCM sensor by all deposition techniques. The contact angles of the 5c-coated QCM sensors revealed that the soaking method was more efficient than the other methods for the coating of the QCM sensor with molecules of 5c.
AFM images of the QCM sensors were obtained to evalu-ate the changes in their surface morphologies after deposition. The AFM in semicontact mode was applied to characterize the formation of the 5c-coated QCM sensors on the gold surface by different coating techniques. AFM images of the bare crys-tal surface and the 5c-coated QCM sensors obtained by the soaking, spin-coating, and drop-casting methods are given in Fig.S16. The average surface roughness increased with each layer, from 1.51 nm for the bare crystal surface to 3.60 nm for the 5c-coated QCM sensor obtained by the soaking technique, 4.14 nm for the 5c-coated QCM sensor obtained by the spin-coating technique, and 13.63 nm for the 5c-coated QCM sen-sor obtained by the drop-casting technique. The increase of peak-to-peak height (bare crystal surface 19.53 nm, soaking method 51.48 nm, spin-coating method 55.82 nm, and drop-casting method 231.86 nm) indicated the formation of 5c-coated QCM sensors on the bare crystal surface by each tech-nique. Thus, the bond formation between disulfide moieties of 5c molecules and gold on the bare crystal surface resulted in the formation of 5c-coated QCM sensors. However, the bare crystal surface seems to be almost smooth, whereas some Fig. 2 Calibration curves for the
response of the 5c-coated quartz crystal microbalance sensor toward alanine enantiomers in different concentrations
Table 3 Limit of detection (LOD), sensitivity (S), and time constant (τ) of the 5c-coated quartz crystal microbalance sensor obtained by the soaking method for alanine enantiomers
Enantiomer LOD (μM) S (Hz/μM) τads(s)a τdes(s)b D-Alanine 85.4 0.019 67.8 36.7 L-Alanine 60.9 0.028 36.2 40.5
a
The adsorption process of 1000μM solutions of alanine enantiomers
b
large peaks occurred on the QCM surfaces after the coating by each method. Indeed, different surface morphologies were obtained from each coating method. The surface obtained by the soaking method (Fig.S16b) has some large peaks that are not the same height and width. This means that the QCM surface may have been irregularly coated with 5c molecules or they may have overlapped each other. In the case of the spin-coating method, the surface of the 5c-coated QCM sensor (Fig.S16c) was smooth locally. This indicates that 5c mole-cules were scattered on the QCM sensor surface equally but there are some gaps locally. In the case of the drop-casting method, the surface of the 5c-coated QCM sensor (Fig.S16d) had no uniform morphology. There were some high and large peaks in several regions of the surface.
Effect of mass loading
We also examined the effect of 5c content on chiral discrim-ination. Accordingly, solutions of 5c in different concentra-tions were used to prepare the QCM sensors with various mass loadings of 5c on the QCM surface. These QCM sensors including 5c in different amounts were used with alanine en-antiomers; the results are given in FigS17. The monitoring of a change in the chiral discrimination factors on change of the 5c content on the QCM sensor demonstrated that the 5c con-tent plays an important role in the sensing process. The opti-mum mass loading of 5c on the QCM sensor was 103 Hz for the chiral discrimination of alanine enantiomers. The sensor with a mass loading of 109 Hz exhibited much lower chiral discrimination than the 103-Hz sensor although the mass load-ing of the sensors was similar. This may have resulted from different scattering of 5c molecules on the sensor surface. Above this mass loading (235 and 317 Hz), the chiral discrim-ination decreased gradually. It is considered that the decrease in chiral discrimination may have resulted from overlapping of 5c molecules on the QCM sensor. In the case of a mass loading of 71 Hz, the chiral discrimination was also low com-pared with that of the 5c-coated QCM sensor, which has a mass loading of 103 Hz. This is attributed to the lack of suf-ficient 5c molecules on the QCM surface for sufsuf-ficient adsorp-tion of alanine molecules.
