Contents lists available atScienceDirect
Food Research International
journal homepage:www.elsevier.com/locate/foodresRicinodendron heudelotii (Baill.) Heckel stem barks and seed extracts, a
native food plant from Africa: Characterization by NMR and
HPLC-DAD-ESI-MS
n
Stefania Sut
a, Stefano Dall'Acqua
b, Kouadio Bene
c, Serena Barbon di Marco
b,
Kouadio Ibrahime Sinan
d, Mohamad Fawzi Mahomoodally
e, Marie Carene Nancy Picot-Allain
e,
Gokhan Zengin
d,⁎aDAFNAE, Department of Agronomy, Food, Natural Resources, Animals and Environment, Agripolis Campus, University of Padova, 35020 Legnaro (PD), Italy bDepartment of Pharmaceutical and Pharmacological Sciences, University of Padova, Via Marzolo 5, 35131 Padova, Italy
cLaboratoire de Botanique et Phytothérapie, Unité de Formation et de Recherche Sciences de la Nature, 02 BP 801 Abidjan 02, Université Nangui Abrogoua, Abidjan, Cote
d’Ivoire
dDepartment of Biology, Science Faculty, Selcuk Universtiy, Campus, Konya, Turkey
eDepartment of Health Sciences, Faculty of Science, University of Mauritius, 230 Réduit, Mauritius
A R T I C L E I N F O
Keywords: African wood-oil nut Gallic acid derivatives Seed
Stem bark Antioxidant Glucosidase
A B S T R A C T
Ricinodendron heudelotii (Baill.) Heckle is used as food ingredient and in the African traditional medicine. In the present study inhibitory activity onα-amylase, α-glucosidase, acetylcholinesterase, butyrylcholinesterase, and tyrosinase of ethyl acetate, methanol, and water extracts of R. heudelotii seeds and stem bark were assessed. Stem bark extracts exhibited significant antioxidant properties. Ethyl acetate extract of seed had great inhibitory potential againstα-glucosidase, acetylcholinesterase, and butyrylcholinesterase. Nuclear magnetic resonance (NMR) and high-performance liquid chromatography with electrospray ionization mass spectrometry (HPLC-DAD-ESI-MSn) analysis revealed the presence of catechin and gallic acid derivatives in bark while fatty acid in
seeds. Multivariate analysis of obtained data was performed showing a clear separation between seed and stem bark. Obtained results indicate R. heudelotii stem bark as new starting materials for the development of novel pharmaceutical formulations.
1. Introduction
Throughout the ages, plants have played crucial roles in the ex-istence and survival of mankind. Man has used plants as food, to shelter from bad weather, for clothing, and as medicine to treat and/or manage human ailments. Therapeutic use of plants is one of the most ancient approach for medical uses and many plant species have been used to develop drugs (Cragg & Newman, 2013; Harvey, Edrada-Ebel, & Quinn, 2015; Newman & Cragg, 2016). Past decades have witnessed a re-surgence in interest towards medicinal plants, depicted by an increase in the number of scientific publications focusing on the biological properties of traditionally used medicinal plants. This effort endeavours to explore the possible pharmacological activities of underexplored medicinal plants (Majolo, de Oliveira Becker Delwing, Marmitt, Bustamante-Filho, & Goettert, 2019; Vyas, Kothari, & Kachhwaha, 2019; Zovko Končić & Bljajić, 2019). In this sense, the present study
was designed to evaluate the antioxidant and enzyme inhibitory prop-erties of the seed and stem bark of Ricinodendron heudelotii (Baill.) Heckle.
Ricinodendron heudelotii, also known as African wood-oil nut tree, is a large fast growing tree belonging to the Euphorbiaceae family, grows in the tropical forest of Africa and can reach up to 40 m height and 120 cm large (Assanvo, Gogoi, Dolui, & Baruah, 2015). The almond of the seed, well-known for its unique taste, is used as a thickener or condiment in soup and stews (Assanvo & Baruah, 2015). Several plant parts or preparation of R. heudelotii have been used in traditional medicine for the management of multiple human ailments, and have been studied for pharmacological activities.
Male Sprague-Dawley rats fed with R. heudelotii almond oil showed reduced cholesterol and triglyceride levels compared with the control group fed with standard diet (Tchankou Leudeu, Tchiégang, Barbé, Nicolas, & Guéant, 2009). A paste made from seed was directly applied
https://doi.org/10.1016/j.foodres.2019.108877
Received 28 August 2019; Received in revised form 27 November 2019; Accepted 30 November 2019
⁎Corresponding author.
E-mail address:gokhanzengin@selcuk.edu.tr(G. Zengin).
Available online 05 December 2019
0963-9969/ © 2019 Elsevier Ltd. All rights reserved.
to the tooth to relieve toothache (Ashu Agbor & Naidoo, 2015). R. heudelotii almond has a high protein content (30%) and high levels of polyunsaturated fatty acids, such as omega-3 fatty acids. Heudeloti-none, 1,2-dihydroheudelotinol, E-ferulic acid octacosylate, 3-methyl-methylorsellinate, and lupeol were identified from R. heudelotii stem bark and roots (Kimbu, Keumedjio, Sondengam, & Connolly, 1991).
The methanol extract of R. heudelotii leaves (IC5031.73 µg/ml)
ex-hibited strong antifungal (Cryptococcus neoformans) activity (Ogbole, Segun, & Fasinu, 2018). The leaves and latex of R. heudelotii were used as purgative and anthelmintic (Assanvo et al., 2015). The leaves of the plant were used to treat dysentery (Momeni et al., 2010).
The bark of the tree was used against coughs, sexual and fertility problems, labour and menstrual pain, intestinal problems, as antidote and to increase breast milk in lactating mothers (Assanvo et al., 2015; Momeni et al., 2010). The stem bark was reported to be used against stomach ulcer and to heal wounds.
This study aimed to establish possible bioactivities of R. heudelotii stem bark and seeds extracts in thefield of antioxidant assays, on key enzymes related to diabetes type II, Alzheimer’s disease, and epidermal hyperpigmentation problems. Although preliminary these data may reveal that some of the constituents or the phytocomplex of R. heudelotii may present significant effects in the in vitro assays. In order to depict these effects detailed phytochemical fingerprinting should be obtained. Due to the large number of phytoconstituents that are present in plants different extract process can be performed and the solvent selection can plays a significant role in the extraction of bioactive secondary meta-bolites. In the light of the above statement, the present study has at-tempted to compare the bioactivity of different extracts (ethyl acetate, methanol and water) of the stem bark and seed of R. heudelotii. In ad-dition, the antioxidant and phytochemical profiles of the different ex-tracts of R. heudelotii stem bark and seed has been established. To study the overall composition of starting material methanol extracts of both bark and seeds were subjected to phytochemical analysis, due to the fact that methanol as a solvent, due to its amphyphylic nature, is able to extract both lipophilic and hydrophilic constituents of plant material. It is expected that data collected from this study will provide additional data on the chemical composition and biological activities of R. heu-delotii stem bark and seed.
2. Materials and methods 2.1. Collection of plant material
Sampling of the plant species was done in Gontougo region (Nioumassi) of Ivory Coast in the year 2018. Botanical authentication of the plant was done by the botanist Dr. Kouadio Bene (Laboratoire de Botanique et Phytothérapie, Université Nangui Abrogoua, Abidjan, Côte d'Ivoire). The stem barks and seeds were dried at room temperature (in shade, about 10 days). A voucher specimen of the plant material is deposited at Selcuk University, Science Faculty. These materials were then powdered by using a laboratory mill.