Repeatability and durability of the sensor
It is well known that the repeatability and the durability of a sensor are very important parameters in sensing studies. To examine the repeatability of the 5c-coated QCM sensor (mass loading of 109 Hz obtained by the soaking method), it was exposed to a solution of alanine enantiomer (1000μM) at least five times; the recordedΔF and ΔR are given in Figs.S18and
S19, respectively. After every adsorption process, desorption was performed with distilled water. The results showed that the differences in the responses of the QCM sensor remained
almost constant after each process. This demonstrated that the 5c-coated QCM sensor showed superior reproducibility in sensing of alanine enantiomers.
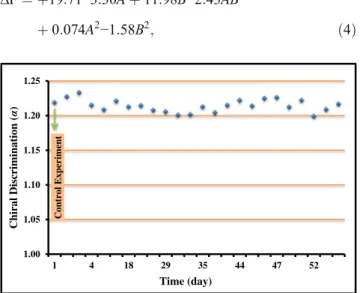
To measure the durability of the 5c-coated QCM sensor, frequency changes were recorded after the QCM sensor had been exposed to a solution of each alanine enantiomer for different times. The results (Fig. 3) show that there was a significant difference in chiral discrimination of alanine enan-tiomers for 2 months. This implies that the 5c-coated QCM sensor can be used for a long time in chiral recognition studies.
Enantioselectivity studies of racemic mixtures
Enantioselectivity of alanine racemic mixtures was evaluated by RSM to optimize two parameters: the volume fraction of theDenantiomer in a racemic mixture and the concentration of the racemic mixture. The independent variables are symbol-ized asA and B. The responses of the newly prepared 5c--coated QCM sensor (with a mass loading of 103 Hz) toward racemic mixtures were determined from the output response variables. Other factors, such as stirring rate, temperature, and circulation rate of the racemic mixtures, were held constant. The levels of the independent variables are given in TableS1. CCD was used to investigate the combined effect of the two independent variables by 11 sets of experiments including three replications at the center points. The responses of the QCM sensor toward racemic mixtures under various experi-mental conditions are given in TableS2. A series of statistical analysis as an ANOVA was examined to check the accuracy of the fitted model. A statistical model describing the responses of the QCM sensor toward racemic mixtures and under vari-ous experimental conditions was obtained by a response sur-face quadratic model fitting method with Design-Expert ac-cording to the following equation:
ΔF ¼ þ19:71−3:30A þ 11:98B−2:43AB þ 0:074A2−1:58B2; ð4Þ Control Experiment 1.00 1.05 1.10 1.15 1.20 1.25 1 4 18 29 35 44 47 52 ( ) Time (day) Chiral Discrimination
Fig. 3 The durability of the 5c-coated QCM sensor toward alanine enantiomers (alanine concentration 1000μM)
whereΔF is the frequency change of the sensor, and A and B are independent variables as mentioned earlier. TableS2gives predicted results for frequency changes of the sensor for race-mic mixtures according to Eq.4.
A quadratic model is suggested for racemic mixtures in TableS3. Additionally, ANOVA results for the response sur-face quadratic model for the sensing of racemic mixtures are given in TableS4. TheF value of 3647.26 implies that the model is significant for responses of the QCM sensor toward racemic mixtures. Adequate precision measures the signal-to-noise ratio, and a ratio greater than 4 is desirable [57,58]. Hence, in the quadratic models for responses of the QCM sensor toward racemic mixtures, the ratio of 180.660 indicated an adequate signal for this model to be used to navigate the design space. Thep value (probability greater than F) is less than 0.0500, indicating that the model terms are significant because a value greater than 0.1000 indicates that the model terms are not significant [59]. Furthermore, the lack-of-fitF value of 25.57 implies significance for the sensing of racemic mixtures. The low coefficient of variation indicates high pre-cision and good reliability of the experimental values [60].
Moreover, the predicted R2of 0.9973 is in reasonable agreement with the adjustedR2of 0.9995 because of a differ-ence less than 0.2. Such good agreement implies good predict-ability of the model [60,61]. There is good agreement be-tween the experimental and predicted values of the response of the QCM sensor toward racemic mixtures (Fig.S20a). The internally standardized residuals and normal probability plots for the response of the QCM sensor toward racemic mixtures were obtained, and the points that were obtained data
consistently appear on a trend line that indicated that errors are distributed normally (Fig.S20b) [62].