2.2. Protocols for extraction
Maceration was used to prepare ethyl acetate and methanol ex-tracts. Briefly, 5 g dried plant samples were mixed 100 ml of solvents for 24 h at the room temperature. The solvents were removed using a rotary evaporator under vacuum at 40 °C. Regarding water extracts, traditional infusion method was used, as such 5 g dried plant samples were boiled in 100 ml of water for 20 min. The water extracts were lyophilized. The extracts were stored at refrigerated conditions (+4 °C) until analysis.
2.3. Profile of bioactive compounds
The total phenolic content (TPC) and totalflavonoid content (TFC)
of R. heudelotii extracts were determined spectrophotometrically using Folin-Ciocalteu and aluminium chloride methods, respectively (Uysal et al., 2017). The results were expressed as equivalents of gallic acid (GAE) (in case of TPC) and rutin (RE) (in case of TFC).
2.4. Chemical characterization of methanolic extracts
HPLC-DAD-ESI-MSn was used through a Chromatograph Agilent 1260 (Santa Clara, CA, USA) equipped with 1260 autosampler, column oven and diode array detector (DAD). After column, theflow was se-parated by a“T” connectors: half of the liquid was split to DAD and half to Agilent/Varian MS-500 ion trap mass spectrometer. Separation was achieved using a Agilent Eclipse XDB C-18 (3x150 mm, particle size 3,5 µm) as stationary phase. The sample injection volume was 20μl, the flow was 0,4 ml/min and the temperature was set at 40 °C. UV–vis spectra were acquired in the range of 190–640 nm. The mobile phase was 0,1% formic acid in water (A) and acetonitrile (B). A gradient program was used starting with 90%A then in 30 min going to 100%B, being isocratic up to 35 min than at 36 going back to 90%A and re-equilibrate up to 40 min.
MS spectra were collected in the m/z 100–2000 range, using ESI ion source operating in negative ion mode. Fragmentation of the ionic species was obtained using the turbo data dependent scanning (TDDS) instrument function.
DAD detector was used to estimate the amount of PACs,flavonoids and galloylated derivatives. As reference compounds, catechin (Sigma Aldrich, St. Louis, MO, USA), rutin (Sigma Aldrich) and gallic acid (Sigma Aldrich) were used. The chromatograms were monitored atλ 350 nm forflavonoids, while at 280 nm for galloylated derivatives and PACs. Compounds quantification was obtained with the method of ca-libration curve: catechin was used as external standard for PACs quantification, rutin was used for flavonoid and gallic acid for galloy-lated derivatives. Calibration curves were as follows: y = 6,914x − 19,041 (R2 = 0,99888) for catechin; y = 28,506x − 19,135 (R2 = 0,99916) for rutin;
y = 69,692x + 62,196 (R2= 0,99932) for gallic acid.
For NMR analysis 50 mg of the stem bark and seeds methanol ex-tract were dissolved in 1 ml of deuterated methanol and sonicated for 5 min. Sample was centrifuged and supernatant was used for NMR measurement. Bruker Avance III 400 MHz spectrometer was used. Standard Bruker library experiments were used to acquire H, HSQC-DEPT, HMBC, COSY and decoupled 13C experiments.
2.5. Determination of antioxidant and enzyme inhibitory effects
Estimation of anti-enzymatic activity of R. heudelotii extracts was done following methods previously described by (Uysal et al., 2017). Measurement of the antioxidant potential of R. heudelotii extracts using FRAP (ferric reducing antioxidant power), ABTS(2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid), CUPRAC (cupric reducing an-tioxidant capacity), DPPH (2,2-diphenyl-1-picrylhydrazyl), phospho-molybdenum and metal chelating assays were as reported previously (Uysal et al., 2017).
2.6. Statistical analysis
One-way ANOVA was done to determine any differences between the different extraction methods following by Tukey’s test. p < 0.05 were assigned to be statistically significant. Pearson’s linear correlation were employed to recognize any relationship between phytochemical contents and the observed biological activities. Besides, Partial least squares discriminant analysis (PLS-DA) analysis was done to evaluate the effect of the factors “organ type” and “solvent” respectively. Lastly, principal component (PCA) were performed to classify the performed extraction methods. The statistical procedures were performed by R software v. 3.5.1.
3. Results and discussion
Quantification of phenolic and flavonoid content from the different extracts of R. heudelotii seeds and stem bark was presented inTable 1. The stem bark extracts presented higher phenolic andflavonoid content compared to the seed extracts. Among the stem bark extracts, the me-thanol extract possessed highest phenolic (194.59 mg GAE/g) and fla-vonoid (8.12 mg RE/g) content.
Over the past few years, there has been an upsurge of interest in the pharmacological potential of medicinal plants which might be due to their polyphenol compounds, in particular to flavonoids ( Llorent-Martínez et al., 2017; Mollica et al., 2017; Zengin, Bulut, Mollica, Picot-Allain, & Mahomoodally, 2018; Zengin, Ceylan, et al., 2017; Zengin, Locatelli, et al., 2017). From this perspective, some biological activities including the antioxidant activity of R. heudelotii extracts was evaluated using the total antioxidant capacity or phosphomolybdenum, radical scavenging (DPPH and ABTS), reducing power, and metal chelating assays. Generally, the stem bark extracts were more active antioxidants compared to the seed extracts (Table 2). Besides, it was noted that the methanol stem bark extract exhibited highest total antioxidant capacity (3.86 mmol trolox equivalent (TE)/g), radical scavenging (487.30 and 1043.42 mg TE/g, for DPPH and ABTS), and reducing power (752.85 and 1230.96 mg TE/g, for FRAP and CUPRAC). It has been postulated that phenolic and flavonoid content of plant extracts was positively correlated to antioxidant activity (Farasat, Khavari-Nejad, Nabavi, & Namjooyan, 2014; Kefayati, Motamed, Shojaii, Noori, & Ghods, 2017; Khorasani Esmaeili, Mat Taha, Mohajer, & Banisalam, 2015). Accord-ingly, Pearson’s correlation analysis showed that TPC and TFC was correlated to DPPH, ABTS, FRAP, CUPRAC and phosphomolybdenum (Fig. 1).Momeni et al. (2010)have previously studied the antioxidant activity of the ethyl acetate and methanol/dichloromethane extracts of R. heudelotii leaves and reported low activity (Momeni et al., 2010). These findings highlight the importance of extraction solvent choice suggesting that the more active constituents for the considered tests are less lipophilic. However, the methanol extract of R. heudelotii,
possessing highest phenolic andflavonoid content, was not identified as the most potent metal chelator. FromTable 2, it was shown that the water extract (69.69 mg ethylenediaminetetraacetat equivalent (EDTAE)/g) of R. heudelotii seed was the most active chelating agent. Likewise, among the stem bark extracts, the water extract (58.78 mg EDTAE/g) shown pronounced metal chelating activity compared to the ethyl acetate (19.17 mg EDTAE/g) and methanol (26.67 mg EDTAE/g) extracts. The role of transition metal, such as iron, in oxidative stress has been extensively reported, through its involvement in the Fenton reaction generating hydroxyl radicals which favours oxidative stress.