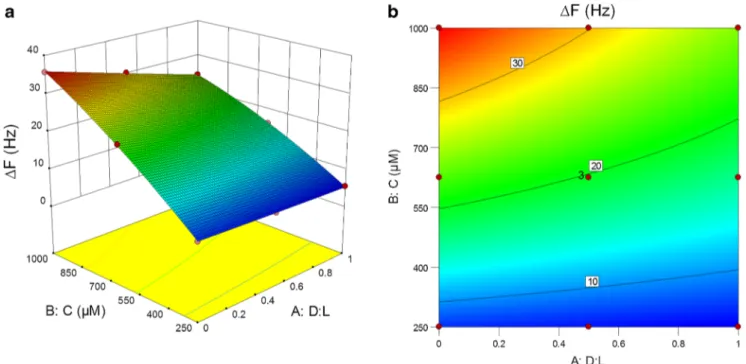
The three-dimensional surface, which is curvilinear be-cause of the quadratic model, and contour graphs for the re-sponse surface are shown in Fig.4. The two parameters men-tioned earlier for each model were plotted on thex and y axes, with the response of the QCM sensor toward racemic mixtures on thez axis. The three-dimensional surface and contour plots describe the effect of the parameters on the sensor response. The volume fractions of theDenantiomer in the racemic mix-ture and the concentrations of the racemic mixmix-tures were the most important factors. The responses of the QCM sensor toward racemic mixtures increased because of an increase in the concentration of racemic mixtures at constant ratios of the Denantiomer to theLenantiomer. Figure4also indicates that the responses of the QCM sensor toward racemic mixtures for each concentration remained unchanged for theDandL enan-tiomers individually. Moreover, the response toward the L enantiomer increased better gradually than that toward theD enantiomer. Indeed, it was understood that chiral discrimina-tion increased on increase in the concentradiscrimina-tion of the racemic mixtures. Consequently, the 5c-coated QCM sensor exhibited selective chiral recognition of the alanine enantiomers.
Conclusion
A remarkable chiral recognition was observed for only alanine enantiomers among the amino acid enantiomers tested. The 5c-coated QCM sensor showed the highest selective chiral
Fig. 4 a Three-dimensional surface and b contour plots for frequency changes of the 5c-coated quartz crystal microbalance sensor toward alanine racemic mixtures
recognition for alanine enantiomers over the other amino acid enantiomers. The size-fit concept, the three-dimensional struc-tures of the molecules, steric effects, and complexation inter-action between moieties of the sensible film layer and analyte molecules such as hydrogen-bonding interactions were major factors during the sensing process. Moreover, the chiral rec-ognition ability of this effective sensor was investigated to-ward various concentrations of amino acid enantiomers. The sensitivity (S) is 0.028 Hz/μM, the LOD is 60.9 μM, and the time constant (τ) is 36.2 s for 1000 μM L-alanine solution. Selectivity studies of racemic mixtures revealed that the enantioselective recognition of alanine enantiomers was achieved by a QCM sensor with the calix[4]arene derivative 5c having (R)-2-phenylglycinol moieties. Thus, we produced a new QCM sensor for alanine enantiomers having outstand-ing properties, such as real-time, sensitive, effective, and se-lective chiral detection, durability and easy recovery with dis-tilled water.
Acknowledgements We thank the Technical Research Council of Turkey (TUBITAK grant number 115Z249) and the Research Foundation of Selçuk University (SUBAP grant number 16401003), Konya, Turkey, and for financial support of this work produced from FT’s Ph.D. thesis.
Compliance with ethical standards
Conflict of interest The authors declare that they have no competing interests.
References
1. Yoshio O, Eiji Y. Polysaccharide derivatives for chromatographic separation of enantiomers. Angew Chem Int Ed. 1998;37(8):1020– 43. https://doi.org/10.1002/(SICI)1521-3773(19980504)37: 8<1020::AID-ANIE1020>3.0.CO;2-5.
2. Gawley RE, Aubé J. Practical aspects of asymmetric synthesis. In: Principles of asymmetric synthesis. 2nd ed. Oxford: Elsevier; 2012. p. 63–95.https://doi.org/10.1016/B978-0-08-044860-2.00002-7. 3. Dewick PM. Medicinal natural products: a biosynthetic approach.
2nd ed. Chichester: Wiley; 2001.
4. Gasparrini F, Pierini M, Villani C, Filippi A, Speranza M. Induced-fit in the gas phase: conformational effects on the enantioselectivity of chiral tetra-amide macrocycles. J Am Chem Soc. 2008;130(2): 522–34.https://doi.org/10.1021/ja073287+.
5. Sambasivan S, Kim D-s, Ahn KH. Chiral discrimination of α-amino acids with a C2-symmetric homoditopic receptor. Chem Commun. 2010;46(4):541–3.https://doi.org/10.1039/B919957H. 6. Bi Q, Dong S, Sun Y, Lu X, Zhao L. An electrochemical sensor
based on cellulose nanocrystal for the enantioselective discrimina-tion of chiral amino acids. Anal Biochem. 2016;508:50–7.https:// doi.org/10.1016/j.ab.2016.05.022.
7. Ilisz I, Péter A, Lindner W. State-of-the-art enantioseparations of natural and unnatural amino acids by high-performance liquid chro-matography. Trends Anal Chem. 2016;81:11–22.https://doi.org/10. 1016/j.trac.2016.01.016.
8. Sánchez-Hernández L, Bernal JL, Nozal MJ, Toribio L. Chiral analysis of aromatic amino acids in food supplements using
subcritical fluid chromatography and Chirobiotic T2 column. J Supercrit Fluids. 2016;107:519–25. https://doi.org/10.1016/j. supflu.2015.06.027.
9. Yu X, Yao Z-P. Chiral differentiation of amino acids through binuclear copper bound tetramers by ion mobility mass spectrom-etry. Anal Chim Acta. 2017;981:62–70.https://doi.org/10.1016/j. aca.2017.05.026.
10. Tabakcı M, Tabakcı B, Yılmaz M. Design and synthesis of new chiral calix[4]arenes as liquid phase extraction agents forα-amino acid methylesters and chiralα-amines. J Incl Phenom Macrocycl Chem. 2005;53(1–2):51–6. https://doi.org/10.1007/s10847-005-0697-8.
11. Erdemir S. Synthesis of novel chiral Schiff base and amino alcohol derivatives of calix[4]arene and chiral recognition properties. J Mol Struct. 2012;1007:235–41.https://doi.org/10.1016/j.molstruc.2011. 10.053.
12. Zhang X, Chen S, Xu P, Yu Q, Dai Z. Synthesis of new chiral fluorescent sensors and their applications in enantioselective dis-crimination. Tetrahedron Lett. 2017;58(29):2850–5.https://doi. org/10.1016/j.tetlet.2017.06.025.
13. Gao G, Lv C, Li Q, Ai L, Zhang J. Enantiomeric discrimination of α-hydroxy acids and N-Ts-α-amino acids by1
H NMR spectrosco-py. Tetrahedron Lett. 2015;56(48):6742–6.https://doi.org/10.1016/ j.tetlet.2015.10.060.
14. Yin X, Ding J, Zhang S, Kong J. Enantioselective sensing of chiral amino acids by potentiometric sensors based on optical active polyaniline films. Biosens Bioelectron. 2006;21(11):2184–7.
https://doi.org/10.1016/j.bios.2005.10.010.
15. Fu YQ, Luo JK, Nguyen NT, Walton AJ, Flewitt AJ, Zu XT, et al. Advances in piezoelectric thin films for acoustic biosensors, acoustofluidics and lab-on-chip applications. Prog Mater Sci. 2017;89:31–91.https://doi.org/10.1016/j.pmatsci.2017.04.006. 16. Sayin S, Ozbek C, Okur S, Yilmaz M. Preparation of the
ferrocene-substituted 1,3-distalp-tert-butylcalix[4]arene based QCM sensors array and utilization of its gas-sensing affinities. J Organomet Chem. 2014;771:9–13.https://doi.org/10.1016/j.jorganchem.2014. 06.004.