In addition to study on antioxidant properties, the inhibitory ac-tivity in vitro of the different extracts of R. heudelotii seeds and stem bark on α-amylase, α-glucosidase, tyrosinase, acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) was investigated. As shown in Table 3, a low inhibitory action (0.16–0.77 mmol acarbose aquivalent (ACAE)/g) was recorded againstα-amylase. Highest inhibition against α-glucosidase was recorded for the ethyl acetate (1.26 mmol ACAE/g) and methanol (1.25 mmol ACAE/g) extracts of R. heudelotii seeds. The water extracts of both R. heudelotii seeds and stem bark showed not inhibitory activity againstα-glucosidase. These two enzymes are key targets in the management of diabetes type II which is characterized by elevated post prandial glucose level. The prevalence of diabetes type II, termed as“the epidemic of modern age”, is steadily increasing in almost every country, affecting approximately 422 million individuals world-wide (Roglic, 2016). The search for new drugs has been partly fuelled by the side effects (such as abdominal discomforts and flatulence) of currently used medications as acarbose, miglitol, and voglibose (Zengin et al., 2015).α-Amylase and α-glucosidase remain the two main targets in the management of diabetes type II. Being carbohydrate hydrolyzing enzymes, they hydrolyse ingested polysaccharides producing mono-saccharides in the form of glucose available for absorption, thereby increasing blood glucose level. The effect of R. heudelotii extracts on tyrosinase was presented inTable 3. Tyrosinase has been extensively studied for the development of depigmenting products. Indeed, tyr-osinase is key regulatory enzyme responsible for the synthesis of mel-anin, a pigment protecting the human body from harmful UV lights (Mukherjee et al., 2018). However, overproduction of melanin has been related to epidermal hyperpigmentation conditions, such as age spots, melasma and freckles. Additionally, it has been shown that tyrosinase is related to brain neuromelanin formation and thesefindings support the involvement of tyrosinase in Parkinson's disease (Wang, Wang, Xia, Sui, & Si, 2019). In this study, it was reported that the ethyl acetate (150.34 mg kojic acid equivalent (KAE)/g) and methanol (153.12 mg KAE/g) extracts of R. heudelotii stem bark showed pronounced in-hibitory action against tyrosinase. The ethyl acetate (130.30 mg KAE/g) and methanol (135.29 mg KAE/g) extracts of R. heudelotii seed were significantly (p < 0.05) less active that the equivalent stem bark ex-tracts. The water extracts of both R. heudelotii stem bark (68.29 mg KAE/g) and seed (88.27 mg KAE/g) were least active against tyrosinase. Apart from Parkinson’s disease, another neurodegenerative disease af-fecting the elderly portion of the global population, is Alzheimer’s disease. This condition is characterized by the progressive decline of Table 1
Total bioactive components of Ricinodendron heudelotii extracts*.
Parts/Solvents Total phenolic content (mg GAE/g)
Totalflavonoid content (mg RE/g) Seeds-EA 6.99 ± 0.80e 0.21 ± 0.03d Seeds-MeOH 14.37 ± 0.28d 0.12 ± 0.03d Seeds-Water 16.19 ± 0.27d 0.13 ± 0.05d Stem Barks-EA 128.34 ± 2.13c 5.03 ± 0.02c Stem Barks-MeOH 194.59 ± 4.17a 8.12 ± 0.20a Stem Barks-Water 150.284 ± 1.79b 6.20 ± 0.21b
* Values expressed are means ± S.D. of three parallel measurements. EA: Ethyl acetate; MeOH: Methanol; GAE: Gallic acid equivalent; RE: Rutin equivalent. Different letters on the same columns indicate a significant differ-ence (p < 0.05).
Table 2
Antioxidant properties of Ricinodendron heudelotii extracts.*
Parts/Solvents DPPH (mg TE/g) ABTS (mg TE/g) CUPRAC (mg TE/g) FRAP (mg TE/g) Phosphomolybdenum (mmol TE/g) Metal chelating (mg EDTAE/g) Seeds-EA 5.31 ± 0.83d 47.77 ± 3.47e 38.73 ± 0.86d 16.59 ± 0.18e 0.26 ± 0.03c 26.28 ± 1.10c Seeds-MeOH 3.19 ± 0.13d 104.37 ± 0.98d 47.85 ± 2.06d 17.64 ± 0.45e 0.85 ± 0.08c 15.65 ± 1.52d Seeds-Water 10.32 ± 1.53d 169.35 ± 16.79c 52.03 ± 0.45d 40.02 ± 0.47d 0.51 ± 0.04c 69.69 ± 0.19a Stem Barks-EA 407.02 ± 2.80b 714.10 ± 16.04b 678.62 ± 23.85c 448.88 ± 13.46c 3.03 ± 0.44b 19.17 ± 1.54d Stem Barks-MeOH 487.30 ± 1.56a 1043.42 ± 35.08a 1230.96 ± 17.94a 752.85 ± 1.28a 3.86 ± 0.28a 26.67 ± 2.27c Stem Barks-Water 360.573 ± 17.15c 737.91 ± 8.04b 823.57 ± 8.09b 509.03 ± 5.81b 2.70 ± 0.11b 58.78 ± 1.18b
* Values expressed are means ± S.D. of three parallel measurements; EA: Ethyl acetate; MeOH: Methanol; ABTS: 2,2 ′-azino-bis(3-ethylbenzothiazoline-6-sul-phonic acid; CUPRAC: cupric reducing antioxidant capacity; DPPH: 2,2-diphenyl-1-picrylhydrazyl; FRAP: ferric reducing antioxidant power; TE: Trolox equivalent; EDTAE: EDTA equivalent. Different letters on the same columns indicate a significant difference (p < 0.05).
cognitive and memory function and is estimated to affect 35 million individuals worldwide (Dinda, Dinda, Kulsi, Chakraborty, & Dinda, 2019). Cholinesterase enzymes have been targeted for the front-line management of Alzheimer’s disease. In the present study, the inhibitory action of R. heudelotii extracts on AChE and BChE was reported (Table 3). The role of AChE in neurotransmission regulation and thus in Alzheimer’s disease is long-established and well-defined. During normal neurotransmission, acetyl choline, released from the pre-synaptic neuron into the synaptic cleft, binds to acetyl choline receptors on the post-synaptic membrane triggering the relay of the signal from the nerve and the signal transmission is terminated by AChE, which hy-drolyses acetyl choline at the post-synaptic membrane (Masondo, Stafford, Aremu, & Makunga, 2019). Based on the cholinergic deficit
hypothesis, the inhibition of AChE enhances neurotransmission in Alzheimer’s disease patients. In the late stages of Alzheimer’s disease, AChE activity has been shown to decrease to 85%, while BChE in-creased up to two-fold, becoming the predominant cholinesterase en-zyme in the brain (Omar, Scott, Hamlin, & Obied, 2017). However, the exact physiological mechanism of BChE in Alzheimer’s disease remains unclear. From Table 3, it was observed that ethyl acetate extract (4.55 mg galantamine equivalent (GALAE)/g) of R. heudelotii seed and methanol extract (4.48 mg GALAE/g) of R. heudelotii stem bark were the most potent inhibitor of AChE. Strong BChE inhibition was recorded for the ethyl acetate extract (4.72 mg GALAE/g) of R. heudelotii seed. The water extracts of R. heudelotii seed and stem bark were poor in-hibitors of AChE and BChE.
Fig. 1. Pearson’s linear correlation considering TPC and TFC values together with each biological activities.