17. Baldini L, Sansone F, Faimani G, Massera C, Casnati A, Ungaro R. Self-assembled Chiral dimeric capsules from difunctionalized N,C-linked peptidocalix[4]arenes: scope and limitations. Eur J Org Chem. 2008;5:869–86.https://doi.org/10.1002/ejoc.200700943. 18. Pirondini L, Dalcanale E. Molecular recognition at the gas–solid
interface: a powerful tool for chemical sensing. Chem Soc Rev. 2007;36(5):695–706.https://doi.org/10.1039/B516256B. 19. Koshets IA, Kazantseva ZI, Shirshov YM, Cherenok SA,
Kalchenko VI. Calixarene films as sensitive coatings for QCM-based gas sensors. Sensors Actuators B Chem. 2005;106(1):177– 81.https://doi.org/10.1016/j.snb.2004.05.054.
20. Miah M, Pavey KD, Gun'ko VM, Sheehan R, Cragg PJ. O b s e r v a t i o n o f t r a n s i e n t a l k a l i m e t a l i n c l u s i o n i n oxacalix[3]arenes. Supramol Chem. 2004;16(3):185–92.https:// doi.org/10.1080/10610270310001644473.
21. Wang C, He X-W, Chen L-X. A piezoelectric quartz crystal sensor array self assembled calixarene bilayers for detection of volatile organic amine in gas. Talanta. 2002;57(6):1181–8.https://doi.org/ 10.1016/S0039-9140(02)00193-5.
22. Sharma K, Cragg P. Calixarene based chemical sensors. Chem Senses. 2011;1(9):1–18.
23. Hao R-Z, Song H-B, Zuo G-M, Yang R-F, Wei H-P, Wang D-B, et al. DNA probe functionalized QCM biosensor based on gold nanoparticle amplification for Bacillus anthracis detection. Biosens Bioelectron. 2011;26(8):3398–404.https://doi.org/10. 1016/j.bios.2011.01.010.
24. Jearanaikoon P, Prakrankamanant P, Leelayuwat C, Wanram S, Limpaiboon T, Promptmas C. The evaluation of loop-mediated isothermal amplification-quartz crystal microbalance
(LAMP-QCM) biosensor as a real-time measurement of HPV16 DNA. J Virol Methods. 2016;229:8–11.https://doi.org/10.1016/j.jviromet. 2015.12.005.
25. Karaseva N, Ermolaeva T, Mizaikoff B. Piezoelectric sensors using molecularly imprinted nanospheres for the detection of antibiotics. Sensors Actuators B Chem. 2016;225:199–208.https://doi.org/10. 1016/j.snb.2015.11.045.
26. Pei Z, Saint-Guirons J, Käck C, Ingemarsson B, Aastrup T. Real-time analysis of the carbohydrates on cell surfaces using a QCM biosensor: a lectin-based approach. Biosens Bioelectron. 2012;35(1):200–5.https://doi.org/10.1016/j.bios.2012.02.047. 27. Sauerbrey G. Use of quartz vibrator for weighing thin layers and as
a microbalance. Z Phys. 1959;155:206–22.https://doi.org/10.1007/ BF01337937.
28. Fakhrullin RF, Vinter VG, Zamaleeva AI, Matveeva MV, Kourbanov RA, Temesgen BK, et al. Quartz crystal microbalance immunosensor for the detection of antibodies to double-stranded DNA. Anal Bioanal Chem. 2007;388(2):367–75.https://doi.org/ 10.1007/s00216-007-1230-2.
29. Lee S-W, Hinsberg WD, Kanazawa KK. Determination of the vis-coelastic properties of polymer films using a compensated phase-locked oscillator circuit. Anal Chem. 2002;74(1):125–31.https:// doi.org/10.1021/ac0108358.
30. Arnau A, Sogorb T, Jiménez Y. Circuit for continuous motional series resonant frequency and motional resistance monitoring of quartz crystal resonators by parallel capacitance compensation. Rev Sci Instrum. 2002;73(7):2724–37.https://doi.org/10.1063/1. 1484254.
31. Nwankwo E, Durning CJ. Impedance analysis of thickness-shear mode quartz crystal resonators in contact with linear viscoelastic media. Rev Sci Instrum. 1998;69(6):2375–84.https://doi.org/10. 1063/1.1148963.
32. Su X-L, Li Y. A QCM immunosensor for Salmonella detection with simultaneous measurements of resonant frequency and motional resistance. Biosens Bioelectron. 2005;21(6):840–8.https://doi.org/ 10.1016/j.bios.2005.01.021.