Table 3
Enzyme inhibitory effects of Ricinodendron heudelotii extracts.*
Parts/Solvents AChE (mg GALAE/g) BChE (mg GALAE/g) Tyrosinase (mg KAE/g) Alpha-Amylase (mmol ACAE/g) Alpha-Glucosidase (mmol ACAE/g) Seeds-EA 4.55 ± 0.01a 4.72 ± 0.16a 130.30 ± 0.33c 0.42 ± 0.04c 1.26 ± 0.00a Seeds-MeOH 3.77 ± 0.11c 3.61 ± 0.11c 135.29 ± 0.68b 0.55 ± 0.02b 1.25 ± 0.00a Seeds-Water 1.01 ± 0.02d 2.20 ± 0.11d 68.29 ± 3.48e 0.16 ± 0.00e na Stem Barks-EA 4.27 ± 0.02b 4.25 ± 0.07b 150.34 ± 0.12a 0.49 ± 0.05bc 1.14 ± 0.01b Stem Barks-MeOH 4.48 ± 0.02a 4.43 ± 0.04b 153.12 ± 0.57a 0.77 ± 0.02a na Stem Barks-Water 1.03 ± 0.10d na 88.27 ± 0.96d 0.24 ± 0.01d na
* Values expressed are means ± S.D. of three parallel measurements; AChE: acetylcholinesterase; BChE: butyrylcholinesterase; GALAE: Galantamine equivalent; KAE: Kojic acid equivalent; ACAE: Acarbose equivalent; na: not active. Different letters on the same columns indicate a significant difference (p < 0.05).
In order to assess the chemical composition of the two plant mate-rials, methanol extracts were chosen due to the ability of methanol to dissolve both the hydrophilic and lipophilic constituents. Furthermore in the light of bioassays MeOH extract present most promising results. Thus the stem bark and seeds extracts were used to perform more de-tailed phytochemical investigations using LC-DAD-MS and NMR mea-surements.
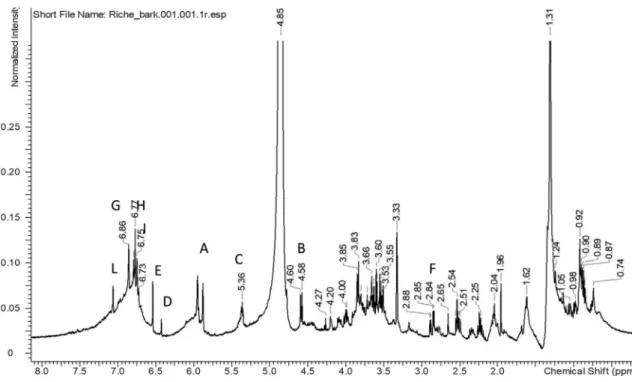
1H NMR spectrum of R. heudelotii bark extract was characterized by
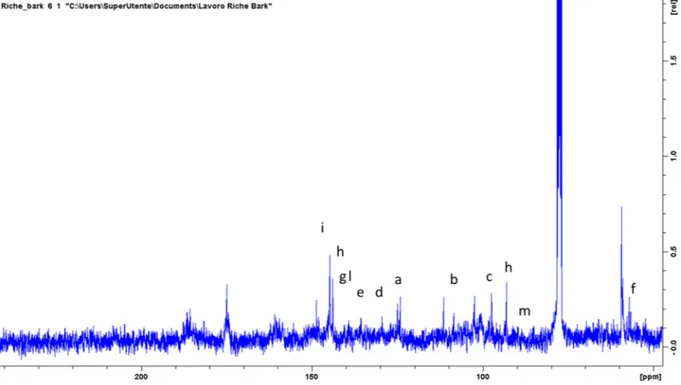
significant signals in the aromatic region suggesting the presence of phenolic derivatives. Also signals due to sugar portion are clearly visible in the spectrum region in the range ofδ 3.00–5.00 ppm as well as numerous aliphatic proton signals are detected in the most shielded region of the spectrum. Tentative assignments of some of the most in-tense signals were obtained by combining the data obtained by het-eronuclear 2D experiments (HSQC-DEPT and HMBC) as well as homonuclear coupling revealed by COSY. Exemplificative spectra are reported inFigs. 2–4.
The analysis of spectra allowed the identification of at least three different constituents as summarised inTable 4. Most abundant com-pounds appear to be gallocatechin gallate, gallocatechin, catechin and gallic acid moieties, with the presence of sugar residues (Fig. 5).
In order to increase the identification of constituents in R. heudelotii extracts, HPLC-DAD-ESI-MSnwas performed. Several peaks were
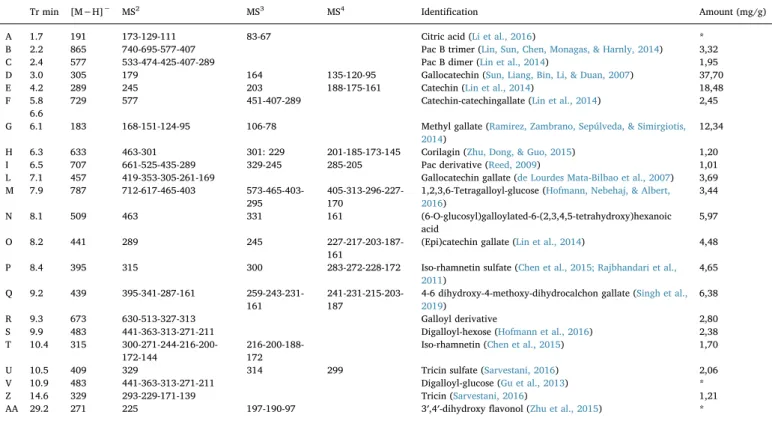
de-tected (Fig. 6) and fragmentation scheme were ascribable to catechin and gallocatechin derivatives as observed by NMR. Nevertheless, other classes of constituents were identified, namely procyanidin oligomers and galloyl derivatives of sugars. Identified compounds and their amount in the extract were summarised inTable 5.
1H NMR spectrum of R. heudelotii seed extract sample was obtained
in deuterated methanol and was characterized by signals supporting the presence of fatty acid and acylglycerol derivatives: spectra are reported inFig. 7. As observed inTable 6, signals ascribable to the presence of mono, diglyceride and triglyceride derivatives were present. HPLC-DAD-MS confirms the obtained data and only traces of phenolic Fig. 3.13C NMR spectrum of the stem bark, the signals used for compounds assignment are indicated with same letters as inTable 4.
compounds were detectable but were not quantified. Main ion peaks were identified as lipid derivatives and main peaks that were tenta-tively identified are reported inTable 7.
Composition of the bark extract revealed the presence of phenolic
compounds such as gallic acid and catechin derivatives also in high amount and thesefindings can at least justify the observed activities. Previously published studied revealed a positive link between con-sumption of catechin and gallocatechin gallate, a predominant Table 4
NMR assignments of the main constituents present in the Ricinodendron heudelotii stem bark (Davis, Cai, Davies, & Lewis, 1996; Le Gall, Colquhoun, & Defernez, 2004; Yuan et al., 2014).
Compounds H HSQC HMBC and notes
Gallocatechin Gallate
A H-6/8 5.95–5.96 a 94.1/96.0 155.5 (C-8a), 101.0 (C-4a), 94, 96 (C-6/8) B H-2 4.58 d J = 7,46 b 80.2 27.7 (C-4), 66.5 (C-3), 130.0 (C-1)
C H-3 5.38 m c 69.3 cosy copuling with 4.58
D H-2′/6′ 6.42 d 105.3 132.0 (C-4) E H-2″/6″ 7.04 e 108.7 167 (carbonyl function), 138.1 (C-4″) F H-4 2.80/2.76 dd f 27.7 99.0 (C-4a), 80.2 (C-2), 66.4 (C-3) Catechin G H-2′ 6.86 d J = 1.71 g 114.1 144.2/145.0 (C-4′/C-3′), 80.7 (C-2) H H-5′ 6.78 J = 8.07 h 113.8 129.8 (C-1′) I H-6′ 6.74 J = 1.7, 8.07 I 117.9 144.2/145.0 (C-4′/C-3′), 80.7 (C-2) Gallic acid derivatives
L H-2 7.05 s l 108.0
M OCH3 3.85 m 55.6 145.0
Gallocatechin
N H-3 3.97 h 67.3
Letters indicate the peaks in the reported spectra of 1H and 13C.
8 5 7 6 2 3
O
1 4 1' 6' 2' 5' 3' 4'O
O
1'' 2'' 6'' 3'' 5'' 4''O
H
O
H
OH
OH
OH
OH
O
H
R
O
OH
OH
OH
OH
O
H
R
O
H
O
O
OH
OH
Fig. 5. structure of the most abundant constituents identified by NMR.
flavonoid in tea with the increased of human plasma antioxidant ca-pacity (Higdon & Frei, 2003). Other authors, (Grzesik, Naparło, Bartosz, & Sadowska-Bartosz, 2018) reported that catechin and gallocatechin gallate has a remarkable antioxidant activities in FRAP and ABTS as-says. Considering the structure of these phenolics, previous data re-vealed that the reactivity in the FRAP assay of is related to the presence of free ortho-hydroxyl group in the A ring. Similarly (Lu, Nie, Belton,
Tang, & Zhao, 2006) studied structure–activity relationship analysis of antioxidant ability and neuroprotective effect of gallic acid derivatives suggesting that antioxidant efficiency was closely related with the presence of the phenolic hydroxyl groups at para-position to the car-boxylic group. In addition to their antioxidant properties, several stu-dies reported inhibitory effects of such compounds on some key en-zyme. Indeed natural antioxidants have gained increase interest owing Table 5
Identification and quantification of phytochemicals present in the Ricinodendron heudelotii stem bark.
Tr min [M−H]− MS2 MS3 MS4 Identification Amount (mg/g)
A 1.7 191 173-129-111 83-67 Citric acid (Li et al., 2016) * B 2.2 865 740-695-577-407 Pac B trimer (Lin, Sun, Chen, Monagas, & Harnly, 2014) 3,32 C 2.4 577 533-474-425-407-289 Pac B dimer (Lin et al., 2014) 1,95 D 3.0 305 179 164 135-120-95 Gallocatechin (Sun, Liang, Bin, Li, & Duan, 2007) 37,70 E 4.2 289 245 203 188-175-161 Catechin (Lin et al., 2014) 18,48 F 5.8
6.6
729 577 451-407-289 Catechin-catechingallate (Lin et al., 2014) 2,45
G 6.1 183 168-151-124-95 106-78 Methyl gallate (Ramirez, Zambrano, Sepúlveda, & Simirgiotis, 2014)
12,34
H 6.3 633 463-301 301: 229 201-185-173-145 Corilagin (Zhu, Dong, & Guo, 2015) 1,20 I 6.5 707 661-525-435-289 329-245 285-205 Pac derivative (Reed, 2009) 1,01 L 7.1 457 419-353-305-261-169 Gallocatechin gallate (de Lourdes Mata-Bilbao et al., 2007) 3,69 M 7.9 787 712-617-465-403
573-465-403-295
405-313-296-227-170
1,2,3,6-Tetragalloyl-glucose (Hofmann, Nebehaj, & Albert, 2016) 3,44 N 8.1 509 463 331 161 (6-O-glucosyl)galloylated-6-(2,3,4,5-tetrahydroxy)hexanoic acid 5,97 O 8.2 441 289 245 227-217-203-187-161
(Epi)catechin gallate (Lin et al., 2014) 4,48
P 8.4 395 315 300 283-272-228-172 Iso-rhamnetin sulfate (Chen et al., 2015; Rajbhandari et al., 2011) 4,65 Q 9.2 439 395-341-287-161 259-243-231-161 241-231-215-203-187
4-6 dihydroxy-4-methoxy-dihydrocalchon gallate (Singh et al., 2019)
6,38
R 9.3 673 630-513-327-313 Galloyl derivative 2,80
S 9.9 483 441-363-313-271-211 Digalloyl-hexose (Hofmann et al., 2016) 2,38 T 10.4 315
300-271-244-216-200-172-144
216-200-188-172
Iso-rhamnetin (Chen et al., 2015) 1,70
U 10.5 409 329 314 299 Tricin sulfate (Sarvestani, 2016) 2,06 V 10.9 483 441-363-313-271-211 Digalloyl-glucose (Gu et al., 2013) *
Z 14.6 329 293-229-171-139 Tricin (Sarvestani, 2016) 1,21
AA 29.2 271 225 197-190-97 3′,4′-dihydroxy flavonol (Zhu et al., 2015) *
*Not quantified.
to the role of reactive oxygen species in the pathogenesis of multiple diseases, such as Alzheimer’s disease, diabetes type II, cancer, and cardiovascular complications (Liguori et al., 2018). In a previous study, (Rasoolijazi, Joghataie, Roghani, & Nobakht, 2007) previously eval-uated the effect of gallocatechin gallate on behavioral abnormalities in a model of Alzheimer’s disease in rat. They obtained an attenuation of the spatial memory and the psychomotor behaviour in rat Alzheimer’s disease model upon intraperitoneal injection of gallocatechin gallate. (Srividhya, Gayathri, & Kalaiselvi, 2012) obtained a good docking complex between the ligand (gallocatechin gallate) and AChE. In a recent study, (He, Xu, Yang, & Wang, 2018) demonstrated the protec-tion ability of gallocatechin gallate against oxidative stress-induced neurodegeneration and the strong capacity to reduce ROS production. Gallocatechin gallate acted to be an reducing agent postprandial blood glucose levels in vivo (Forester, Gu, & Lambert, 2012). In fact the au-thors exploring the mechanisms of the green tea phenolic compound gallocatechin gallate action on the digestion and postprandial effects of oral carbohydrates in mice reported that gallocatechin gallate caused a blood glucose levels decreased in starch-fed mice arising from inhibi-tion of pancreatic alpha-amylase activity.