33. Singh AK, Singh M. Molecularly imprinted Au-nanoparticle com-posite-functionalized EQCM sensor forL-serine. J Electroanal Chem. 2016;780:169–75.https://doi.org/10.1016/j.jelechem.2016. 09.021.
34. Mirmohseni A, Shojaei M, Farbodi M. Application of a quartz crystal nanobalance to the molecularly imprinted recognition of phenylalanine in solution. Biotechnol Bioprocess Eng. 2008;13(5):592–7.https://doi.org/10.1007/s12257-008-0028-1. 35. Nakanishi T, Yamakawa N, Asahi T, Osaka T, Ohtani B, Uosaki K.
Enantioselective adsorption of phenylalanine onto self-assembled monolayers of 1,1‘-binaphthalene-2,2‘-dithiol on gold. J Am Chem Soc. 2002;124(5):740–1.https://doi.org/10.1021/ja012084x. 36. Bodenhofer K, Hierlemann A, Seemann J, Gauglitz G,
Koppenhoefer B, Gopel W. Chiral discrimination using piezoelec-tric and optical gas sensors. Nature. 1997;387(6633):577–80. 37. Yılmaz A, Tabakcı B, Tabakcı M. New diamino derivatives of
p-tert-butylcalix[4]arene for oxyanion recognition: synthesis and complexation studies. Supramol Chem. 2009;21(6):435–41.
https://doi.org/10.1080/10610270802165969.
38. Gutsche CD, Dhawan B, No KH, Muthukrishnan R. Calixarenes. 4. The synthesis, characterization, and properties of the calixarenes fromp-tert-butylphenol. J Am Chem Soc. 1981;103(13):3782–92.
https://doi.org/10.1021/ja00403a028.
39. Ovsyannikov A, Solovieva S, Antipin I, Ferlay S. Coordination polymers based on calixarene derivatives: structures and properties. Coord Chem Rev. 2017;352(Suppl C):151–86.https://doi.org/10. 1016/j.ccr.2017.09.004.
40. Kostyukevych KV, Khristosenko RV, Pavluchenko AS, Vakhula AA, Kazantseva ZI, Koshets IA, et al. A nanostructural model of ethanol adsorption in thin calixarene films. Sensors Actuators B
Chem. 2016;223:470–80.https://doi.org/10.1016/j.snb.2015.09. 123.
41. Nikoleli G-P, Nikolelis DP, Evtugyn G, Hianik T. Advances in lipid film based biosensors. Trends Anal Chem. 2016;79:210–21.https:// doi.org/10.1016/j.trac.2016.01.021.
42. Temel F, Özçelik E, Türe AG, Tabakcı M. Sensing abilities of functionalized calix[4]arene coated QCM sensors towards volatile organic compounds in aqueous media. Appl Surf Sci. 2017;412: 238–51.https://doi.org/10.1016/j.apsusc.2017.03.258.
43. Su WC, Zhang WG, Zhang S, Fan J, Yin X, Luo ML, et al. A novel strategy for rapid real-time chiral discrimination of enantiomers using serum albumin functionalized QCM biosensor. Biosens Bioelectron. 2009;25(2):488–92.https://doi.org/10.1016/j.bios. 2009.06.040.
44. Temel F, Tabakcı M. Calix[4]arene coated QCM sensors for detec-tion of VOC emissions: methylene chloride sensing studies. Talanta. 2016;153:221–7.https://doi.org/10.1016/j.talanta.2016. 03.026.
45. Long GL, Winefordner JD. Limit of detection a closer look at the IUPAC definition. Anal Chem. 1983;55(7):712A–24A.https://doi. org/10.1021/ac00258a724.
46. Koshets IA, Kazantseva ZI, Belyaev AE, Kalchenko VI. Sensitivity of resorcinarene films towards aliphatic alcohols. Sensors Actuators B Chem. 2009;140(1):104–8.https://doi.org/10.1016/j.snb.2009. 04.014.