The present work focused on the evaluation of the antioxidant and enzyme inhibitory properties of three extracts of R. heudelotii seed and stem bark. As observed in the univariate statistical approach, generally a significant difference was observed in the extracts in terms of anti-oxidant and enzyme inhibitory activities. In order to determine the source of variation of the obtained data, multivariate analysis, espe-cially PLS discriminant analysis, was performed. Partial Least Squares Discriminant Analysis is an exploratory multivariate regression model attempting tofind the optimal linear combinations of variables which best discriminate the sample groups. It is used to obtain pertinent
information contained in two data sets (X = predictor quantitative matrix, Y = the categorical response matrix) and shows a very sa-tisfying predictive performance (Barker & Rayens, 2003). Based on PLS-DA statistical approach, the hypothesis that plant part (seed, and stem bark) and solvents (ethyl acetate, methanol, and water) had significant effect on enzyme inhibitory potential and antioxidant activities tested. Results of supervised multivariate analysis were shown inFig. 8. PLS-DA model allowed a clear differentiation between seed and stem bark as well as solvents (Fig. 8A & C). In the separation, stem bark and water were on the negative side of thefirst axis respectively while seeds and both ethyl acetate and methanol were on the positive side. The loadings plot showed the variables characterizing each obtained samples classes (Fig. 8G & I); more specifically, AChE, amylase, tyrosinase and anti-oxidant activities recorded the highest value in stem bark extracts in contrast to seeds extracts that possessed the best activities of glucosi-dase and BChE enzyme inhibition as well as metal chelating ability. For the second factor, water extracts exhibited the highest content of metal chelating ability whereas, methanol and ethyl acetate exhibited the highest capacity in the other assays. On the other hand, the variable importance for projection was explored; in this study, variables with variable important in projection (VIP) values was higher than 1 were considered to contribute significantly for the discrimination. As shown inFig. 8B&Dfive biological activities essentially antioxidant activities (ABTS, DPPH, FRAP, CUPRAC and phosphomolybdenum (PPBD)) were accountable for most of the difference between the plant part. Also, the performance values (classification error rate based on the prediction distance, such as, the centroids distance and 5-fold cross-validation (CV) repeated times of PLS-DA models were given inFig. 8F&H. Based on the data, the studied biological activities did not allow di fferentia-tion between methanol and ethyl acetate. To provide certain dis-crimination, PLS analysis was done. One and two principal components in PLS-DA were noted as a better accuracy.
The extracts were also classified based on the interaction of the plant part and solvents. Therefore, unsupervised PCA and clustered image map using Euclidean as similarity and ward as linkage rule were carried out. The factorial plane of PCA formed by thefirst two com-ponents, that summarized 93.95% of the total variability, revealed differences between samples (Fig. 8J). In the heat-map built on thefirst two component of PCA, three main clusters that included the water extracts as one group, the methanol and ethyl acetate extracts (stem bark) as a second group and the methanol and ethyl acetate extracts (seeds) as the third group could be identified (Fig. 8L).
4. Conclusion
The present study investigated antioxidant activity using different tests and enzyme inhibitory action of different extracts obtained from R. heudelotii seeds and stem bark. Results indicate that, the stem bark extracts possessed higher phenolic andflavonoid contents as expected. Table 6
NMR assignments of the main constituents present in the Ricinodendron heudelotii seeds (Davis et al., 1996).
Signals H Tentative assignment A 0.93 t (overlapped)
0.86 0.98
Fatty acid terminal methyl group
B 1.30–1.35 m Aliphatic CH2 of fatty acid C 1.63 m CH2 of fatty acid
D 2.03 m CH2 of fatty acid vicinal to one double bond E 2.28–2.35 CH2 of fatty acid vicinal to carboxy function F 2.79 CH2 vicinal to two double bond
G 3.60–3.65 Glycoerol protons in 1 Monoacyl derivatives H 4.12–4.26 Glycerol protons in Diacyl derivatives I 4.38–5.26 Glycerol signals in Trygliceride
L 5.36 Olefinic proton of double bonds of unsaturated fatty acids
M 6.00–7.50 minor peaks suggesting the presence of phenolic constituents
Table 7
Identification of phytochemicals present in the Ricinodendron heudelotii seed extract (based onBlumhorst, Venkitasubramanian, & Collison, 2011).
Tr min [M−H]− MS2 MS3 MS4 Identification
A 1.7 341 255-179-143 Caffeic acid derivative
C 10.3 187 125(100)-169 97 95-93-80-69-67 Gallic acid monohydrate (Kang, Price, Ashton, Tapsell, & Johnson, 2016) E 14.5 329 171 153-127-125-97 Hydroxy trimetoxyflavanone (Reed, 2009)
F 15.9 329 309-292-243-211-171-129 Hydroxy trimetoxyflavanone (Reed, 2009) Tr [M+H]+ 9.5 285 247 229 209 229 Margaric acid 12.7 325 307 305 289 233 220 205 160 148 Heptadecanoic 9.9 341 323 285 271 Stearic acid 14.2 367 349 333 293 275 Gadoleic acid 11.7 369 351 333 283 213 Arachicic acid 13.5 425 407 385 274 Lignoceric acid 395 Fucosterol
In accordance with studies reporting higher antioxidant activity of phenolic/flavonoid rich extracts, the stem bark extracts showed higher antioxidant activity for radical scavenging and reducing potential, particularly the methanol stem bark extract. High metal chelating ac-tivity was measured for the water extracts, especially the seed water extract. Regarding enzyme inhibition, all extracts exhibited low activity againstα-amylase, while the methanol and ethyl acetate extracts of R. heudelotii seeds showed highest inhibition againstα-glucosidase. The ethyl acetate and methanol extracts of R. heudelotii stem bark were potent inhibitors of tyrosinase. AChE and BChE were effectively in-hibited by ethyl acetate seed extract. Chemical investigation on R. heudelotii seeds revealed that, as expected, the extract was composed mainly by fatty acid derivatives, while bark can be considered a valu-able source of gallocatechin and catechin derivatives. This study has established valuable baseline data on the biological activity of the seed and stem bark of R. heudelotii. It is to be noted that there was a positive relationship between high phenolic/flavonoid contents and antioxidant activity. However, high phenolic/flavonoid contents were not related to enzyme inhibitory capacity. It can be argued that the observed enzyme inhibitory action was related to the presence of secondary metabolites which could interact with the enzyme molecule, thereby hindering substrate binding. Multivariate data analysis allowed to establish dif-ferences and similarities beneath several samples. This approach can suggest and indicate potential differences of the part of the plant for potential uses due to the different response in the considered bioassays. Thus this methodology considering a panel of relatively simple che-mical/enzymatic assays and the general chemical analysis can offer a system to show potential similarities or differences of several plant extracts as we showed for example for Ricinodendron heudelotii. CRediT authorship contribution statement
Stefania Sut: Conceptualization, Data curation, Investigation,
Writing - original draft.Stefano Dall’Acqua: Conceptualization, Data curation, Investigation, Writing - original draft. Kouadio Bene: Investigation, Methodology. Serena Barbon di Marco: Conceptualization, Data curation, Investigation, Writing - original draft. Kouadio Ibrahime Sinan: Conceptualization, Data curation, Methodology, Software. Mohamad Fawzi Mahomoodally: Conceptualization, Data curation, Formal analysis, Writing - original draft, Writing - review & editing.Marie Carene Nancy Picot-Allain: Writing - original draft. Gokhan Zengin: Conceptualization, Data curation, Formal analysis, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors declare that there is no conflict. References
Ashu Agbor, M., & Naidoo, S. (2015). Ethnomedicinal plants used by traditional healers to treat oral health problems in Cameroon. Evidence-based Complementary and Alternative Medicine: eCAM, 2015, 649832.
Assanvo, E. F., & Baruah, S. D. (2015). Synthesis and properties of Ricinodendron heudelotii oil based hybrid alkyd–acrylate latexes via miniemulsion polymerization. Progress in Organic Coatings, 86, 25–32.
Assanvo, E. F., Gogoi, P., Dolui, S. K., & Baruah, S. D. (2015). Synthesis, characterization, and performance characteristics of alkyd resins based on Ricinodendron heudelotii oil and their blending with epoxy resins. Industrial Crops and Products, 65, 293–302. Barker, M., & Rayens, W. (2003). Partial least squares for discrimination. Journal of
Chemometrics, 17(3), 166–173.
Blumhorst, M. R., Venkitasubramanian, P., & Collison, M. W. (2011). Direct determina-tion of glycidyl esters of fatty acids in vegetable oils by LC–MS. Journal of the American Oil Chemists' Society, 88(9), 1275–1283.
Chen, Y., Yu, H., Wu, H., Pan, Y., Wang, K., Jin, Y., & Zhang, C. (2015). Characterization and quantification by LC-MS/MS of the chemical components of the heating products of theflavonoids extract in Pollen Typhae for transformation rule exploration. Molecules, 20(10), 18352–18366.
Cragg, G. M., & Newman, D. (2013). Natural products: A continuing source of novel drug
Fig. 8. Multivariate statistical analysis on biological activities of Ricinodendron heudelotii (Baill.) Heckel. A&C: PLS-DA score plots. B&D: discriminant biological activities identified by Variable Important in Projection (VIP). E, F&H: validation of PLS-DA discriminant models by the classification error rate estimation and the Area under the curve evaluation. G&I: The loading plot displaying the obtained clusters in which the biological activity had a maximum level of activity. J&K: PCA score plot and percentage of variance explained for each component. L: Heat-map and HCA analyses of biological activities.
leads. Journal Biochimica et Biophysica Acta -General Subjects, 1830(6), 3670–3695. Davis, A. L., Cai, Y., Davies, A. P., & Lewis, J. (1996). 1H and 13C NMR assignments of
some green tea polyphenols. Journal Magnetic Resonance in Chemistry, 34(11), 887–890.
de Lourdes Mata-Bilbao, M., Andrés-Lacueva, C., Roura, E., Jáuregui, O., Torre, C., & Lamuela-Raventós, R. M. (2007). A new LC/MS/MS rapid and sensitive method for the determination of green tea catechins and their metabolites in biological samples. Journal of Agricultural and Food Chemistry, 55(22), 8857–8863.
Dinda, B., Dinda, M., Kulsi, G., Chakraborty, A., & Dinda, S. (2019). Therapeutic poten-tials of plant iridoids in Alzheimer's and Parkinson's diseases: A review. European Journal of Medicinal Chemistry, 169, 185–199.
Farasat, M., Khavari-Nejad, R.-A., Nabavi, S. M. B., & Namjooyan, F. (2014). Antioxidant activity, total phenolics andflavonoid contents of some edible green seaweeds from Northern Coasts of the Persian Gulf. Iranian Journal of Pharmaceutical Research, 13(1), 163–170.
Forester, S. C., Gu, Y., & Lambert, J. D. (2012). Inhibition of starch digestion by the green tea polyphenol,(−)-epigallocatechin-3-gallate. Molecular Nutrition & Food Research, 56(11), 1647–1654.
Grzesik, M., Naparło, K., Bartosz, G., & Sadowska-Bartosz, I. (2018). Antioxidant prop-erties of catechins: Comparison with other antioxidants. Food Chemistry, 241, 480–492.
Gu, D., Yang, Y., Bakri, M., Chen, Q., Xin, X., & Aisa, H. A. (2013). A LC/QTOF–MS/MS application to investigate chemical compositions in a fraction with protein tyrosine phosphatase 1B inhibitory activity from Rosa rugosaflowers. Phytochemical Analysis, 24(6), 661–670.
Harvey, A. L., Edrada-Ebel, R., & Quinn, R. (2015). The re-emergence of natural products for drug discovery in the genomics era. Nature Reviews Drug discovery, 14(2), 111. He, J., Xu, L., Yang, L., & Wang, X. (2018). Epigallocatechin gallate is the most effective
catechin against antioxidant stress via hydrogen peroxide and radical scavenging activity. Medical Science Monitor, 24, 8198.
Higdon, J. V., & Frei, B. (2003). Tea catechins and polyphenols: Health effects, meta-bolism, and antioxidant functions. Critical Reviews in Food Science and Nutrition, 43(1), 89–143.
Hofmann, T., Nebehaj, E., & Albert, L. (2016). Antioxidant properties and detailed polyphenol profiling of European hornbeam (Carpinus betulus L.) leaves by multiple antioxidant capacity assays and high-performance liquid chromatography/multistage electrospray mass spectrometry. Industrial Crops and Products, 87, 340–349. Kang, J., Price, W. E., Ashton, J., Tapsell, L. C., & Johnson, S. (2016). Identification and
characterization of phenolic compounds in hydromethanolic extracts of sorghum wholegrains by LC-ESI-MSn. Food Chemisty, 211, 215–226.
Kefayati, Z., Motamed, S. M., Shojaii, A., Noori, M., & Ghods, R. (2017). Antioxidant activity and phenolic andflavonoid contents of the extract and subfractions of Euphorbia splendida Mobayen. Pharmacognosy Research, 9(4), 362–365.
Khorasani Esmaeili, A., Mat Taha, R., Mohajer, S., & Banisalam, B. (2015). Antioxidant activity and total phenolic andflavonoid content of various solvent extracts from in vivo and in vitro grown Trifolium pratense L. (Red Clover). BioMed Research International, 2015, 11.
Kimbu, S. F., Keumedjio, F., Sondengam, L. B., & Connolly, J. D. (1991). Two dinordi-terpenoids from Ricinodendron heudelotii. Phytochemistry, 30(2), 619–621. Le Gall, G., Colquhoun, I. J., & Defernez, M. (2004). Metabolite profiling using 1H NMR
spectroscopy for quality assessment of green tea, Camellia sinensis (L.). Journal of Agricultural and Food Chemistry, 52(4), 692–700.
Li, S., Lin, Z., Jiang, H., Tong, L., Wang, H., & Chen, S. (2016). Rapid identification and assignation of the active ingredients in fufang banbianlian injection using HPLC-DAD-ESI-IT-TOF-MS. Journal of Chromatographic Science, 54(7), 1225–1237.
Liguori, I., Russo, G., Curcio, F., Bulli, G., Aran, L., Della-Morte, D., ... Abete, P. (2018). Oxidative stress, aging, and diseases. Clinical Interventions in Aging, 13, 757–772. Lin, L.-Z., Sun, J., Chen, P., Monagas, M. J., & Harnly, J. M. (2014). UHPLC-PDA-ESI/
HRMS n profiling method to identify and quantify oligomeric proanthocyanidins in plant products. Journal of Agricultural and Food Chemistry, 62(39), 9387–9400. Llorent-Martínez, E. J., Zengin, G., Fernández-de Córdova, M. L., Bender, O., Atalay, A.,
Ceylan, R., ... Guler, G. O. (2017). Traditionally used Lathyrus species: Phytochemical composition, antioxidant activity, enzyme inhibitory properties, cytotoxic effects, and in silico studies of L. czeczottianus and L. nissolia. Frontiers in Pharmacology, 8, 83. Lu, Z., Nie, G., Belton, P. S., Tang, H., & Zhao, B. (2006). Structure–activity relationship analysis of antioxidant ability and neuroprotective effect of gallic acid derivatives. Neurochemistry International, 48(4), 263–274.
Majolo, F., de Oliveira Becker Delwing, L. K., Marmitt, D. J., Bustamante-Filho, I. C., & Goettert, M. I. (2019). Medicinal plants and bioactive natural compounds for cancer treatment: Important advances for drug discovery. Phytochemistry Letters, 31, 196–207.
Masondo, N. A., Stafford, G. I., Aremu, A. O., & Makunga, N. P. (2019).