47. Fu Y, Finklea HO. Quartz crystal microbalance sensor for organic vapor detection based on molecularly imprinted polymers. Anal Chem. 2003;75(20):5387–93.https://doi.org/10.1021/ac034523b. 48. Grate JW, Snow A, Ballantine DS, Wohltjen H, Abraham MH,
McGill RA, et al. Determination of partition coefficients from sur-face acoustic wave vapor sensor responses and correlation with gas-liquid chromatographic partition coefficients. Anal Chem. 1988;60(9):869–75.https://doi.org/10.1021/ac00160a010. 49. Meng R, Kang J. Determination of the stereoisomeric impurities of
sitafloxacin by capillary electrophoresis with dual chiral additives. J Chromatogr A. 2017;1506:120–7. https://doi.org/10.1016/j. chroma.2017.05.010.
50. Li Z-T, Ji G-Z, Zhao C-X, Yuan S-D, Ding H, Huang C, et al. Self-assembling calix[4]arene [2]catenanes. Preorganization, conforma-tion, selectivity, and efficiency. J Org Chem. 1999;64(10):3572–84.
https://doi.org/10.1021/jo9824100.
51. Jeong H, Park K, Yoo J-C, Hong J. Structural heterogeneity in polymeric nitric oxide donor nanoblended coatings for controlled release behaviors. RSC Adv. 2018;8(68):38792–800.https://doi. org/10.1039/C8RA07707J.
52. Häkkinen H. The gold–sulfur interface at the nanoscale. Nat Chem. 2012;4:443.https://doi.org/10.1038/nchem.1352.
53. Zhang X, Yu Q, Lu W, Chen S, Dai Z. Synthesis of new chiral fluorescent sensors and their applications in enantioselective dis-crimination. Tetrahedron Lett. 2017;58(41):3924–7.https://doi. org/10.1016/j.tetlet.2017.08.077.
54. Memon FN, Memon S. Sorption and desorption of basic dyes from industrial wastewater using calix[4]arene based impregnated mate-rial. Sep Sci Technol. 2014;50(8):1135–46.https://doi.org/10.1080/ 01496395.2014.965831.
55. Mutihac L, Lee JH, Kim JS, Vicens J. Recognition of amino acids by functionalized calixarenes. Chem Soc Rev. 2011;40(5):2777– 96.https://doi.org/10.1039/C0CS00005A.
56. Yuan Y, Lee TR. Contact angle and wetting properties. In: Bracco G, Holst B, editors. Surface science techniques. Berlin: Springer; 2013. p. 3–34.https://doi.org/10.1007/978-3-642-34243-1_1. 57. Mannan S, Fakhru'l-Razi A, Alam MZ. Optimization of process
parameters for the bioconversion of activated sludge by Penicillium corylophilum, using response surface methodology. J Environ Sci. 2007;19(1):23–8. https://doi.org/10.1016/S1001-0742(07)60004-7.
58. Noordin MY, Venkatesh VC, Sharif S, Elting S, Abdullah A. Application of response surface methodology in describing the per-formance of coated carbide tools when turning AISI 1045 steel. J Mater Process Technol. 2004;145(1):46–58. https://doi.org/10. 1016/S0924-0136(03)00861-6.
59. Körbahti BK, Tanyolaç A. Electrochemical treatment of simulated textile wastewater with industrial components and Levafix Blue CA reactive dye: optimization through response surface methodology. J Hazard Mater. 2008;151(2–3):422–31.https://doi.org/10.1016/j. jhazmat.2007.06.010.
60. Amini M, Younesi H, Bahramifar N, Lorestani AAZ, Ghorbani F, Daneshi A, et al. Application of response surface methodology for optimization of lead biosorption in an aqueous solution by
Aspergillus niger. J Hazard Mater. 2008;154(1):694–702.https:// doi.org/10.1016/j.jhazmat.2007.10.114.
61. Silva JP, Sousa S, Gonçalves I, Porter JJ, Ferreira-Dias S. Modelling adsorption of acid orange 7 dye in aqueous solutions to spent brewery grains. Sep Purif Technol. 2004;40(2):163–70.
https://doi.org/10.1016/j.seppur.2004.02.006.
62. Lee K, Hamid S. Simple response surface methodology: investiga-tion on advance photocatalytic oxidainvestiga-tion of 4-chlorophenoxyacetic acid using UV-active ZnO photocatalyst. Materials. 2015;8(1):339– 54.https://doi.org/10.3390/ma8010339.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.