Acetylcholinesterase inhibitors from southern African plants: An overview of eth-nobotanical, pharmacological potential and phytochemical research including and beyond Alzheimer's disease treatment. South African Journal of Botany, 120, 39–64. Mollica, A., Zengin, G., Locatelli, M., Stefanucci, A., Macedonio, G., Bellagamba, G., ...
Ayileka, A. (2017). An assessment of the nutraceutical potential of Juglans regia L. leaf powder in diabetic rats. Food and Chemical Toxicology, 107, 554–564.
Momeni, J., Djialeu Ntchatchoua, W. P., Fadimatou, Akam, M. T., & Ngassoum, M. B.
(2010). Antioxidant activities of some cameroonian plants extracts used in the treatment of intestinal and infectious diseases. Indian Journal of Pharmaceutical Sciences, 72 (1), 140–144.
Mukherjee, P. K., Biswas, R., Sharma, A., Banerjee, S., Biswas, S., & Katiyar, C. K. (2018). Validation of medicinal herbs for anti-tyrosinase potential. Journal of Herbal Medicine, 14, 1–16.
Newman, D. J., & Cragg, G. M. J. J. (2016). Natural products as sources of new drugs from 1981 to 2014. Journal of Natural Products, 79(3), 629–661.
Ogbole, O. O., Segun, P. A., & Fasinu, P. S. (2018). Antimicrobial and antiprotozoal ac-tivities of twenty-four Nigerian medicinal plant extracts. South African Journal of Botany, 117, 240–246.
Omar, S. H., Scott, C. J., Hamlin, A. S., & Obied, H. K. (2017). The protective role of plant biophenols in mechanisms of Alzheimer's disease. The Journal of Nutritional Biochemistry, 47, 1–20.
Rajbhandari, R., Peng, N., Moore, R., Arabshahi, A., Wyss, J. M., Barnes, S., & Prasain, J. K. (2011). Determination of cranberry phenolic metabolites in rats by liquid chro-matography–tandem mass spectrometry. Journal of Agricultural and Food Chemistry, 59(12), 6682–6688.
Ramirez, J., Zambrano, R., Sepúlveda, B., & Simirgiotis, M. (2014). Antioxidant proper-ties and hyphenated HPLC-PDA-MS profiling of chilean Pica mango fruits (Mangifera indica L. cv. piqueño). Molecules, 19 (1), 438–458.
Rasoolijazi, H., Joghataie, M. T., Roghani, M., & Nobakht, M. (2007). The beneficial effect of (-)-epigallocatechin-3-gallate in an experimental model of Alzheimer’s disease in rat: A behavioral analysis. Iranian Biomedical Journal, 11(4), 237–243.
Reed, K. A. (2009). Identification of phenolic compounds from peanut skin using HPLC-MSn. Virginia: Tech.
Roglic, G. (2016). WHO Global report on diabetes: A summary. International Journal of Noncommunicable Diseases 1(1), 3.
Sarvestani, A. K. (2016). Metabolomic profiling of lignocellulosic biomass process streams. Michigan State University. Chemistry.
Singh, D., Siew, Y.-Y., Chong, T.-I., Yew, H.-C., Ho, S. S.-W., Lim, C. S. E.-S., ... Koh, H.-L. (2019). Identification of phytoconstituents in Leea indica (Burm. F.) Merr. leaves by high performance liquid chromatography micro time-of-flight mass spectrometry. Molecules, 24(4), 714.
Srividhya, R., Gayathri, R., & Kalaiselvi, P. (2012). Impact of epigallo catechin-3-gallate on acetylcholine-acetylcholine esterase cycle in aged rat brain. Neurochemistry International, 60(5), 517–522.
Sun, J., Liang, F., Bin, Y., Li, P., & Duan, C. (2007). Screening non-colored phenolics in red wines using liquid chromatography/ultraviolet and mass spectrometry/mass spec-trometry libraries. Molecules, 12(3), 679–693.
Tchankou Leudeu, B. C., Tchiégang, C., Barbé, F., Nicolas, B., & Guéant, J.-L. (2009). Ricinodendron heutelotii (Bail.) or Tetracarpidium conophorum Müll. oils fed to male rats lower blood lipids. Nutrition Research, 29 (7), 503–509.
Uysal, S., Zengin, G., Locatelli, M., Bahadori, M. B., Mocan, A., Bellagamba, G., ... Aktumsek, A. (2017). Cytotoxic and enzyme inhibitory potential of two Potentilla species (P. speciosa L. and P. reptans Willd.) and their chemical composition. Frontiers in Pharmacology, 8, 290.
Vyas, S., Kothari, S. L., & Kachhwaha, S. (2019). Nootropic medicinal plants: Therapeutic alternatives for Alzheimer’s disease. Journal of Herbal Medicine, 100291.
Wang, R., Wang, G., Xia, Y., Sui, W., & Si, C. (2019). Functionality study of lignin as a tyrosinase inhibitor: Influence of lignin heterogeneity on anti-tyrosinase activity. International Journal of Biological Macromolecules, 128, 107–113.
Yuan, Y., Song, Y., Jing, W., Wang, Y., Yang, X., & Liu, D. (2014). Simultaneous de-termination of caffeine, gallic acid, theanine,(−)-epigallocatechin and (−)-epi-gallocatechin-3-gallate in green tea using quantitative 1H NMR spectroscopy. Journal of Analytical Methods, 6(3), 907–914.
Zengin, G., Bulut, G., Mollica, A., Picot-Allain, C. M. N., & Mahomoodally, M. F. (2018). In vitro and in silico evaluation of Centaurea saligna (K. Koch) Wagenitz—An endemic folk medicinal plant. Computational Biology and Chemistry, 73, 120–126.
Zengin, G., Ceylan, R., Katanić, J., Mollica, A., Aktumsek, A., Boroja, T., ... Aumeeruddy-Elalfi, Z. (2017). Combining in vitro, in vivo and in silico approaches to evaluate nutraceutical potentials and chemicalfingerprints of Moltkia aurea and Moltkia coerulea. Food and Chemical Toxicology, 107, 540–553.
Zengin, G., Guler, G. O., Aktumsek, A., Ceylan, R., Picot, C. M. N., & Mahomoodally, M. F. (2015). Enzyme inhibitory properties, antioxidant activities, and phytochemical profile of three medicinal plants from Turkey. Advances in Pharmacological Sciences, 2015, 8.
Zengin, G., Locatelli, M., Stefanucci, A., Macedonio, G., Novellino, E., Mirzaie, S., Dvorácskó, S., Carradori, S., Brunetti, L., & Orlando, G. (2017). Chemical char-acterization, antioxidant properties, anti-inflammatory activity, and enzyme inhibi-tion of Ipomoea batatas L. leaf extracts. Internainhibi-tional Journal of Food Properties, 20 (sup2), 1907–1919.
Zhu, M., Dong, X., & Guo, M. (2015). Phenolic profiling of Duchesnea indica combining macroporous resin chromatography (MRC) with HPLC-ESI-MS/MS and ESI-IT-MS. Molecules, 20(12), 22463–22475.
Zovko Končić, M., & Bljajić, K. (2019). Chapter 42 - traditional herbal products used for the management of diabetes in croatia: Linking traditional use with α-glucosidase-inhibitory activity. In R. R. Watson, & V. R. Preedy (Eds.). Bioactive food as dietary interventions for diabetes (pp. 647–664). (2nd ed.). Academic Press.